Resonancia magnética de perfusión para diferenciar gliomas de bajo y alto grado en la primera consulta
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | Prospective, consecutive | ||
| Patient characteristics and setting | The study reported 89 patients with suspected LGGs with absent or faint enhancement, mean age 39.6 ± 12.6 y.o., in a neurological institute in Italy, between 2006 and 2009. | ||
| Index tests | DSC MR perfusion (Max rCBV) Pre‐specified rCBV threshold: 1.5 Study‐determined rCBV threshold: 1.29 | ||
| Target condition and reference standard(s) | Resection | ||
| Flow and timing | Interval period between MR perfusion and resection: 1‐45 days | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 1.5 T MRI scanner Use of contrast preload: N/A Post‐processing algorithm: Arterial input function (unpublished information, confirmed by authors) 3 ROI placed on areas of maximal CBV and normalised with an identical ROI positioned on the contralateral healthy white matter | ||
| Notes | The author provided individual patient data and clarification of study method. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective, consecutive | ||
| Patient characteristics and setting | The study reported 25 adults with non‐ and minimally enhancing radiologically suspected LGGs, 22‐70 y.o., in a university hospital in Sweden from May 2010 and November 2012. | ||
| Index tests | DSC MR perfusion: single shot gradient‐echo EPI (Mean CBV, CBF, kapp) DCE MR perfusion (Mean CBV, CBF, ktrans), performed prior to DSC MR perfusion Pre‐specified rCBV/Ktrans threshold: None Study‐determined rCBV/Ktrans threshold: None | ||
| Target condition and reference standard(s) | Resection (N = 15), Biopsy (N = 5) | ||
| Flow and timing | Interval period between MR perfusion and resection: <1 ‐ 10 months | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 3 T MRI scanner Use of contrast preload: Yes (as DCE MR perfusion) Post‐processing algorithm: Arterial input function | ||
| Notes | The author provided individual patient data and clarification of study method. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective, consecutive | ||
| Patient characteristics and setting | The study reported 129 patients with primary brain tumours, 11‐84 y.o., in a university hospital in Spain, from Feb 2004 ‐ April 2009. | ||
| Index tests | DSC MR perfusion: single shot gradient‐echo EPI (Mean rCBV) Pre‐specified rCBV threshold: None Study‐determined rCBV threshold: None | ||
| Target condition and reference standard(s) | Resection (N = 15), Biopsy (N = 3) | ||
| Flow and timing | Interval period between MR perfusion and biopsy/resection: 5‐103 days | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 1.5 T MRI scanner Use of contrast preload: No Post‐processing algorithm: Gamma variate function | ||
| Notes | The author provided individual patient data and clarification of study method. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective, consecutive | ||
| Patient characteristics and setting | The study reported 169 patients with brain tumour, 1‐18 y.o., in a university hospital in France, from Oct 2006 to Apr 2013. The author provided individual data on 4 patients with solid and non‐enhancing gliomas which were selected for the review. | ||
| Index tests | DSC MR perfusion: gradient echo EPI (Max rCBV) Pre‐specified rCBV threshold: None Study‐determined rCBV threshold: None | ||
| Target condition and reference standard(s) | Resection and biopsy (not specified per case) | ||
| Flow and timing | Interval period between MR perfusion and biopsy/resection: 1 week | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 1.5 T MRI scanner Use of contrast preload: No Post‐processing algorithm: Arterial input function | ||
| Notes | The author provided individual patient data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective, consecutive | ||
| Patient characteristics and setting | The study reported 35 patients with WHO Grade II‐IV gliomas, 8‐91y.o., in a university hospital in Japan, from May 2009 to June 2013. | ||
| Index tests | DSC MR perfusion: gradient echo EPI (Max rCBV) Pre‐specified rCBV threshold: None Study‐determined rCBV threshold: 5.66 | ||
| Target condition and reference standard(s) | Resection in majority (unpublished data, confirmed by authors) | ||
| Flow and timing | Interval period between MR perfusion and resection: Within 2 weeks (unpublished data, confirmed by authors) | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 3 T MRI scanner Use of contrast preload: Yes Post‐processing algorithm: Arterial input function | ||
| Notes | The author provided individual patient data and clarification of study method. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective, consecutive | ||
| Patient characteristics and setting | The study reported individual data on 21 adults with suspected supratentorial nonenhancing LGG, 23‐60 y.o., in a university hospital in Brazil, from Feb 2001‐2004. | ||
| Index tests | DSC MR perfusion: spin‐echo EPI (Mean rCBV for homogeneous tumour, Max rCBV for heterogeneous tumour) Pre‐specified rCBV threshold: None Study‐determined rCBV threshold: None for LGG vs HGG (1.2 for differentiating diffuse astrocytoma histology subtype) | ||
| Target condition and reference standard(s) | Resection | ||
| Flow and timing | Interval period between MR perfusion and resection: 2 days (unpublished data, confirmed by author) | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 1.5 T MRI scanner Use of contrast preload: No Post‐processing algorithm: Gamma variate function | ||
| Notes | The author provided clarification of study method. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective, patient sampling not reported | ||
| Patient characteristics and setting | The study reported individual data on 17 patients with supratentorial gliomas, 14‐67 y.o., in a university hospital in Japan. | ||
| Index tests | DSC MR perfusion: gradient echo EPI (Max rCBV) Pre‐specified rCBV threshold: None Study‐determined rCBV threshold: None | ||
| Target condition and reference standard(s) | Resection (N = 14), Biopsy (N = 3) | ||
| Flow and timing | Interval period between MR perfusion and biopsy/resection: within 10 days | ||
| Comparative | |||
| MR perfusion acquisition and analysis | 1.5 T MRI scanner Use of contrast preload: No Post‐processing algorithm: Area under the curve | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was tumour grading based on histopathological assessment or WHO criteria only? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
CBF: cerebral blood flow, CBV: cerebral blood volume, DCE: dynamic contrast‐enhanced, DSC: dynamic susceptibility, EPI: Echo planar images, HGG: high‐grade gliomas, LGG: low‐grade gliomas, MR: magnetic resonance, MRI: magnetic resonance imaging, rCBV: CBV ratio of tumour, ROI: region of interest, y.o.: years old.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Unclear/long interval period between index test and reference test | |
| Unclear/long interval period between index test and reference test | |
| Unable to select solid and non‐enhancing gliomas | |
| Unclear/long period between index test and reference test | |
| Unclear/long period between index test and reference test | |
| No 2 x 2 table can be derived | |
| Insufficient sample (no solid non‐enhancing HGGs) | |
| Unclear/long interval period between index test and reference test | |
| Unclear/long period between index test and reference test | |
| Insufficient sample (no solid non‐enhancing HGGs) | |
| Unable to select solid and non‐enhancing gliomas | |
| Unclear/long interval period between index test and reference test |
HGG: high‐grade gliomas
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 rCBV ‐ Law Threshold Show forest plot | 7 | 115 |
| Test 1  rCBV ‐ Law Threshold. | ||
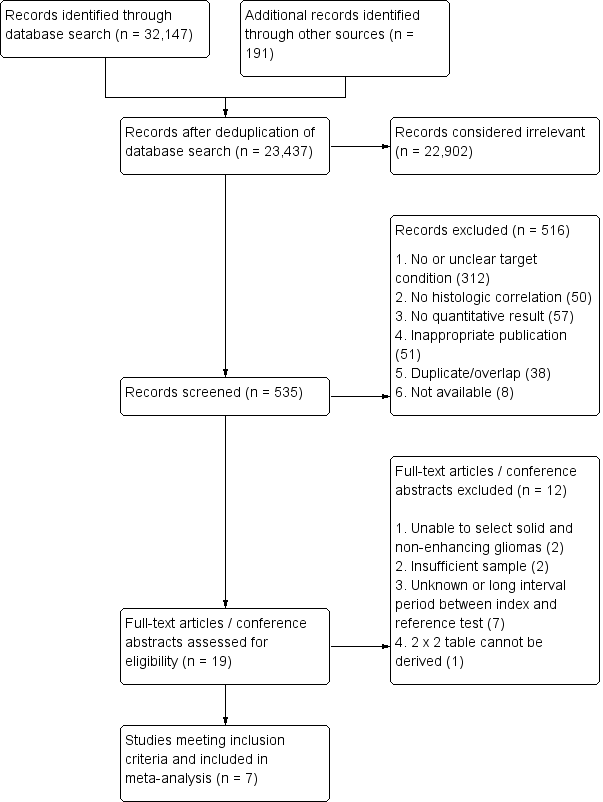
Diagram shows the clinical management algorithm for patients with infiltrative glioma. The role of the index test (MRP) for differentiating LGGs and HGGs at first presentation is shown with alternative tests (MRS, DWI, PET). These advanced MRI techniques are also used to identify progression or recurrence during interval scanning and are included, although they are outside the scope of this review. *May or may not be offered, depending on institutional/regional practice.
Abbreviations: LGG: Low‐grade glioma, HGG: High‐grade glioma,MRP: Magnetic resonance perfusion, MRS: magnetic resonance spectroscopy, DWI: Diffusion‐weighted imaging, PET: Positron emission tomography

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study using QUADAS 2 tool, applied on study design and included patient data

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
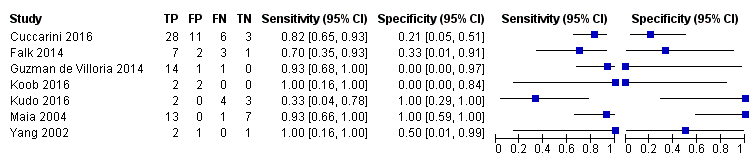
Coupled forest plots of included studies using rCBV threshold of < 1.75 for differentiating low grade gliomas from high‐grade gliomas.
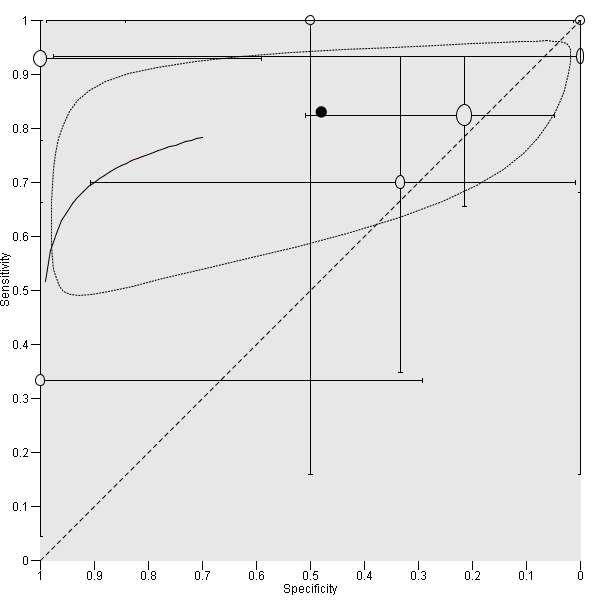
Summary ROC Plot of DSC MR perfusion using rCBV threshold of 1.75 for differentiating low grade gliomas from high‐grade gliomas. In this review, a positive test or rCBV < 1.75 implied an LGG diagnosis, while a negative test or rCBV > 1.75 suggested an HGG diagnosis. In the SROC plot, each study is represented by an open circle with emanating lines, representing the sensitivity and specificity with their confidence intervals. The size of the open circle is proportional to the study sample size. The shaded circle represents the pooled sensitivity and specificity surrounded by a 95% confidence ellipse (dotted line), which in this case is 0.830 (95% CI 0.657, 0.926) and 0.479 (95% CI 0.086, 0.900), respectively.

rCBV ‐ Law Threshold.
| Population | Almost all adults | |||||
| Setting | Mostly university hospitals, employing exclusively 1.5T or 3T MRI scanners | |||||
| Index test | Dynamic susceptibility contrast MR perfusion (commonly gradient echo rather than spin echo sequence acquisition), usually without contrast preload, typically using arterial input function or gamma variate function post‐processing algorithms, and preferentially using region‐of‐interest method to obtain Max rCBV values (CBV ratio of tumour: contralateral normal appearing white matter) | |||||
| Importance | For solid and non‐enhancing brain tumours with low rCBV, patients with no or little neurologic deficit may opt for conservative management over surgery to avoid early neurologic disability. Meanwhile, patients with high rCBV could favour early treatment for better tumour control. | |||||
| Reference standard | All with histologic examinations, majority with resection. | |||||
| Studies | Mostly prospective cross sectional studies (no case‐control studies) | |||||
| Positive Test | Summary accuracy | No. of study participants / selected patients | Prevalence | Implications | Quality of studies | Comments |
| rCBV threshold <1.75 indicates LGG | Sensitivity (proportion of LGG detected by MR perfusion) 0.83 (0.66, 0.93) (proportion of HGG detected by MR perfusion) 0.48 (0.09 to 0.90) | 392 patients / 115 with solid non‐enhancing Grade II‐IV gliomas who underwent tissue sampling within 2 months of MR perfusion (7 studies) | In a hypothetical population of solid and non‐enhancing Grade II‐IV gliomas, the prevalence of LGGs and HGGs is 72% and 28%, respectively. | Given 100 patients with solid and non‐enhancing infiltrative gliomas, 72 will have LGG and 28 with HGG. Of 72 patients with LGG, it is expected 12 patients will be misclassified to have HGG (but this could potentially be between 5 to 24 patients) and may undergo surgery, thus risking early neurologic deterioration. Meanwhile, of 28 patients with HGG, 15 will be misclassified to have LGG (but this could be between 3 to 25 patients), which may lead to a delay in treatment that can potentially adversely affect outcomes. | Generally low risk of bias in the patient selection domain, excepting 2 out of 7 studies with unclear patient sampling and inappropriate exclusion of small tumours. High risk of bias in the index test domain, mainly because 6 out of 7 studies did not use a pre‐specified threshold. However this did not affect meta‐analysis as we used a common rCBV threshold of 1.75. Low concerns of applicability for the patient selection, index test and reference standard domains by using patient‐level data. | Low numbers (4 to 48) with target and alternative conditions per study and only 2 studies had >20 patients. In general, individual studies had heterogeneous sensitivity and specificity, both with wide confidence intervals. Only 1 study had low risk of bias and low concern of applicability across all domains. Five studies were considered good quality (i.e., with low risk of bias in the domains of reference standard and flow & timing). Their sensitivity analysis yielded sensitivity 0.80 (95% CI 0.61 to 0.91) and specificity 0.67 (95% CI 0.07 to 0.98). Subgroup analysis showed sensitivity/specificity of [0.92 (95% CI 0.55 to 0.99)/ 0.42 (95% CI 0.02 to 0.95) in astrocytomas and 0.77 (95% CI 0.46 to 0.93)/0.53 (95% CI 0.14 to 0.88) in oligodendrogliomas + oligoastrocytomas. Data were too sparse to investigate any differences across subgroups. |
| HGG: high‐grade glioma, LGG: low‐grade glioma, rCBV: relative cerebral blood volume | ||||||
| WHO Grade | Tumour histology |
| I** | Pilocytic astrocytoma Subependymal giant cell astrocytoma Pleomorphic xanthoastrocytoma Ganglioglioma Ependymoma |
| II | Diffuse astrocytoma Oligodendroglioma Oligoastrocytoma |
| III | Anaplastic astrocytoma Anaplastic oligodendroglioma Anaplastic oligoastrocytoma |
| IV | Glioblastoma multiforme Gliomatosis cerebri |
| * Partial listing and specific to the tumour histology types relevant to this review. **These tumours are included in this table for reference only and are not part of the review. | |
| Included studies | LGG (Grade II) | HGG (Grade III+IV) | DA | OA | OG | AA | AOA | AOG |
| 1.15 ± 0.95 | 1.18 + 0.8 | 1.19 ± 0.76 | 1.12 ± 1.13 | 1.22 ± 0.57 | 1.15 ± 0.53 | 1.33 ± 0.98 | ||
| 1.30 + 0.48 | 1.76 + 0.93 | 1.48 + 0.69 | 1.20 + 0.21 | 1.19 + 0.32 | 2.22 + 1.18 | 0.86 | 1.76 | |
| 1.07 + 0.79 | 0.75 | 0.98 + 0.29 | 1.24 ±1.33 | 0.75 | ||||
| 0.8 + 0.04 | 0.8 + 0.6 | [0.77] | 0.82 | [0.41] | 1.28 | |||
| 3.1 ± 1.19 | 3.83 ± 2.34 | 2.31 ±1.23 | 3.88 ±.46 | 3.8 + 2.3 | ||||
| 1.16 ± 0.63 | 3.2 ± 0.35 | 0.9 ±.43 | 1.98 ± 0.57 | 1.27 | 3.24 ± 0.37 | 2.99 | ||
| 1.29 ± 0.17 | 1.76 ± 0.08 | 1.29 ± 0.17 | 1.81 | 1.7 | ||||
| LGG: Low‐grade glioma, HGG: high‐grade glioma, DA: diffuse astrocytoma, OA: oligoastrocytoma, OG: oligodendroglioma, AA: anaplastic astrocytoma, AOA: Anaplastic oligoastrocytoma, AOG: anaplastic oligodendroglioma. Nearly all HGGs are Grade III, except for one case of Grade IV/glioblastoma from Cuccarini 2016, with rCBV of 0.3. Bracketed values in Koob 2016 are included for completion but represent unspecified gliomas, with no reported histology. | ||||||||
| Test | No. of studies | No. of participants |
| 1 rCBV ‐ Law Threshold Show forest plot | 7 | 115 |