Liječenje preoperativne anemije željezom
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Prospective randomised blinded placebo‐control trial. | |
| Participants | Pre‐operative patients undergoing surgery for colorectal cancer (n = 60; note only 18 patients anaemic). | |
| Interventions | IV iron sucrose 600 mg in 2 doses versus placebo. | |
| Outcomes | Transfusion rates and amount of blood transfused, recruitment and admission haemoglobin. | |
| Notes | Study has only 9 anaemic patients in each arm of whom many were not iron deficient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocated to either the treatment (iron) group or a placebo group, based on a computer‐generated randomisation sequence provided by the Research and Development Support Unit. To ensure equal numbers of anaemic patients in each treatment group, randomisation was stratified according to pre‐recruitment Hb status: normal (Hb level at least 13.5 g/dL in males and 12/5 g/dL in females), anaemic, or unknown (no test within 2 months of recruitment). Block randomisation was used to ensure similar numbers in each group for each subset." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation codes were sealed in sequentially numbered opaque envelopes which were secured within a locked store room in a dedicated research unit." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Although the investigator administering the infusion was not blinded to the treatment group, this was concealed from the patient by using an opaque sheath to cover the drug‐giving set." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The chief investigator and clinicians involved in perioperative care also remained blinded to the treatment group for the duration of the trial." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: It appears there was no loss to follow up among people with anaemia. |
| Selective reporting (reporting bias) | Unclear risk | Comment: None identified. |
| Other bias | Unclear risk | Comment: None identified. |
| Methods | Prospective randomised control trial, open label. | |
| Participants | Anaemic pre‐operative patients with menorrhagia who were due to undergo surgery (n = 76; note only 56 patients > 80% compliance are included in analysis, Hb < 90 g/L). | |
| Interventions | IV iron sucrose (dose according to Ganzoni’s formula for cumulative iron deficit) versus oral iron succinylate (dose 80 mg per day for 3 weeks). | |
| Outcomes | Recruitment and admission haemoglobin. | |
| Notes | The study took place between December 2005 and January 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation table... [to] randomly assign patients." |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation was determined by one of the authors not directly involved in patient care." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: Open label study, no blinding but unlikely to influence the change in haemoglobin. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: No blinding but objective measurement of haemoglobin unlikely to be influenced. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Participants who had > 80% compliance were included in the analysis" Comment: Not analysed on intention‐to‐treat basis. Important because oral iron has reportedly poor tolerance and therefore poor compliance. |
| Selective reporting (reporting bias) | Unclear risk | Comment: None identified. |
| Other bias | Unclear risk | Comment: None identified. |
| Methods | Prospective randomised control trial. | |
| Participants | Pre‐operative patients undergoing surgery for colorectal cancer (n = 49; note only 20 patients anaemic). | |
| Interventions | Oral ferrous sulphate 200 mg TDS versus standard care. | |
| Outcomes | Transfusion rates and amount of blood transfused, pre‐treatment and pre‐operative haemoglobin. | |
| Notes | Did not exclude non‐anaemic patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study does not explain how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomised (by telephone to a distant centre)." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The clinical team (surgeons, nurses, anaesthetists) were |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The collection of data was performed by a research fellow not involved in the direct care of the patient." |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Two patients from each group were deemed unsuitable for resective surgery at Comment: No incomplete outcome data was reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: None identified. |
| Other bias | Unclear risk | Comment: None identified. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomised controlled trial. All anaemic patients given iron therapy with no control arm. Non‐anaemic patients were randomised but since they did not have pre‐operative anaemia this study is excluded from the review. | |
| Study included all patients, anaemic and non‐anaemic, and has not stratified results to allow analysis of only those patients with pre‐operative anaemia. | |
| Study specifically excluded patients with previous anaemia therefore no patients in this study had pre‐operative anaemia. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who received a blood transfusion Show forest plot | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| Analysis 1.1  Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 1 Proportion of patients who received a blood transfusion. | ||||
| 2 Haemoglobin levels pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 2 Haemoglobin levels pre‐treatment (g/dL). | ||||
| 3 Haemoglobin levels at end of treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 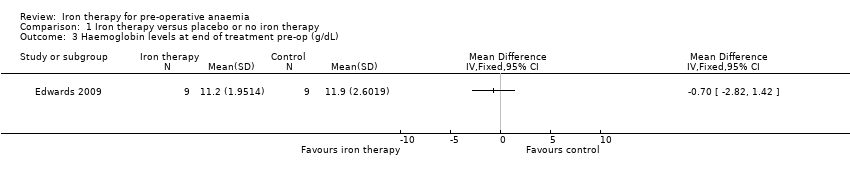 Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 3 Haemoglobin levels at end of treatment pre‐op (g/dL). | ||||
| 4 Haemoglobin levels after treatment post‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 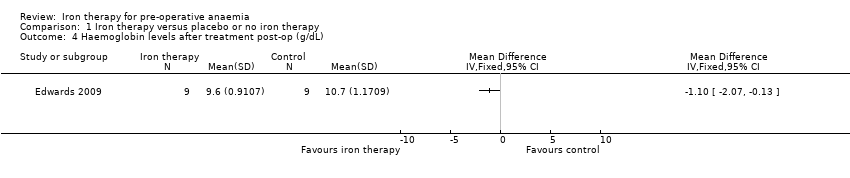 Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 4 Haemoglobin levels after treatment post‐op (g/dL). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Haemoglobin level pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 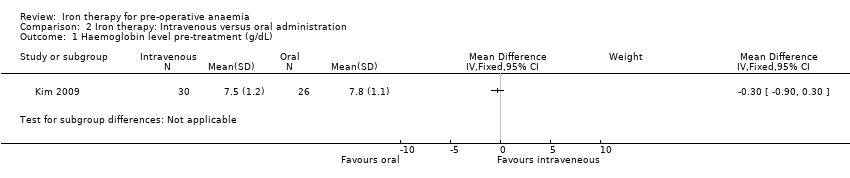 Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 1 Haemoglobin level pre‐treatment (g/dL). | ||||
| 2 Haemoglobin level post‐treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 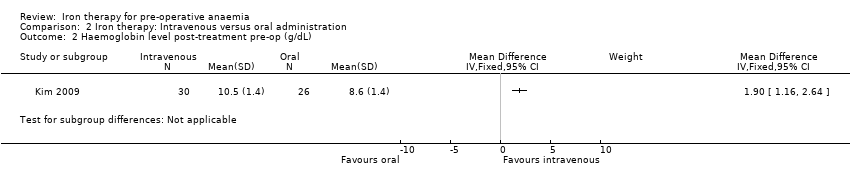 Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 2 Haemoglobin level post‐treatment pre‐op (g/dL). | ||||
| 3 Ferritin level pre‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 3 Ferritin level pre‐treatment (ɥg/L). | ||||
| 4 Ferritin level post‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 4 Ferritin level post‐treatment (ɥg/L). | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Three studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 1 Proportion of patients who received a blood transfusion.

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 2 Haemoglobin levels pre‐treatment (g/dL).

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 3 Haemoglobin levels at end of treatment pre‐op (g/dL).

Comparison 1 Iron therapy versus placebo or no iron therapy, Outcome 4 Haemoglobin levels after treatment post‐op (g/dL).
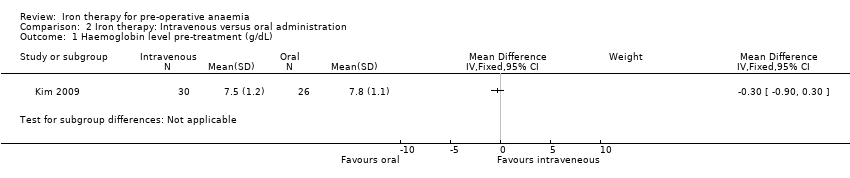
Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 1 Haemoglobin level pre‐treatment (g/dL).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 2 Haemoglobin level post‐treatment pre‐op (g/dL).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 3 Ferritin level pre‐treatment (ɥg/L).

Comparison 2 Iron therapy: Intravenous versus oral administration, Outcome 4 Ferritin level post‐treatment (ɥg/L).
| Iron therapy versus placebo or no iron therapy for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Iron therapy | |||||
| Proportion of patients who received a blood transfusion | 635 per 1000 | 356 per 1000 | RR 0.56 | 38 | ⊕⊕⊝⊝ | |
| Amount of blood transfused per patient (in units) | Data from two small studies could not be combined as they were skewed and reported as medians and ranges. One RCT in 18 people reported a difference in medians of 0 (interquartile range: 1) with iron therapy. Another RCT in 20 people reported a median difference of 1 unit with iron therapy (range 0 to 2). | ‐ | 38 | ⊕⊕⊝⊝ | It is not possible to combine the data because they are skewed. These are the raw data. | |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in either of the two studies available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 11.9 g/dL (SD 2.6) | mean 11.2 g/d L(SD 1.95) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intervention groups was 0.7 g/dL lower | 18 | ⊕⊕⊝⊝ | Data from one study; the raw data are presented. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only two small randomised control trials and a subset of anaemic patients resulting in a very small number of participants. | ||||||
| Iron therapy: Intravenous versus oral administration for pre‐operative anaemia | ||||||
| Patient or population: Patients with pre‐operative anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron therapy | Intravenous iron therapy | |||||
| Proportion of patients who received a blood transfusion | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Amount of blood transfused per patient (in units) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Post‐operative morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Any validated measure of quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the one study available. |
| Haemoglobin levels at end of treatment pre‐op (g/dL) | mean 8.6 g/dL (SD 1.4) | mean 10.5 g/dL (SD 1.4) | The mean haemoglobin levels at end of treatment pre‐op (g/dl) in the intravenous group was | 56 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study excluded those with less than 80% compliance with therapy and compliance was lower in the oral administration group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who received a blood transfusion Show forest plot | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 2 Haemoglobin levels pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Haemoglobin levels at end of treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Haemoglobin levels after treatment post‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Haemoglobin level pre‐treatment (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Haemoglobin level post‐treatment pre‐op (g/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Ferritin level pre‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Ferritin level post‐treatment (ɥg/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

