Extracción temprana versus tardía del stent ureteral después del trasplante de riñón
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011455.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 29 enero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Riñón y trasplante
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Draft the protocol: CHW, SAH, MLN
-
Study selection: CHW, SAH, ERT
-
Extract data from studies: CHW, SAH, ERT
-
Enter data into RevMan: CHW, SAH, ERT
-
Carry out the analysis: CHW, SAH, ERT
-
Interpret the analysis: CHW, SAH, ERT
-
Draft the final review: CHW, SAH, ERT
-
Disagreement resolution: MLN
-
Update the review: CHW, SAH
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge, UK.
-
Newcastle University, UK.
-
NHS Blood and Transplant (NHSBT), UK.
Declarations of interest
-
Emily Thompson: none known
-
Sarah Hosgood: none known
-
Michael Nicholson: none known
-
Colin Wilson: none known
Acknowledgements
The authors would like to acknowledge the help and support given by Cochrane Kidney and Transplant Group and the referees for their comments and feedback. The research was funded by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or NHSBT.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jan 29 | Early versus late ureteric stent removal after kidney transplantation | Review | Emily R Thompson, Sarah A Hosgood, Michael L Nicholson, Colin H Wilson | |
| 2015 Jan 05 | Early versus late ureteric stent removal after kidney transplantation | Protocol | Colin H Wilson, Sarah A Hosgood, Michael L Nicholson | |
Differences between protocol and review
There were no identified studies that utilised the PC method of stent placement and therefore this subgroup analysis that was included in the protocol could not be included. Only two studies included examined in any detail the incidence of idiosyncratic stent complications (e.g. bladder irritation, haematuria, encrustation) and therefore a robust meta‐analysis could not be performed.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Device Removal [*adverse effects];
- Foreign Bodies [etiology];
- Incidence;
- Kidney Transplantation [*adverse effects];
- Postoperative Complications [epidemiology, *etiology, prevention & control];
- Randomized Controlled Trials as Topic;
- Stents [*adverse effects];
- Time Factors;
- *Ureter;
- Urinary Bladder;
- Urinary Tract Infections [epidemiology, *etiology, prevention & control];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO
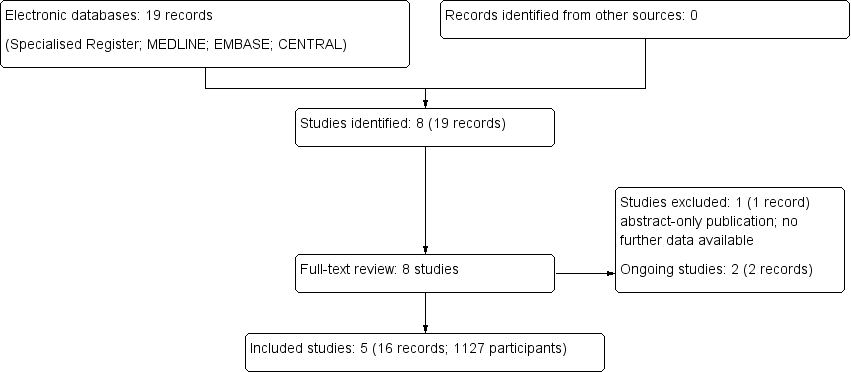
Flow chart of study selection

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
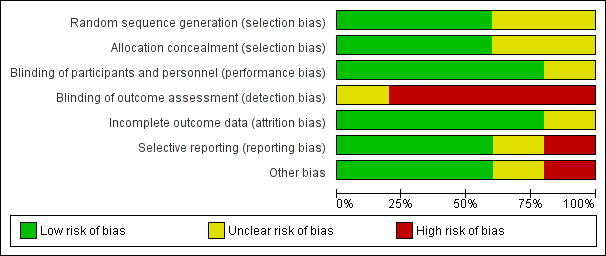
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Major urological complications, Outcome 1 Major urological complications.

Comparison 2 Urinary tract infection, Outcome 1 Urinary tract infection.
| Early versus late ureteric stent removal after kidney transplantation | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with late removal | Risk with early removal | ||||
| Major urological complications: all stents | Study population | RR 1.87 | 1127 (5) | ⊕⊕⊕⊕ | |
| 12 per 1,000 | 23 per 1,000 | ||||
| Major urological complications: bladder indwelling stents | Study population | RR 1.67 | 539 (3) | ⊕⊕⊕⊕ | |
| 15 per 1,000 | 24 per 1,000 | ||||
| Major urological complications: per‐urethral stents | Study population | RR 1.51 | 588 (2) | ⊕⊕⊕⊕ | |
| 10 per 1,000 | 15 per 1,000 | ||||
| Urinary tract infection: all stents | Study population | RR 0.49 | 1126 (5) | ⊕⊕⊕⊝ | |
| 185 per 1,000 | 91 per 1,000 | ||||
| Urinary tract infection: bladder indwelling stents | Study population | RR 0.45 | 539 (3) | ⊕⊕⊕⊝ | |
| 209 per 1,000 | 94 per 1,000 | ||||
| Urinary tract infection: per‐urethral stents | Study population | RR 0.60 | 587 (2) | ⊕⊕⊝⊝ | |
| 164 per 1,000 | 98 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 All studies were unblinded, however, this was unavoidable given the nature of the intervention. The majority of studies provided minimal information on processes of randomisation and allocation 2 Inconsistent definition and variable reporting of urinary tract infection across included studies | |||||
| Study ID | Adverse events |
| Two patients in the late group required re‐stenting due to ureteric stenosis | |
| Three patients in the late group had forgotten stents that were subsequently removed at 12 weeks | |
| Six patients in the early and 5 patients in the late group had acute rejection that required intervention | |
| No adverse events reported | |
| Sixteen patients did not receive their allocated treatment as there were technical difficulties attaching the stent to the catheter. In the early removal group, 1 patient's stent removal was delayed by 1 day because the urethral catheter balloon needed percutaneous needle puncture due to the stent suture There were 5 complications in patients who had early stent removal and these were all related to the percutaneous technique used in which the stent was tied to the catheter |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major urological complications Show forest plot | 5 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.61, 5.71] |
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.52, 5.36] |
| 1.2 Per‐urethral stents | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.03, 74.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urinary tract infection Show forest plot | 5 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.70] |
| 1.2 Per‐urethral stents | 2 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.03] |

