Primjena ljudskog korionskog gonadotropina (hCG) u maternicu žena slabije plodnosti koje idu na medicinski potpomognutu oplodnju
Abstract
Background
Subfertility affects 15% of couples and represents the inability to conceive naturally following 12 months of regular unprotected sexual intercourse. Assisted reproduction refers to procedures involving the in vitro handling of both human gametes and represents a key option for many subfertile couples. Most women undergoing assisted reproduction treatment will reach the stage of embryo transfer (ET) but the proportion of embryos that successfully implant following ET has remained small since the mid‐1990s. Human chorionic gonadotropin (hCG) is a hormone synthesised and released by the syncytiotrophoblast and has a fundamental role in embryo implantation and the early stages of pregnancy. Intrauterine administration of synthetic or natural hCG via an ET catheter during a mock procedure around the time of ET is a novel approach that has recently been suggested to improve the outcomes of assisted reproduction.
Objectives
To investigate whether the intrauterine administration of hCG around the time of ET improves the clinical outcomes in subfertile women undergoing assisted reproduction.
Search methods
We performed a comprehensive literature search of the Cochrane Gynaecology and Fertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PsycINFO, registers of ongoing trials and reference lists of all included studies and relevant reviews (from inception to 10 November 2015), in consultation with the Cochrane Gynaecology and Fertility Group Trials Search Co‐ordinator.
Selection criteria
We included all randomised controlled trials (RCTs) evaluating intrauterine administration of hCG around the time of ET in this review irrespective of language and country of origin.
Data collection and analysis
Two authors independently selected studies, assessed risk of bias, extracted data from studies and attempted to contact the authors where data were missing. We performed statistical analysis using Review Manager 5 in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. We assessed evidence quality using GRADE methods.
Main results
Twelve RCTs investigated the effect of intrauterine administration of hCG for 4038 subfertile women undergoing assisted reproduction. The intra‐cavity hCG (IC‐hCG) was administered in variable doses at different timings before the ET. The source of hCG was from the urine of pregnant women or from cell cultures using recombinant DNA technology.
Most of the studies (9/12) were at high risk of bias in at least one of the seven domains assessed. Common problems were unclear reporting of study methods and lack of blinding. The main limitations in the overall quality of the evidence were high risk of bias and serious imprecision.
For the analyses of live birth and clinical pregnancy, there was considerable heterogeneity (I2 greater than 75%) and we did not undertake a meta‐analysis. Exploration for the sources of heterogeneity identified two key pre‐specified variables as important determinants: stage of ET (cleavage versus blastocyst stage) and dose of IC‐hCG (less than 500 international units (IU) versus 500 IU or greater). We then performed meta‐analysis for these analyses within the subgroups defined by stage of embryo and dose of IC‐hCG.
There was an increase in live birth rate in the subgroup of women having cleavage‐stage ETs with an IC‐hCG dose of 500 IU or greater compared to women having cleavage‐stage ETs with no IC‐hCG (risk ratio (RR) 1.57, 95% confidence interval (CI) 1.32 to 1.87, three RCTs, n = 914, I2 = 0%, moderate quality evidence). In a clinic with a live birth rate of 25% per cycle then the use of IC‐hCG ‐500 IU or greater would be associated with a live birth rate that varies from 33% to 46%. We did not observe a significant effect on live birth in any of the other subgroups.
The was an increase in clinical pregnancy rate in the subgroup of women having cleavage‐stage ETs with an IC‐hCG dose of 500 IU or greater compared to women having cleavage‐stage ETs with no IC‐hCG (RR 1.41, 95% CI 1.25 to 1.58, seven RCTs, n = 1414, I2 = 0%, moderate quality evidence). We did not observe a significant effect on clinical pregnancy in either of the other subgroups.
There was no evidence that miscarriage was influenced by intrauterine hCG administration (RR 1.09, 95% CI 0.83 to 1.43, seven RCTs, n = 3395, I2 = 0%, very low quality evidence).
Other complications reported in the included studies were ectopic pregnancy (three RCTs, n = 915, three events overall), heterotopic pregnancy (one RCT, n = 495, one event), intrauterine death (two RCTs, n = 978, 21 events) and triplets (one RCT, n = 48, three events). There was no evidence of a difference between the groups, but there were too few events to allow any conclusions to be drawn and the evidence was very low quality.
Authors' conclusions
The pregnancy outcome for cleavage‐stage ETs using an IC‐hCG dose of 500 IU or greater is promising. However, given the small size and the variable quality of the trials and the fact that the positive finding was from a subgroup analysis, the current evidence for IC‐hCG treatment does not support its use in assisted reproduction cycles. A definitive large clinical trial with live birth as the primary outcome is recommended. There was no evidence that miscarriage was influenced by intrauterine hCG administration, irrespective of embryo stage at transfer or dose of IC‐hCG. There were too few events to allow any conclusions to be drawn with regard to other complications.
PICO
Laički sažetak
Učinak davanja hormona trudnoće u maternicu žena slabije plodnosti koje idu na medicinski potpomognutu oplodnju
Istraživačko pitanje
Ima li koristi od primjene hormona trudnoće u maternicu u žena slabije plodnosti koje se podvrgavaju postupcima medicinski potpomognute oplodnje (MPO)?
Dosadašnje spoznaje
Smanjena plodnost pogađa 15% parova i podrazumijeva nemogućnost prirodnog začeća kroz 12 mjeseci otkad par ima nezaštićene spolne odnose. MPO podrazumijeva postupke obrade muških (spermija) i ženskih (jajnih stanica) spolnih stanica u laboratoriju. Svrha je te obrade stvaranje zametka (embrija) koji će se prenijeti u maternicu (embriotransfer, ET). To je važna mogućnost za parove sa smanjenom plodnošću koji žele djecu. Većina žena koja prolazi kroz postupak MPO dođe do ET faze, ali udio zametaka koji preživi je nizak još od polovice 1990‐ih. Hormon trudnoće (ljudski korionski gonadotropin) otpušta zametak i ima važnu ulogu u početnim stadijima trudnoće. Davanje prirodnog ili tvorničkog hormona trudnoće u maternicu ženama sa smanjenom plodnošću koje su u postupku MPO novi je pristup. Predložen je za povećavanje preživljavanja zametka.
Obilježja studija
U ovom Cochrane sustavnom pregledu napravljeno je detaljno pretraživanje niza medicinskih baza (od njihovog osnutka do 10. svibnja 2015.). Istraživači su tražili sva randomizirana klinička ispitivanja (kliničke studije u kojima se ljudi nasumično razvrstavaju u jednu od dvije ili više liječenih skupina) koja su istraživala učinke primjene hormona trudnoće u maternicu žena sa slabijom plodnošću koje su u postupku MPO. Pretraživanje literature i kriteriji uključivanja studija nisu bili ograničeni po jeziku na kojem je studija objavljena i državi gdje je provedena. Dva autora neovisno su analizirala studije, procijenili ih, izvukli podatke i pokušali stupiti u kontakt s autorima ukoliko je nedostajalo podataka.
Pronađeno je 12 studija (4038 žena) koje su zadovoljile kriterije uključenja. Prirodni ili tvornički hormon trudnoće davao se ispitanicama u različitim dozama i različitim vremenima prije ET‐a.
Ključni rezultati
Zabilježeno je povećanje stope živorođene djece u analizi nakon završetka studije. Povećanje je zabilježeno u podgrupama koje su uključivale žene kojima je ET napravljen treći dan i koje su primale 500 IJ (internacionalnih jedinica) hormona trudnoće ili više u usporedbi sa ženama kojima je ET napravljen treći dan, a nisu primale hormone trudnoće (umjerena kvaliteta dokaza iz tri studije koje su uključile 914 žena). U klinici u kojoj je stopa živorođene djece bila 25% primjena hormona trudnoće u dozi od 500 IJ ili više povezana je s povećanjem stope živorođene djece u rasponu od 33% do 46%. Nije bilo značajnog učinka na stopu živorođenih u ostalim podskupinama (npr. niže doze hormona trudnoće).
Primjena hormona trudnoće u maternicu nije utjecala na stopu pobačaja, bez obzira na stanje razvoja zametka ili dozu hormona (jako niska kvaliteta dokaza iz sedam studija koje su uključivale 3395 žena). Druge zabilježene komplikacije su ektopične trudnoće (zametak se razvija izvan maternice), heterotopične trudnoće (zametak se razvija unutar i izvan maternice), smrt zametka u maternici i trojke. Nisu zabilježeni dokazi o razlikama među skupinama, ali bilo je premalo takvih događaja da bi se mogao izvući zaključak i kvaliteta dokaza bila je jako niska.
Rezultat primjene hormona trudnoće u dozi od 500 IJ ili više za ET koji se provodi treći dan čini se obećavajući. Međutim, kad uzmemo u obzir mali ispitivani uzorak, različitu kvalitetu studija i činjenicu da su pozitivni rezultati zabilježeni samo pri dozama od 500 IJ i više, trenutni dokazi nisu dovoljni da bi se hormonska terapija uvela u stalnu upotrebu. Preporučuje se provođenje novih, velikih istraživanja u kojima će stopa živorođene djece biti glavni rezultat.
Kvaliteta dokaza
Kvaliteta dokaza je vrlo niska do umjerena.
Authors' conclusions
Summary of findings
| Intrauterine administration of hCG for women undergoing assisted reproduction | |||||
| Population: women undergoing assisted reproduction | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Intrauterine administration of hCG | ||||
| Live birth ‐ cleavage stage: hCG < 500 IU | 495 per 1000 | 376 per 1000 | RR 0.76 | 280 | ⊕⊝⊝⊝ |
| Live birth ‐ cleavage stage: hCG ≥ 500 IU | 247 per 1000 | 388 per 1000 | RR 1.57 | 914 | ⊕⊕⊕⊝ |
| Live birth ‐ blastocyst stage: hCG ≥ 500 IU | 366 per 1000 | 337 per 1000 | RR 0.92 | 1666 | ⊕⊕⊕⊝ |
| Pregnancy ‐ cleavage stage: hCG < 500 IU | 579 per 1000 | 509 per 1000 (405 to 637) | RR 0.88 (0.70 to 1.10) | 280 | ⊕⊝⊝⊝ |
| Pregnancy ‐ cleavage stage: hCG ≥ 500 IU | 321 per 1000 | 453 per 1000 (401 to 507) | RR 1.41 (1.25 to 1.58) | 1414 (7 studies) | ⊕⊕⊕⊝ |
| Pregnancy ‐ blastocyst stage: hCG ≥ 500 IU | 430 per 1000 | 408 per 1000 (370 to 455) | RR 0.95 (0.86 to 1.06) | 1991 (3 studies) | ⊕⊕⊕⊝ |
| Miscarriage Follow‐up: mean 40 weeks | 48 per 1000 | 52 per 1000 (40 to 68) | RR 1.09 (0.83 to 1.43) | 3395 (7 studies) | ⊕⊝⊝⊝ |
| Other complications | Other complications reported in the included studies were ectopic pregnancy (3 studies, n = 915, 3 events overall), heterotopic pregnancy (1 study, n = 495, 1 event), intrauterine death (2 studies, n = 978, 21 events) and triplets (1 study, n = 48, 3 events). There were too few events to allow any conclusions to be drawn | ⊕⊝⊝⊝ | |||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded two levels due to very serious risk of bias: lack of blinding of participants and personnel, no clear description of allocation concealment and premature termination of the study following interim analysis. | |||||
Background
Description of the condition
Subfertility is defined as the inability of a couple to conceive spontaneously following 12 months of regular unprotected sexual intercourse. It is estimated that 15% of couples are affected by subfertility of different causes (female factor, male factor, unexplained). Assisted reproduction refers to procedures involving the in vitro (in a laboratory dish) handling of both human gametes (sperm and eggs) with the objective of establishing a pregnancy (Zegers‐Hochschild 2009). The most vulnerable step of assisted reproduction is the embryo transfer (ET) as it involves a radical change in the embryo's environment, which makes it prone to demise (Schoolcraft 2001). Most women undergoing assisted reproduction treatment will reach the stage of ET due to important improvements in ovarian stimulation protocols and laboratory technology but the proportion of embryos that successfully implant following ET has remained small (less than one third) since the mid‐1990s (Kupka 2014).
The process of implantation involves a reciprocal interaction between the embryo and endometrium, culminating in a small reception‐ready phase of the endometrium during which implantation can occur. This interaction is dependent on the temporal differentiation of endometrial cells to attain uterine receptivity. Implantation failure is thought to occur as a consequence of impairment of the embryo developmental potential or impairment of uterine receptivity, or both, and the embryo‐uterine dialogue (Diedrich 2007).
Many interventions have been attempted, with varying degrees of success, before ET (endometrial injury (Nastri 2012), dummy ET (Mansour 1990), endometrial preparation (Derks 2009), peri‐implantation (heparin (Akhtar 2013), aspirin (Siristatidis 2011)), during ET (ultrasound guidance (Brown 2010), cervical mucous removal (Craciunas 2014)), and after ET (fibrin sealant, bed rest (Abou‐Setta 2014)) in order to optimise the embryo‐endometrial interaction and improve outcomes.
Description of the intervention
Human chorionic gonadotropin (hCG) is a hormone synthesised and released by the syncytiotrophoblast. It stimulates ovarian production of progesterone during the first trimester of pregnancy. Intrauterine administration of synthetic or natural hCG around the time of ET is a novel approach that has been suggested to improve the outcomes of assisted reproduction treatment based on the fundamental role of hCG in embryo implantation and the early stages of pregnancy (Cole 2010). The intervention involves the intrauterine administration of hCG via an ET catheter during a mock procedure (a trial of the actual ET without using an embryo, performed to assess the difficulty of the ET) using the lowest volume of medium before the conventional ET. The hCG can be released in different points inside the uterine cavity (close to the internal cervical os, mid‐cavity or near the fundus) within minutes, hours or days before the actual ET. The hCG sources for medical treatments include extraction from the urine of pregnant women (natural) or from cell cultures using recombinant DNA technology (rhCG).
How the intervention might work
The hCG may promote peritrophoblastic immune tolerance, which facilitates trophoblast invasion by inducing an increase in endometrial T‐cell apoptosis (Kayisli 2003). It also supports trophoblast apposition (the first stage of implantation, loose alignment of the trophoblast to the decidua) and adhesion (second stage of implantation, closer attachment of the trophoblast to the decidua) to the endometrium by regulating proteins involved in implantation (Racicot 2014). Intrauterine injection of urinary hCG alters endometrial secretory parameters (Licht 1998), while cell proliferation and migration are increased in the presence of hCG (Bourdiec 2013).
Why it is important to do this review
Subfertility affects a relatively large proportion of couples and assisted reproduction treatments remain costly and stressful. All the effort should be directed towards increasing the success rates of infertility treatment and primary research should be translated into clinical practice in an efficient and timely manner. Intrauterine administration of hCG around the time of ET has the potential to improve the outcome of assisted reproduction treatments and randomised and non‐randomised trials have reported varying results (Mansour 2011; Rebolloso 2013).
One meta‐analysis assessed the efficacy of intrauterine injection of hCG before ET in assisted reproductive cycles, but improvements could be made to the methods of analysis (Ye 2015). Different studies have evaluated variable circumstances of intrauterine hCG administration in terms of stage of the embryo at transfer (cleavage versus blastocyst), source of hCG (urine versus recombinant), dose of hCG, embryo processing (fresh versus frozen‐thawed) and number of embryos transferred, leading to real uncertainties about the role of the intervention.
Objectives
To investigate whether the intrauterine administration of hCG around the time of ET improves the clinical outcomes in subfertile women undergoing assisted reproduction.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) evaluating intrauterine administration of hCG around the time of ET in this review irrespective of language and country of origin. We planned to include only data from the first phase of cross‐over RCTs in meta‐analyses.
Types of participants
Subfertile women undergoing in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) followed by ET.
Types of interventions
RCTs comparing intrauterine administration of hCG around the time of ET versus any other active intervention, no intervention or placebo were eligible for inclusion.
Types of outcome measures
Primary outcomes
-
Live birth (the delivery of a live foetus after 24 completed weeks of gestational age) rate per woman or couple randomised.
-
Miscarriage (the loss of the pregnancy before 24 completed weeks of gestational age) rate per woman or couple randomised.
Secondary outcomes
-
Clinical pregnancy (the presence of a gestational sac on ultrasound scan) rate per woman or couple randomised.
-
Complication rate per woman or couple randomised, including ectopic pregnancy, intrauterine growth restriction, foetal or congenital defects, pelvic infection or other adverse events, reported as an overall complication rate or as individual outcomes, or both (as reported by individual studies).
Search methods for identification of studies
We sought all published and unpublished RCTs of intrauterine hCG administration around the time of ET in consultation with the Cochrane Gynaecology and Fertility Group Trials Search Co‐ordinator. The search dates were from the inception of the databases to 10 November 2015 without any language restriction.
Electronic searches
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying RCTs, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, Chapter 6, Section 6.4.11). We combined the EMBASE and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
The search terms used for the Cochrane Gynaecology and Fertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL and PsycINFO are presented in the Appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6).
We searched the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) and ClinicalTrials.gov for ongoing and registered trials. We searched OpenGrey (www.opengrey.eu/) and Google Scholar (scholar.google.co.uk/) for grey literature. We handsearched the abstracts published following major conferences (e.g. the American Society for Reproductive Medicine (ASRM), European Society of Human Reproduction and Embryology (ESHRE)) in the last five years to find additional studies not yet published in full.
Searching other resources
We screened the references lists of all included studies and relevant reviews to identify further articles for possible inclusion.
Data collection and analysis
We used Review Manager 5 for input of data and statistical analysis (RevMan 2012), in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two authors (LC and NT) independently screened the title, abstract and keywords for each publication to exclude the studies that were irrelevant for the objective of this review. We retrieved the remaining publications in full text and the same two authors appraised them independently to identify the RCTs suitable for inclusion. There was no disagreement related to study eligibility. We documented the selection process with a PRISMA flow chart.
Data extraction and management
Two authors (LC and NT) independently extracted data using a pre‐designed and pilot‐tested data extraction form. For studies with multiple publications, we used the main RCT report as the reference and we supplemented it with additional data from secondary publications. We attempted to contact authors where published data were insufficient. There were no disagreements. One author (LC) entered data into Review Manager 5 (RevMan 2012), and a second author (NT) checked the data against the data extraction form.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' assessment tool to assess the included studies for: selection, performance, detection, attrition, reporting and other bias. There were no disagreements. We included the 'Risk of bias' table in the 'Characteristics of included studies' table, describing the judgements in detail.
Measures of treatment effect
All outcomes were dichotomous. We calculated Mantel‐Haenszel risk ratios (RRs) with 95% confidence intervals (CI) using the numbers of events in the intervention and control groups of each study. For outcomes with event rates below 1%, we used the Peto one‐step odds ratio (OR) method to calculate the combined outcome with 95% CI.
Unit of analysis issues
We performed analysis per woman or couple randomised for live birth, clinical pregnancy, miscarriage and complication rates. We counted multiple live births (twins, triplets) as a single live birth event. We performed a secondary analysis for miscarriage per clinical pregnancy to broaden the understanding of the treatment effect.
If a study included multiple treatment arms based on hCG dose, we planned to split the control group proportionally with the experimental groups in order to avoid analysing control participants in duplicate.
Dealing with missing data
We attempted to contact the authors of the RCTs to obtain missing data in order to perform analyses on an intention‐to‐treat basis. In the case of unobtainable data, we planned imputation of individual values to be undertaken for the live birth rate only. We assumed that live births had not occurred in participants without a reported outcome. For other outcomes, we analysed only the available data.
Assessment of heterogeneity
We identified heterogeneity by visual inspection of forest plots and by using a standard Chi2 test with significance set at P value < 0.1. We used the I2 statistic to estimate the total variation across RCTs that was due to heterogeneity, where I2 greater than 50% indicated substantial heterogeneity.
Assessment of reporting biases
We conducted a comprehensive search to minimise the potential impact of publication bias and other reporting biases. We planned to use a funnel plot to explore the possibility of small‐study effects when the number of included RCTs exceeded 10.
Data synthesis
We combined the data from similar RCTs comparing similar treatments using a random‐effects model. We displayed an increase in the odds of an outcome to the right of the centre line and a decrease in the odds of an outcome to the left of the centre line. For comparisons where there was considerable clinical, methodological or statistical heterogeneity (I2 greater than 75%), we did not combine RCTs results in a meta‐analysis. Where data were incomplete and could not be presented in the analyses, we reported available data in narrative form.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to investigate the efficacy of intrauterine hCG administration around the time of ET depending on:
-
stage of the embryo at transfer (cleavage versus blastocyst);
-
source of intra‐cavity hCG (IC‐hCG) (urine versus recombinant);
-
embryo processing (fresh versus frozen‐thawed);
-
number of embryos transferred.
If we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. Factors considered included treatment indication, age of the women, ovarian stimulation protocol, response to ovarian stimulation, timing of IC‐hCG administration, IC‐hCG dose and volume of infused medium, method of IC‐hCG administration (i.e. type of catheter), embryo quality, endometrial thickness, source of oocytes (i.e. donated, own) and ET difficulty. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We performed sensitivity analysis to examine the stability and robustness of the results for the primary outcomes in relation to the following eligibility and analysis factors.
-
Inclusion of RCTs without high risk of bias.
-
Publication type (abstract versus full text).
-
Use of a random‐effects model.
-
Calculation of OR.
-
Imputation of outcomes.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro software. This table evaluated the overall quality of the body of evidence for the main review outcomes (live birth rate, miscarriage and clinical pregnancy rate) using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified, documented and incorporated judgements about evidence quality (high, moderate or low) into reporting of results for each outcome.
Results
Description of studies
Results of the search
We performed the systematic search on 10 November 2015 and identified 516 publications (499 from databases and 17 from other sources). Nineteen articles were potentially relevant and we assessed these in full text. We included 12 articles, excluded five articles and two articles await classification. See Figure 1 for detailed search results.
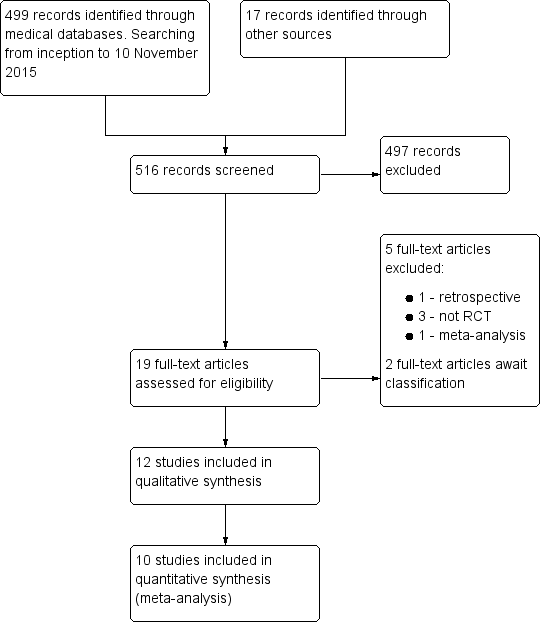
Study flow diagram.
Included studies
Types of studies
All 12 included studies were parallel‐arm RCTs. One study had two experimental arms (IC‐hCG 500 IU versus IC‐hCG 1000 IU versus control) (Janati 2013), one study had two phases with three experimental arms (phase one: IC‐hCG 100 IU versus IC‐hCG 200 IU versus control; and phase two: IC‐hCG 500 IU versus control) (Mansour 2011), and one study had two experimental arms at two different timings (IC‐hCG 500 IU versus control two days prior to ET; IC‐hCG 500 IU versus control on the day of ET) (Wirleitner 2015a). Six studies were as full text articles (Aaleyasin 2015; Hong 2014; Mansour 2011; Santibañez 2014; Wirleitner 2015a; Zarei 2014), and six studies were abstracts (Cambiaghi 2013; Janati 2013; Kokkali 2014; Leao 2013; Singh 2014; Wirleitner 2015b).
Six studies did not report funding (Aaleyasin 2015; Cambiaghi 2013; Hong 2014; Janati 2013; Leao 2013; Wirleitner 2015a), and six studies reported internal funding (Kokkali 2014; Mansour 2011; Santibañez 2014; Singh 2014; Wirleitner 2015b; Zarei 2014). None of the studies reported external funding.
Participants
Participants were couples/women recruited prior to undergoing assisted reproductive treatment for different subfertility causes. The number of participants varied between 36 (Leao 2013) and 1186 (Wirleitner 2015a). The studies were conducted in Iran, Brazil, USA, Greece, Egypt, Mexico, India and Austria.
Interventions
Most of the studies compared intrauterine administration of urine hCG 500 IU with controls. One study had two additional arms with lower doses (IC‐hCG 100 and 200 IU) (Mansour 2011), and one study had an additional arm with higher dose (IC‐hCG 1000 IU) (Janati 2013). One study used rhCG 250 μg (equivalent of 6500 IU) (Zarei 2014), and one study used intra‐cavity rhCG (IC‐rhCG) 40 μL (equivalent to 500 IU) (Singh 2014).
Nine studies administered the IC‐hCG within minutes before ET (Aaleyasin 2015; Hong 2014; Janati 2013; Kokkali 2014; Mansour 2011; Santibañez 2014; Singh 2014; Wirleitner 2015b; Zarei 2014), ranging from less than three minutes (Hong 2014) up to 12 minutes (Zarei 2014), and two studies administered the IC‐hCG six hours before ET (Cambiaghi 2013; Leao 2013). One study had four groups (two experimental and two controls) at two different timings (two days before ET and three minutes before ET) (Wirleitner 2015a).
For the control groups, six studies administered the same volume of transfer media (Hong 2014), culture media (Aaleyasin 2015; Singh 2014; Wirleitner 2015a; Wirleitner 2015b), or normal saline (Zarei 2014), without hCG and six studies did not administer anything prior to ET (Cambiaghi 2013; Janati 2013; Kokkali 2014; Leao 2013; Mansour 2011; Santibañez 2014).
Outcomes
Seven studies reported on one of our pre‐defined primary outcomes: live birth (Aaleyasin 2015; Mansour 2011; Singh 2014; Wirleitner 2015a; Wirleitner 2015b) and miscarriage (Aaleyasin 2015; Hong 2014; Janati 2013; Mansour 2011; Singh 2014; Wirleitner 2015a; Wirleitner 2015b).
Twelve studies reported on one of our pre‐defined secondary outcomes: clinical pregnancy (Aaleyasin 2015; Cambiaghi 2013; Hong 2014; Janati 2013; Kokkali 2014; Leao 2013; Mansour 2011; Santibañez 2014; Singh 2014; Wirleitner 2015a; Wirleitner 2015b; Zarei 2014), and complications (Aaleyasin 2015; Mansour 2011; Santibañez 2014; Zarei 2014).
Studies awaiting classification
Two studies await classification (Badehnoosh 2014; Bhat 2014). These studies reported interim outcomes (implantation rate and fertilisation rate) and it was unclear whether they also collected data on clinical outcomes that might be relevant to our review. We emailed the authors of these studies in February 2016, asking for more information on the methods and outcome measures of their studies.
Excluded studies
We excluded five studies due to retrospective design (Jeong 2013), non‐randomisation (Li 2013; Rebolloso 2013; Riboldi 2013), and meta‐analysis (Ye 2015).
Risk of bias in included studies
Figure 2 shows the 'Risk of bias' graph and Figure 3 shows the 'Risk of bias'. See the Characteristics of included studies table for rationales behind each judgement.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
All included studies were RCTs. The randomisation technique was adequate in 10 studies (Aaleyasin 2015; Cambiaghi 2013; Hong 2014; Janati 2013; Kokkali 2014; Mansour 2011; Santibañez 2014; Singh 2014; Wirleitner 2015a; Zarei 2014), which we classified at low risk of bias. Two studies lacked adequate randomisation description and we classified them at unclear risk of bias (Leao 2013; Wirleitner 2015b).
Allocation concealment
Three studies mentioned adequate allocation concealment and we classified them at low risk of bias (Aaleyasin 2015; Hong 2014; Kokkali 2014). Nine studies lacked a description of methods of allocation concealment and we classified them at unclear risk of bias (Cambiaghi 2013; Janati 2013; Leao 2013; Mansour 2011; Santibañez 2014; Singh 2014; Wirleitner 2015a; Wirleitner 2015b; Zarei 2014).
Blinding
Four studies documented blinding of participants or personnel (or both) and we classified them at low risk of bias (Aaleyasin 2015; Hong 2014; Wirleitner 2015b; Zarei 2014). We classified the remaining studies at high risk of bias (Cambiaghi 2013; Janati 2013; Kokkali 2014; Leao 2013; Mansour 2011; Santibañez 2014; Singh 2014; Wirleitner 2015a).
The outcome measurement was not likely to be influenced by lack of blinding; hence, we classified all studies at low risk of bias.
Incomplete outcome data
Five studies followed up all participants and reported the results adequately (Aaleyasin 2015; Hong 2014; Santibañez 2014; Singh 2014; Wirleitner 2015b). We classified these at low risk of bias. We classified six studies at unclear risk of bias (Cambiaghi 2013; Janati 2013; Kokkali 2014; Leao 2013; Mansour 2011; Wirleitner 2015a). One study reported large numbers of participants lost to follow‐up and we classified this at high risk of bias (Zarei 2014).
Selective reporting
Five studies reported on all relevant outcomes and we classified them at low risk of bias (Aaleyasin 2015; Mansour 2011; Singh 2014; Wirleitner 2015a; Wirleitner 2015b). All studies reported on clinical pregnancy, but, if there were no reports on live birth, we classified them at unclear risk of bias (Cambiaghi 2013; Hong 2014; Janati 2013; Kokkali 2014; Leao 2013; Santibañez 2014; Zarei 2014).
Other potential sources of bias
We classified six studies at low risk of other potential bias because groups appeared to be comparable at baseline and we could not identify any other sources of bias (Aaleyasin 2015; Santibañez 2014; Singh 2014; Wirleitner 2015a; Wirleitner 2015b; Zarei 2014). We classified four studies at unclear risk of bias because they did not report on baseline characteristics between groups (probably due to availability as abstract only) (Cambiaghi 2013; Janati 2013; Kokkali 2014), or reported a large number of participants who declined to participate after randomisation for various reasons (Hong 2014). We classified two studies at high risk of bias due to lack of reporting of participant numbers in each study group (Leao 2013), and due to performing interim analysis that changed the study protocol and ended the study prematurely (Mansour 2011)
The overall birth rate in the control groups in Mansour 2011 was 47%, whereas the control group live birth rate ranged from 25% to 39% in the other included studies. The reason for this was unclear. The mean age of women in Mansour 2011 was under 30 years, but this was also the case in Aaleyasin 2015, which reported a control group live birth rate of only 25%.
Effects of interventions
Note: One study included three experimental arms based on intrauterine hCG dose and we regarded and analysed them as three separate comparisons (Mansour 2011). We split the control group proportionally with the experimental groups in order to avoid analysing control participants in duplicate. One study investigated intrauterine hCG administration at two different timings (day three versus day five administration) and we regarded and analysed them as two separate comparisons (Wirleitner 2015a).
Two of the comparisons had considerable heterogeneity (I2 greater than 75%) and we did not perform a global meta‐analysis, as pre‐specified in the protocol (Craciunas 2015) (Analysis 1.1; Analysis 1.4).
Exploration for the sources of heterogeneity in these analyses identified two key pre‐specified variables as important determinants: stage of ET (cleavage versus blastocyst stage) and dose of IC‐hCG (less than 500 IU versus 500 IU or greater). When we subgrouped the data according to these variables, there was evidence of significant differences between the subgroups. We then performed meta‐analysis within the subgroups defined by stage of embryo and dose of hCG.
Primary outcomes
Live birth (Analysis 1.1)
Five studies with eight experimental arms reported on live birth (Aaleyasin 2015; Mansour 2011; Singh 2014; Wirleitner 2015a; Wirleitner 2015b) (Analysis 1.1).
Subgroup analysis
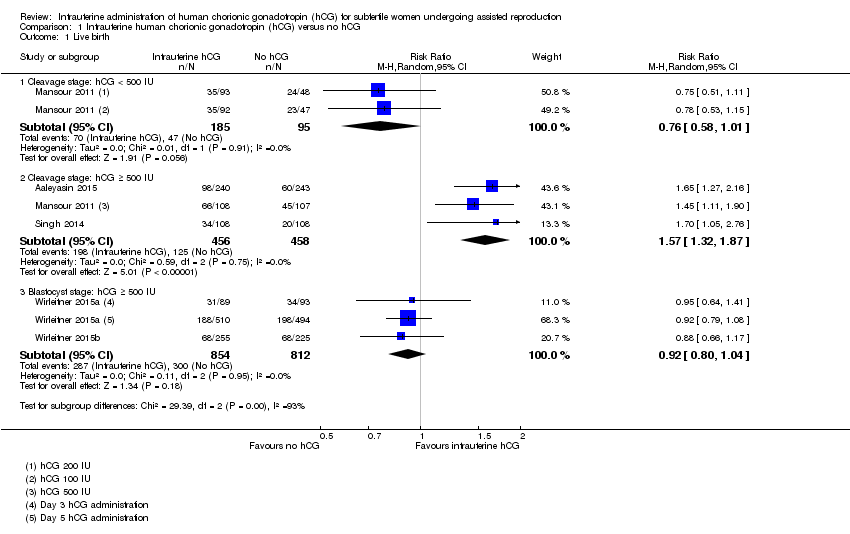
The forest plot displayed the studies based on the embryo stage at transfer and the hCG dose (Figure 4). The test for subgroup differences indicated a considerable difference between the subgroups (Chi2 = 29.39, degrees of freedom (df) = 2, P value ≤ 0.00001, I2 = 92.3%).
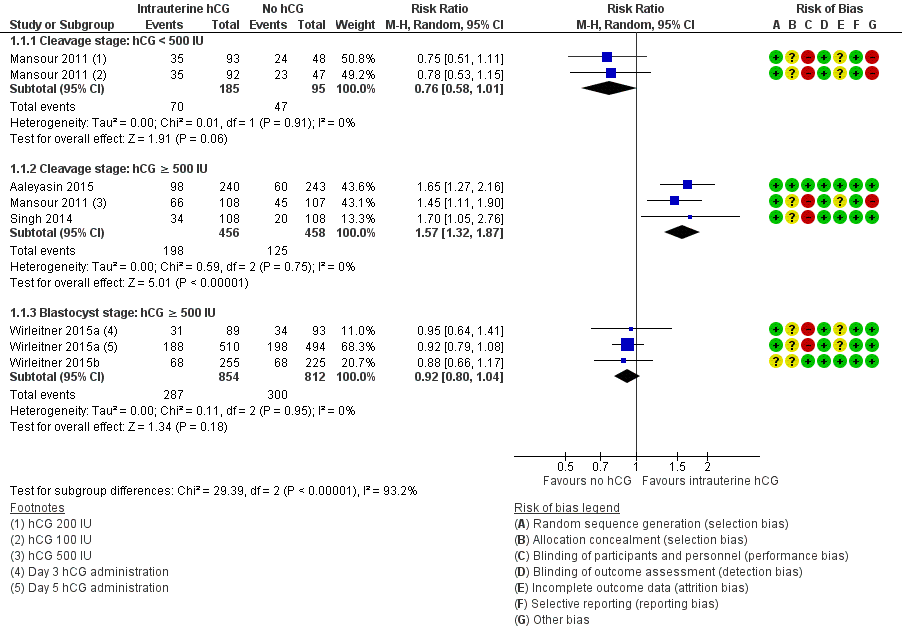
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.1 Live birth.
-
Cleavage stage: IC‐hCG less than 500 IU versus no IC‐hCG: one RCT with two experimental arms contributed to the calculation of the combined outcome (Mansour 2011). The heterogeneity was insignificant (Chi2 = 0.01, df = 1, P value = 0.91, I2 = 0%) and there was no evidence of a difference between the groups in live birth rates (RR 0.76, 95% CI 0.58 to 1.01, one RCT, n = 280, I2 = 0%, very low quality evidence).
-
Cleavage stage: IC‐hCG 500 IU or greater versus no IC‐hCG: three RCTs contributed to the calculation of the combined outcome (Aaleyasin 2015; Mansour 2011; Singh 2014). The heterogeneity was insignificant (Chi2 = 0.59, df = 2, P value = 0.75, I2 = 0%) and the live birth rate was higher in the hCG group (RR 1.57, 95% CI 1.32 to 1.87, three RCTs, n = 914, I2 = 0%, moderate quality evidence). This suggested that in women with a 25% chance of live birth without using IC‐hCG, the live birth rate in women using IC‐hCG 500 IU or greater will be between 33% and 46%.
-
Blastocyst stage: IC‐hCG 500 IU or greater versus no IC‐hCG: two RCTs with three experimental arms contributed to the calculation of the combined outcome (Wirleitner 2015a; Wirleitner 2015b). The heterogeneity was insignificant (Chi2 = 0.11, df = 2, P value = 0.95, I2 = 0%) and there was no evidence of a difference between the groups in live birth rates (RR 0.92, 95% CI 0.80 to 1.04, two RCTs, n = 1666, I2 = 0%, moderate quality evidence).
Data were insufficient to perform the pre‐specified subgroup analyses based on embryo processing and number of embryos transferred.
Sensitivity analyses
Removing the studies with high risk of bias in one or more domains (Mansour 2011; Singh 2014; Wirleitner 2015a) did not alter the results significantly, but it meant that there were no data for one of the comparisons
-
cleavage stage: IC‐hCG less than 500 IU versus no IC‐hCG: no data
-
cleavage stage: IC‐hCG 500 IU or greater versus no IC‐hCG: RR 1.65 (95% CI 1.27 to 2.16, one RCT, n=483)
-
blastocyst stage: IC‐hCG 500 IU or greater versus no IC‐hCG (RR 0.88, 95% CI 0.66 to 1.17, one RCT, n = 480)
Removing the studies available as abstract only (Singh 2014; Wirleitner 2015b) did not alter the results significantly:
-
cleavage stage: IC‐hCG less than 500 IU versus no IC‐hCG (RR 0.76, 95% CI 0.58 to 1.01, one RCT, n = 280, I2 = 0%, very low quality evidence);
-
cleavage stage: IC‐hCG 500 IU or greater versus no IC‐hCG (RR 1.55, 95% CI 1.28 to 1.87, two RCTs, n = 698, I2 = 0%, moderate quality evidence);
-
blastocyst stage: IC‐hCG 500 IU or greater versus no IC‐hCG (RR 0.92, 95% CI 0.80 to 1.07, one RCT, n = 1186, I2 = 0%, moderate quality evidence).
The calculated combined outcome using the fixed‐effect model was similar to random‐effects model for:
-
cleavage stage: IC‐hCG less than 500 IU versus no IC‐hCG (RR 0.76, 95% CI 0.58 to 1.01, one RCT, n = 280, I2 = 0%, very low quality evidence);
-
cleavage stage: IC‐hCG 500 IU or greater versus no IC‐hCG (RR 1.59, 95% CI 1.33 to 1.90, three RCTs, n = 914, I2 = 0%, moderate quality evidence);
-
blastocyst stage: IC‐hCG 500 IU or greater versus no IC‐hCG (RR 0.91, 95% CI 0.80 to 1.04, two RCTs, n = 1666, I2 = 0%, moderate quality evidence).
There was no significant difference between OR and RR:
-
cleavage stage: IC‐hCG less than 500 IU versus no IC‐hCG (OR 0.62, 95% CI 0.38 to 1.03, one RCT, n = 280, I2 = 0%, very low quality evidence);
-
cleavage stage: IC‐hCG 500 IU or greater versus no IC‐hCG (OR 2.10, 95% CI 1.59 to 2.79, three RCTs, n = 914, I2 = 0%, moderate quality evidence);
-
blastocyst stage: IC‐hCG 500 IU or greater versus no IC‐hCG (OR 0.87, 95% CI 0.71 to 1.06, two RCTs, n = 1666, I2 = 0%, moderate quality evidence).
Miscarriage (Analysis 1.2, Figure 5)
Seven studies with 10 experimental arms reported on miscarriage (Aaleyasin 2015; Hong 2014; Mansour 2011; Singh 2014; Wirleitner 2015a; Wirleitner 2015b; Zarei 2014) (Analysis 1.2; Figure 5). The heterogeneity between the studies was unsubstantial (Chi2 = 4.30, df = 9, P value = 0.89, I2 = 0%) and there was no evidence of a difference between the groups in miscarriage rates (RR 1.09, 95% CI 0.83 to 1.43, seven RCTs, n = 3395, I2 = 0%, very low quality evidence).
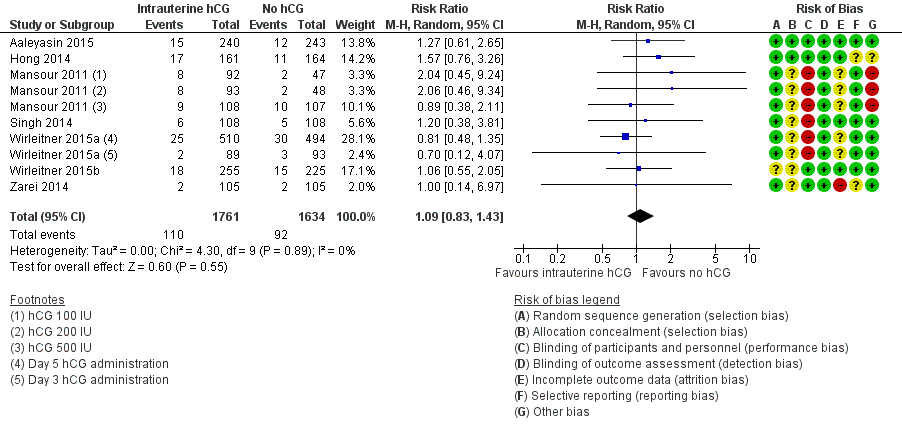
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.2 Miscarriage.
One study investigated IC‐hCG 500 IU and 1000 IU and reported similar miscarriage rates between experimental and control groups, without providing sufficient data to be included in a meta‐analysis (Janati 2013).
Sensitivity analyses
Removing the studies with high risk of bias in one or more domains (Mansour 2011; Singh 2014; Wirleitner 2015a) did not alter the results significantly (RR 1.25 [0.84, 1.87, four studies, n=1498, I2=0%)
Removing the two studies available as abstract only (Singh 2014; Wirleitner 2015b) did not alter the results significantly (RR 1.09, 95% CI 0.80 to 1.48, five RCTs, n = 2699, I2 = 0%, very low quality evidence).
The calculated combined outcome using the fixed‐effect model was similar to that of the random‐effects model (RR 1.10, 95% CI 0.84 to 1.44, seven RCTs, n = 3395, I2 = 0%, very low quality evidence).
There was no significant difference between OR and RR (OR 1.09, 95% CI 0.82 to 1.46, seven RCTs, n = 3395, I2 = 0%, very low quality evidence).
Secondary analysis per clinical pregnancy (Analysis 1.3)
There was no evidence of a difference between the groups in miscarriage rates calculated per clinical pregnancy (RR 1.00, 95% CI 0.77 to 1.30, seven RCTs, n = 1450, I2 = 0%, very low quality evidence) (Analysis 1.3).
Secondary outcomes
Clinical pregnancy (Analysis 1.4)
All included studies reported clinical pregnancy (Analysis 1.4).
Subgroup analysis
The forest plot displayed the studies based on the embryo stage at transfer and the hCG dose (Figure 6). The test for subgroup differences indicated a considerable difference between the subgroups (Chi2 = 28.83, df = 2, P value ≤ 0.00001, I2 = 93.1%).

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.4 Clinical pregnancy.
-
Cleavage stage: IC‐hCG less than 500 IU versus no IC‐hCG: one RCT with two experimental arms contributed to the calculation of the combined outcome (Mansour 2011). The heterogeneity was insignificant (Chi2 = 0.07, df = 1, P value = 0.80, I2 = 0%) and there was no evidence of a difference between the groups in clinical pregnancy rates (RR 0.88, 95% CI 0.70 to 1.10, one RCT, n = 280, I2 = 0%, very low quality evidence).
-
Cleavage stage: IC‐hCG 500 IU or greater versus no IC‐hCG: seven RCTs contributed to the calculation of the combined outcome (Aaleyasin 2015; Cambiaghi 2013; Leao 2013; Mansour 2011; Santibañez 2014; Singh 2014; Zarei 2014). The heterogeneity was insignificant (Chi2 = 3.18, df = 6, P value = 0.79, I2 = 0%) and the clinical pregnancy rate was higher in the hCG group (RR 1.41, 95% CI 1.25 to 1.58, seven RCTs, n = 1414, I2 = 0%, moderate quality evidence).
One study investigated IC‐hCG 500 IU and 1000 IU and reported similar clinical pregnancy rates between experimental and control groups (Janati 2013). One study investigated IC‐hCG 500 IU and reported no evidence of a difference between the groups in clinical pregnancy rates (Kokkali 2014). Data from these two studies were insufficient to be included in meta‐analysis.
-
Blastocyst stage: IC‐hCG 500 IU or greater versus no IC‐hCG: three RCTs with four experimental arms contributed to the calculation of the combined outcome (Hong 2014; Wirleitner 2015a; Wirleitner 2015b). The heterogeneity was insignificant (Chi2 = 2.91, df = 3, P value = 0.41, I2 = 0%) and there was no evidence of a difference between the groups in clinical pregnancy rates (RR 0.95, 95% CI 0.86 to 1.06, three RCTs, n = 1991, I2 = 0%, moderate quality evidence).
Data were insufficient to perform the pre‐defined subgroup analyses based on embryo processing and number of embryos transferred.
Complications (Analysis 1.5)
Four studies with six experimental arms reported complications (Aaleyasin 2015; Mansour 2011; Santibañez 2014; Zarei 2014) (Analysis 1.5).
None of the studies found evidence of a difference between the groups for any of the mentioned complications: ectopic pregnancy (three studies, n = 915, three events overall), heterotopic pregnancy (one study, n = 495, one event), intrauterine death (two studies, n = 978, 21 events), triplets (one study, n = 48, three events). For intrauterine death, the analysis in Figure 7 displays the Peto OR (which is the default setting for this analysis). Mantel‐Haenszel random‐effects RRs were almost identical (RR 0.82, 95% CI 0.34 to 1.94, two studies, n = 978, I2 = 0%).

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.5 Complications: intrauterine death.
Discussion
Summary of main results
This systematic review included 12 RCTs investigating the effect of intrauterine administration of hCG for 4038 subfertile women undergoing assisted reproduction. The IC‐hCG was administered in variable doses at different timings before the ET. The source of hCG was from the urine of pregnant women or from cell cultures using recombinant DNA technology.
Due to considerable heterogeneity (I2 greater than 75%) for several of the comparisons, we did not perform a global meta‐analysis, as pre‐specified in the protocol (Craciunas 2015). Exploration for the sources of heterogeneity identified two key pre‐specified variables as important determinants: stage of ET (cleavage versus blastocyst stage) and dose of IC‐hCG (less than 500 IU versus 500 IU or greater). We then performed meta‐analysis within the subgroups defined by stage of embryo and dose of IC‐hCG.
There was an increase in live birth rate in the subgroup of women having cleavage‐stage ETs with an IC‐hCG dose of 500 IU or greater compared to women having cleavage‐stage ETs with no IC‐hCG. There was no significant effect on live birth in any of the other subgroups.
There was an increase in clinical pregnancy rate in the subgroup of women having cleavage‐stage ETs with an IC‐hCG dose of 500 IU or greater compared to women having cleavage‐stage ETs with no IC‐hCG. There was no significant effect on clinical pregnancy rate in any of the other subgroups.
There was no evidence that miscarriage and complication rates were influenced by IC‐hCG administration, irrespective of embryo stage at transfer or dose of IC‐hCG.
Overall completeness and applicability of evidence
All RCTs reported on clinical pregnancy, which is an important secondary outcome, but only a few RCTs continued the follow‐up until live birth, which is the most important primary outcome.
Most RCTs reported miscarriage rates. RCTs rarely reported complications and adverse events, or their absence.
Data were insufficient to perform all the planned subgroup analyses.
The inclusion criteria for participants assured a broad range of subfertility causes and women's characteristics similar to what is expected in a regular assisted reproduction unit.
Quality of the evidence
We rated most of the studies (9/12) at high risk of bias in at least one of the seven domains assessed. Common problems were unclear reporting of study methods and lack of blinding. Brief reporting of results in studies published as abstracts represent additional potential sources of bias. Six studies did not report funding and six studies reported internal funding. None of the studies reported external funding.
The quality of the evidence as assessed using GRADE was moderate for live birth and clinical pregnancy, which means that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. The quality of the evidence for miscarriage was very low, meaning that we are very uncertain about the estimate. The main limitations in the overall quality of the evidence were high risk of bias and serious imprecision.
Potential biases in the review process
We performed a systematic search in consultation with the Cochrane Gynaecology and Fertility Group Trials Search Co‐ordinator, but we cannot be sure all relevant trials were identified for inclusion. The protocol was pre‐published and followed accordingly (Craciunas 2015). We attempted to contact authors when data were missing, but only one author replied providing clarification and additional data (Mansour 2011). We performed analyses on an intention‐to‐treat basis. Potential bias in the review process was unlikely.
Agreements and disagreements with other studies or reviews
One previously published meta‐analysis concluded that women undergoing IVF/ICSI may benefit from IC‐hCG injection before ET (Ye 2015).
The reported effect of intrauterine hCG administration was consistent within the subgroups of our review, with an apparent different effect based on the stage of embryo at transfer and dose of IC‐hCG.

Study flow diagram.
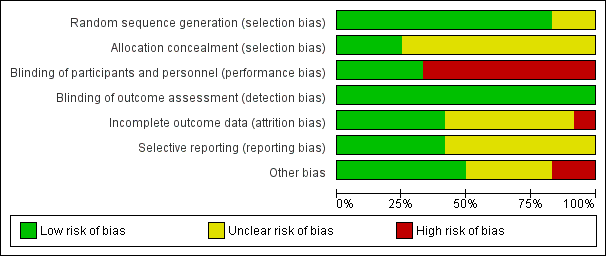
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.1 Live birth.
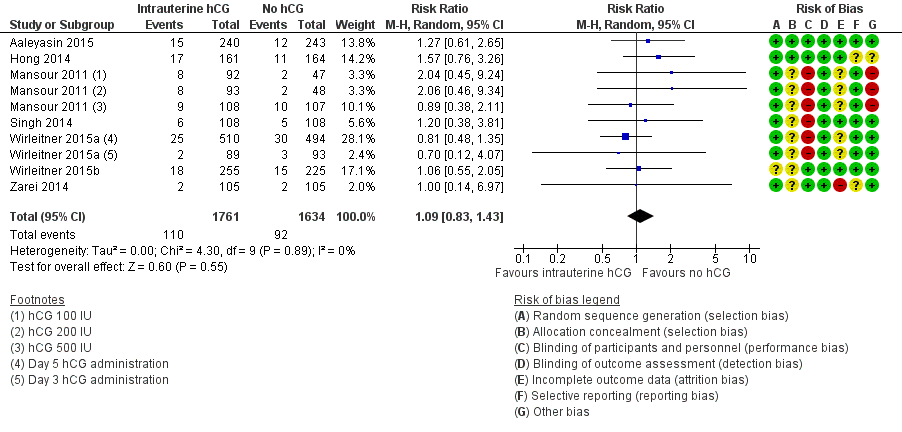
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.2 Miscarriage.

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.4 Clinical pregnancy.
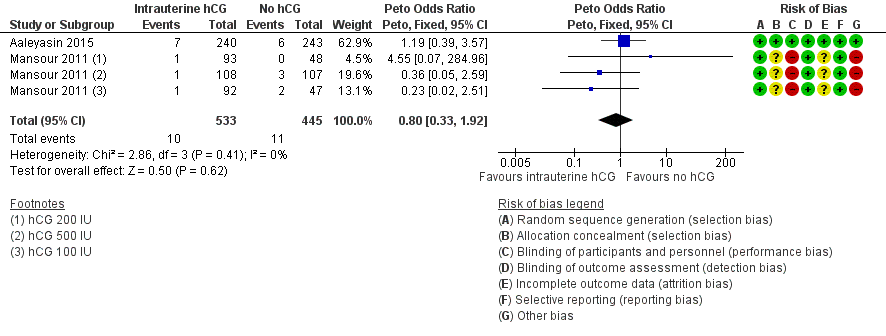
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.5 Complications: intrauterine death.
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 1 Live birth.
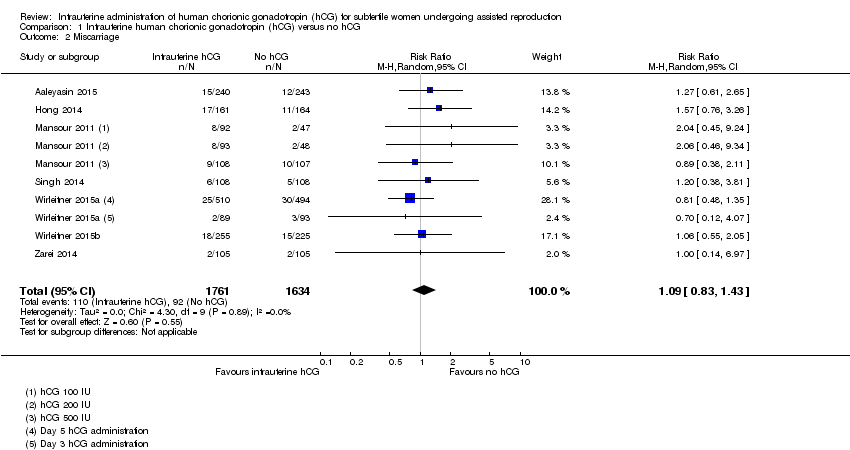
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 2 Miscarriage.
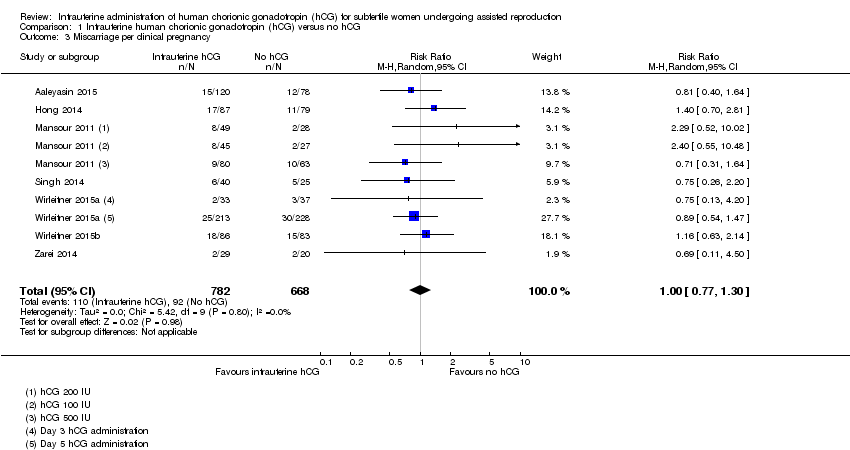
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 4 Clinical pregnancy.

Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 5 Complications: intrauterine death.
| Intrauterine administration of hCG for women undergoing assisted reproduction | |||||
| Population: women undergoing assisted reproduction | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Intrauterine administration of hCG | ||||
| Live birth ‐ cleavage stage: hCG < 500 IU | 495 per 1000 | 376 per 1000 | RR 0.76 | 280 | ⊕⊝⊝⊝ |
| Live birth ‐ cleavage stage: hCG ≥ 500 IU | 247 per 1000 | 388 per 1000 | RR 1.57 | 914 | ⊕⊕⊕⊝ |
| Live birth ‐ blastocyst stage: hCG ≥ 500 IU | 366 per 1000 | 337 per 1000 | RR 0.92 | 1666 | ⊕⊕⊕⊝ |
| Pregnancy ‐ cleavage stage: hCG < 500 IU | 579 per 1000 | 509 per 1000 (405 to 637) | RR 0.88 (0.70 to 1.10) | 280 | ⊕⊝⊝⊝ |
| Pregnancy ‐ cleavage stage: hCG ≥ 500 IU | 321 per 1000 | 453 per 1000 (401 to 507) | RR 1.41 (1.25 to 1.58) | 1414 (7 studies) | ⊕⊕⊕⊝ |
| Pregnancy ‐ blastocyst stage: hCG ≥ 500 IU | 430 per 1000 | 408 per 1000 (370 to 455) | RR 0.95 (0.86 to 1.06) | 1991 (3 studies) | ⊕⊕⊕⊝ |
| Miscarriage Follow‐up: mean 40 weeks | 48 per 1000 | 52 per 1000 (40 to 68) | RR 1.09 (0.83 to 1.43) | 3395 (7 studies) | ⊕⊝⊝⊝ |
| Other complications | Other complications reported in the included studies were ectopic pregnancy (3 studies, n = 915, 3 events overall), heterotopic pregnancy (1 study, n = 495, 1 event), intrauterine death (2 studies, n = 978, 21 events) and triplets (1 study, n = 48, 3 events). There were too few events to allow any conclusions to be drawn | ⊕⊝⊝⊝ | |||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded two levels due to very serious risk of bias: lack of blinding of participants and personnel, no clear description of allocation concealment and premature termination of the study following interim analysis. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.01] |
| 1.2 Cleavage stage: hCG ≥ 500 IU | 3 | 914 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.32, 1.87] |
| 1.3 Blastocyst stage: hCG ≥ 500 IU | 2 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.04] |
| 2 Miscarriage Show forest plot | 7 | 3395 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.83, 1.43] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 7 | 1450 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.30] |
| 4 Clinical pregnancy Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.10] |
| 4.2 Cleavage stage: hCG ≥ 500 IU | 7 | 1414 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.25, 1.58] |
| 4.3 Blastocyst stage: hCG ≥ 500 IU | 3 | 1991 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.06] |
| 5 Complications: intrauterine death Show forest plot | 2 | 978 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.33, 1.92] |

