Entrenamiento con ejercicios de miembros superiores para la EPOC
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 20), never received arm exercise training previously Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Endurance Upper Limb Capacity: unsupported
| |
| Identification | Sponsorship source: Theta Mu Chapter, Sigma Theta Tau, Pittsburgh, Pennsylvania. Country: USA Setting: home‐based training Authors name: Gerene S. Bauldoff Institution: University of Pittsburgh Medical Center Address: University of Pittsburgh Medical Center, Pittsburgh, PA 15213. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation process involved assembling 20 envelopes from which subjects blindly selected 1 that determined their group assignment. |
| Allocation concealment (selection bias) | Low risk | The randomization process involved assembling 20 envelopes from which the subjects blindly selected 1 that determined their group assignment. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | All phone calls, home testing, and training were performed by the same investigator. There was no blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | Unclear risk | Data reported for all outcomes stated (presumed to be complete for all participants but not explicitly stated). |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes stated. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 43) with age > 45 yrs Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Upper Limb Strength
(NB: this could not be used in the pooled analysis as data was not available for the experimental group alone) Respiratory Muscle Strength
Physical Activity Level: Subjective
| |
| Identification | Sponsorship source: the sources of support for this research were The National Institute of Nursing Research R01‐NR08037 and the University of Illinois at Chicago General Clinical Research Center M01‐RR‐13987. Country: USA Setting: lab‐based training Authors name: Margaret K. Covey Institution: University of Illinois at Chicago, USA Email: [email protected] Address: Department of Biobehavioral Health Science, University of Illinois at Chicago, M/C 802, 845 S. Damen Avenue, Chicago, IL, 60612, USA. | |
| Notes | There was a third intervention group who performed upper body resistance training with self‐efficacy training. As this group does not reflect the typical delivery of arm training in a pulmonary rehabilitation programme, the group with arm training and health education only was chosen as the experimental group. However, some data in this paper combined these 2 groups so was not able to be used in the pooled analysis (e.g. upper limb strength where differences between the 2 groups were reported). For dyspnoea, respiratory muscle strength and physical activity level the pooled analysis used a sample size of n = 22, reflecting the experimental group numbers as the paper had reported no significant differences between the 2 upper limb training groups for these outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to group was stratified by gender and disease severity (GOLD stages II, III, and IV) with a software program (biased coin algorithm to ensure equivalent groups)." |
| Allocation concealment (selection bias) | Low risk | Quote: "This was a concealed allocation process." |
| Blinding of participants and personnel (performance bias) | High risk | Whilst subjects were not informed of the intent of the 3 groups, they were aware what group they were in. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Data collectors were blinded to the group assignment." |
| Incomplete outcome data (attrition bias) | High risk | There was a 25% drop‐out rate for the study (n = 7 in each group × 3 groups). |
| Selective reporting (reporting bias) | High risk | Data has been presented across 2 different publications. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 23) Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Endurance Upper Limb Exercise Capacity: unsupported
Respiratory Muscle Strength
| |
| Identification | Country: USA Setting: outpatient and inpatient Authors name: Scott K Epstein. Institution: New England Medical Center and St. Elizabeth's Medical Center, Tufts University. Address: Pulmonary and Critical Care Division, New England Medical Center, Box 369, 750 Washington St, Boston, MA 02111. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | Low risk | The investigators performing the measurements were blinded to the method of training. |
| Incomplete outcome data (attrition bias) | Low risk | Low numbers of drop‐out with reasons provided for incomplete data (i.e. 1 participant withdrew and 2 participants developed respiratory failure). |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes stated. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Severe to very severe, clinically stable COPD (n = 38) Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Peak Upper Limb Exercise Capacity: unsupported
Dyspnoea from questionnaire
Health‐Related Quality of Life
| |
| Identification | Sponsorship source: Alfred Research Trust Small Projects Grant. Country: Australia Setting: outpatient and home programme Authors name: Anne E. Holland Institution: Department of Physiotherapy, Alfred Hospital, Melbourne and University of Melbourne, Melbourne, Victoria, Australia. Email: [email protected] Address: Department of Physiotherapy, Alfred Hospital, Commercial Road, Melbourne, Australia 3004 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Measurements were obtained by an independent data collector blinded to group allocation." |
| Incomplete outcome data (attrition bias) | Low risk | Two participants did not complete the study so overall completion rate was high at 95%. |
| Selective reporting (reporting bias) | Unclear risk | Data not reported for arm fatigue during the arm exercise test. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable, moderate to very severe COPD (n = 12), aged > 50 yrs Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Upper Limb Strength (pulley and bench press)
Endurance Upper Limb Exercise Capacity: Unsupported
| |
| Identification | Country: Brazil Authors name: Daniela Ike | |
| Notes | Study reported in Portugese and required translation to English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | Low risk | Complete data available for all outcomes. |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes stated. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable, mild to very severe COPD (n = 36) Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Health‐Related Quality of Life
Peak Upper Limb Exercise Capacity: unsupported
Upper Limb Strength (shoulder flexion and abduction)
Endurance Upper Limb Exercise Capacity: unsupported
| |
| Identification | Sponsorship source: this study was supported by the Ontario Thoracic Society, West Park Healthcare Centre Foundation, Canada Research Chair Program, and the Swedish Heart and Lung Foundation. Country: Canada Setting: outpatient and inpatient Authors name: Tania Janaudis‐Ferreira Institution: West Park Healthcare Centre, Toronto, ON, Canada Email: [email protected] Address: Department of Physical Therapy, University of Toronto, 160‐500 University Ave, Toronto, ON, Canada, M5G1V7 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly (in blocks of 4) assigned to an intervention or control group. Randomization was stratified according to the presence or absence of the use of supplemental oxygen at rest." |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was kept in opaque envelopes by an investigator who was not involved in the recruitment process. These envelopes were drawn by the trainer after the subjects had completed their pre assessment session, allowing for concealed allocation." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the patients remained unaware of the group allocation." However, the trainers were aware of group allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The outcome assessor and the patients remained unaware of the group allocation." |
| Incomplete outcome data (attrition bias) | Low risk | Data not available in 14% of participants. Reasons were provided, mostly unrelated to the study. |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes stated. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable, severe COPD (n = 26) Baseline characteristics Upper Limb Training Only
Control ‐ No Training
Combined Upper Limb and Lower Limb Training
Lower Limb Training Only
| |
| Interventions | Intervention characteristics 1. Upper Limb Training Only versus No Training Experimental
Control
2. Combined Upper Limb Training and Lower Limb Training versus Lower Limb Training Alone Combined Experimental
Control (Lower Limb only)
| |
| Outcomes | Peak Upper Limb Exercise Capacity: supported
Respiratory Muscle Strength
| |
| Identification | Country: Australia Setting: Outpatient Authors name: Fiona R.Lake Institution: Sir Charles Gairdner Hospital, Perth, Western Australia. Address: Sir Charles Gairdner Hospital, Verdun Street, Nedlands West Australia 6009. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | There is no description of how they performed the allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | Low risk | Data not available in 7% of participants. Reasons were provided, mostly unrelated to the study. |
| Selective reporting (reporting bias) | Unclear risk | Data not reported for resting blood pressure. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable, moderate to severe COPD (n = 34), age > 45 yrs Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Physical Activity Level ‐ Objective
| |
| Identification | Sponsorship source: The National Institute of Nursing Research R01‐NR08037 and the University of Illinois at Chicago General Clinical Research Center M01‐RR‐13987. Country: USA Authors name: Janet L Larson Institution: University of Michigan, Ann Arbor Email: [email protected] Address: University of Michigan, 400 N Ingalls, Ann Arbor, MI 48109, USA | |
| Notes | There was a third intervention group who performed upper body resistance training with self‐efficacy training. As this group does not reflect the typical delivery of arm training in a pulmonary rehabilitation programme, the group with arm training and health education only was chosen as the experimental group. This was the same study as the Covey 2012 study but the Larson 2014 paper includes an objective measure of physical activity in 34 COPD patients (which was not part of the Covey 2012 paper). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by sex and disease severity (Global Initiative on Obstructive Lung Disease stages II, III, and IV) 8 using a customized computer program that blinded investigators to group assignment." |
| Allocation concealment (selection bias) | Low risk | Customized computer program would have concealed the allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Whilst all participants were assigned to an active intervention and were not told which was the experimental group, all participants knew which intervention they were performing. |
| Blinding of outcome assessment (detection bias) | Low risk | The data collectors were blinded to group assignment. |
| Incomplete outcome data (attrition bias) | High risk | 42% participants were not included at 16 weeks. |
| Selective reporting (reporting bias) | High risk | Data has been presented across 2 different publications. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 14) Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Endurance Upper Limb Exercise Capacity: unsupported
Upper Limb Strength
| |
| Identification | Country: Brazil Setting: outpatient (assumed) Authors name: Kamilla Tays Marrara, Institution: Federal University of São Carlos – UFSCar Email: [email protected] Address: Federal University of São Carlos – UFSCar, Av. Filomeno Rispoli no. 179, Parque Santa Marta, CEP: 13564‐200, São Carlos, São Paulo, Brazil | |
| Notes | Data not used in meta‐analysis as only metabolic data presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | 24% drop out in the study. |
| Selective reporting (reporting bias) | Unclear risk | Only metabolic outcomes reported so data not available for meta‐analysis. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 35) Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Endurance Upper Limb Exercise Capacity: unsupported
Peak Upper Limb Exercise Capacity: supported
Respiratory Muscle Strength
| |
| Identification | Sponsorship source: no description Country: USA Setting: outpatient Authors name: Fernando J. Martinez Institution: University of Michigan Medical Center Email: [email protected] Address: 1500 East Medical Center Drive, Ann Arbor MI 48109‐0026 | |
| Notes | Authors contacted for group data but no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | Low risk | Data were not available for 12% of participants but reasons were provided for this, which was unrelated to the study. |
| Selective reporting (reporting bias) | High risk | Mean group data on main outcomes not provided so data not used in meta‐analysis. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | COPD (n = 33) No baseline characteristics provided. | |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Health‐Related Quality of Life
Respiratory Muscle Strength
| |
| Identification | Country: Japan Setting: there is no description of setting. Comments: abstract only so few details Authors name: H. Matsunaga Institution: Nippon Steel Yawata Memorial Hospital, Kitakyushu, Japan, | |
| Notes | Abstract only, no email to contact authors so data cannot be included in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficent information provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Unclear risk | Insufficient information provided. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 38) Baseline characteristics Endurance Training Group
Resistance Training Group
Combined Endurance and Resistance Training Group
Control
| |
| Interventions | Intervention characteristics All groups had exercise training 3 times per week for 8 weeks and performed standard leg endurance and strength training during this period. Experimental (Endurance Training)
Experimental (Resistance Training)
Experimental (Combined Training)
Control
| |
| Outcomes | Endurance Upper Limb Exercise Capacity: supported
Peak Upper Limb Exercise Capacity: Supported
Health‐Related Quality of Life
Respiratory muscle strength
Peak Upper Limb Exercise Capacity: Unsupported
| |
| Identification | Sponsorship source: this article was supported by the Australian Respiratory Council. Country: Australia Setting: outpatient Authors name: Zoe J McKeough Institution: University of Sydney Email: [email protected] Address: Discipline of Physiotherapy, Faculty of Health Sciences, The University of Sydney, 75 East St Lidcombe, NSW 2141, Australia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was via computerised random number generation." |
| Allocation concealment (selection bias) | Low risk | Quote: "...with the sequence concealed using opaque envelopes prepared by an investigator not involved in the study." |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An assessor, blinded to group allocation, performed the outcome measures at baseline and at the end of the intervention period." |
| Incomplete outcome data (attrition bias) | High risk | Quote: "A total of 52 subjects were recruited and 38 (73%) completed the study with 11 in the endurance group, 9 in the strength group, 9 in the combined group, and 9 in the control group". Therefore outcome data was not available in 27%. |
| Selective reporting (reporting bias) | High risk | Data not reported in the paper for respiratory muscle strength but authors have provided this data for the meta‐analysis. Data not reported for upper limb strength although stated in trials registry. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 28) Baseline characteristics Endurance Training Group
Resistance Training Group
Control
| |
| Interventions | Intervention characteristics All groups did lower limb training such as walking over 8 weeks with weekly supervision. Experimental (Endurance Training)
Experimental (Resistance Training)
Control
| |
| Outcomes | Endurance Upper Limb Exercise Capacity: unsupported
Peak Upper Limb Exercise Capacity: supported
Endurance Upper Limb Exercise Capacity: Supported
Activities of Daily Living Function
| |
| Identification | Sponsorship source: supported in part by the Easter Seal Research Foundation of the National Easter Seal Society and National Institutes of Health grant RR00827 from the Division of Research Resources for the Clinical Research Center. Country: USA Setting: outpatient Authors name: Andrew L Ries Institution: Department of Medicine, University of California, San Diego Address: UCSD Medical Center H‐772, 225 Dickinson Street, San Diego, 92103 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not provided. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | 38% of participants did not complete the study. Reasons were given and one may have related to the study. |
| Selective reporting (reporting bias) | Unclear risk | Data from baseline analysis not available. |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable, severe COPD (n = 28) Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Endurance Upper Limb Exercise Capacity: unsupported
Health‐Related Quality of Life
Respiratory Muscle Strength
Hospitalisation index
| |
| Identification | Sponsorship source: none Country: Argentina Setting: Outpatient Authors name: Martin L Sivori Institution: Servicio de Neumonologia Policlinico Bancário. Email: [email protected] Address: Dr Martin L Sivori, Lafayette 124 1872 Avellaneda, Pcia Buenos Aires, Argentina | |
| Notes | Study reported in Spanish and required translation to English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by simple table. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence was not provided. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | 35% did not complete the study. Reasons were given not directly related to study. |
| Selective reporting (reporting bias) | Unclear risk | Data not provided for the quality of life domains (only a total score was given). |
| Other bias | Low risk | Study appears to be free of other sources of risk. |
| Methods | Study design: randomized controlled trial | |
| Participants | Clinically stable COPD (n = 17), age 45 to 75 yrs Baseline characteristics Experimental
Control
| |
| Interventions | Intervention characteristics Experimental
Control
| |
| Outcomes | Dyspnoea from questionnaire
Peak Upper Limb Exercise Capacity: unsupported
| |
| Identification | Country: India Setting: outpatient Authors name: Vaishali Rao Subin Institution: Department of Physiotherapy, Kasturba Medical College Email: [email protected] Address: Ms. Vaishali Rao, Department of Physiotherapy, Kasturba Medical College, Mangalore, India | |
| Notes | A third group was involved and performed upper limb training only but was not included in this review as there was no control group with no training. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used. |
| Allocation concealment (selection bias) | Unclear risk | Block randomisation used but unclear whether it is concealed. |
| Blinding of participants and personnel (performance bias) | High risk | There was no indication that participants or personnel were blind from the knowledge of which intervention the participant was receiving. |
| Blinding of outcome assessment (detection bias) | High risk | There was no indication that there was blinding of the outcome assessor. |
| Incomplete outcome data (attrition bias) | Low risk | Appears to be missing data for 10% of participants only. |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes stated. |
| Other bias | High risk | Block randomisation in an unblinded trial. |
COPD: Chronic Obstructive Pulmonary Disease
FEV₁ ‐ forced expiratory volume in 1 second
yrs: years
L: litres
% pred: percent predicted
no.: number
RM: repetition maximum
lbs: pounds
kg: kilograms
Pimax: maximal inspiratory mouth pressure
cmH₂O: centimetres of water
GOLD: Global Initiative for Chronic Obstructive Lung Disease
wkly: weekly
s: seconds
min: minutes
IQR: interquartile range
RPE: rate of perceived exertion
shld: shoulder
kpm/min: kilopond metre per minute
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention did not fit study criteria | |
| Study design did not fit study criteria | |
| Intervention did not fit study criteria | |
| Comparator group did not fit study criteria | |
| Intervention did not fit study criteria | |
| Outcomes did not fit study criteria | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Editorial ‐ not an original study on upper limb training | |
| No upper limb training intervention | |
| Intervention did not fit study criteria | |
| Intervention did not fit study criteria | |
| Outcomes did not fit study criteria | |
| Study design did not fit study criteria | |
| Outcomes did not fit study criteria | |
| This is an abstract of a full text study that has been included | |
| Outcomes did not fit study criteria | |
| Intervention did not fit study criteria |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: randomized controlled trial |
| Participants | Stable COPD (n = 42), age 40 to 80 years |
| Interventions | Intervention characteristics Experimental
Control
|
| Outcomes | Peak Upper Limb Exercise Capacity: Supported
Upper Limb Strength (hand grip strength)
Activities of Daily Living Function (ADL simulation test)
|
| Notes | This paper was found in the search update conducted on 28 September 2016 and will undergo full review when the next update occurs. |
| Methods | Study design: randomized controlled trial |
| Participants | COPD (n = 39) Baseline characteristics Experimental
Control
|
| Interventions | Intervention characteristics Both groups did lower limb exercise training. Experimental
Control
|
| Outcomes | Dyspnoea from questionnaire
Peak Upper Limb Exercise Capacity: Supported
Endurance Upper Limb Exercise Capacity: Unsupported
|
| Notes | Abstract only so unclear whether the upper limb training is > 4 weeks duration. Unable to contact authors for further information on duration of training. |
COPD: Chronic Obstructive Pulmonary Disease
FEV₁ ‐ forced expiratory volume in one second
L: litres
no.: number
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms of Dyspnoea Show forest plot | 4 | 129 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.02, 0.72] |
| Analysis 1.1  Comparison 1 Upper limb training versus No upper limb training, Outcome 1 Symptoms of Dyspnoea. | ||||
| 1.1 Endurance Training | 2 | 55 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.13, 0.95] |
| 1.2 Resistance Training | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.11, 0.80] |
| 2 Health‐Related Quality of Life Show forest plot | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.31, 0.40] |
| Analysis 1.2  Comparison 1 Upper limb training versus No upper limb training, Outcome 2 Health‐Related Quality of Life. | ||||
| 2.1 Endurance Training | 3 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.47, 0.42] |
| 2.2 Resistance Training | 2 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.43, 0.79] |
| 3 Peak Upper Limb Exercise Capacity: Supported Show forest plot | 3 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.43, 0.77] |
| Analysis 1.3  Comparison 1 Upper limb training versus No upper limb training, Outcome 3 Peak Upper Limb Exercise Capacity: Supported. | ||||
| 3.1 Endurance Training | 3 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.29, 1.16] |
| 3.2 Resistance Training | 2 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.24, 0.52] |
| 4 Peak Upper Limb Exercise Capacity: Unsupported Show forest plot | 4 | 112 | Mean Difference (IV, Random, 95% CI) | 21.23 [‐20.45, 62.92] |
| Analysis 1.4  Comparison 1 Upper limb training versus No upper limb training, Outcome 4 Peak Upper Limb Exercise Capacity: Unsupported. | ||||
| 4.1 Endurance Training | 3 | 69 | Mean Difference (IV, Random, 95% CI) | 10.29 [‐47.37, 67.95] |
| 4.2 Resistance Training | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 33.22 [‐27.12, 93.57] |
| 5 Endurance Upper Limb Exercise Capacity: Supported Show forest plot | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.46, 0.96] |
| Analysis 1.5  Comparison 1 Upper limb training versus No upper limb training, Outcome 5 Endurance Upper Limb Exercise Capacity: Supported. | ||||
| 5.1 Endurance Training | 2 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.23, 1.35] |
| 5.2 Resistance Training | 2 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.35, 1.18] |
| 6 Endurance Upper Limb Exercise Capacity: Unsupported Show forest plot | 6 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.19, 1.13] |
| Analysis 1.6  Comparison 1 Upper limb training versus No upper limb training, Outcome 6 Endurance Upper Limb Exercise Capacity: Unsupported. | ||||
| 6.1 Endurance Training | 4 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.99 [0.32, 1.66] |
| 6.2 Resistance Training | 3 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.31, 0.76] |
| 7 Upper Limb Strength Show forest plot | 2 | 43 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.39, 0.89] |
| Analysis 1.7  Comparison 1 Upper limb training versus No upper limb training, Outcome 7 Upper Limb Strength. | ||||
| 7.1 Resistance Training | 2 | 43 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.39, 0.89] |
| 8 Respiratory Muscle Strength Show forest plot | 5 | 148 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐8.35, 4.94] |
| Analysis 1.8  Comparison 1 Upper limb training versus No upper limb training, Outcome 8 Respiratory Muscle Strength. | ||||
| 8.1 Endurance Training | 4 | 92 | Mean Difference (IV, Random, 95% CI) | ‐3.41 [‐11.02, 4.20] |
| 8.2 Resistance Training | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐9.87, 17.46] |
| 9 Physical Activity Level: Subjective Show forest plot | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.30, 0.30] |
| Analysis 1.9  Comparison 1 Upper limb training versus No upper limb training, Outcome 9 Physical Activity Level: Subjective. | ||||
| 10 Physical Activity Level: Objective Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.68, 2.68] |
| Analysis 1.10  Comparison 1 Upper limb training versus No upper limb training, Outcome 10 Physical Activity Level: Objective. | ||||
| 11 Activities of Daily Living Show forest plot | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.12, 1.47] |
| Analysis 1.11  Comparison 1 Upper limb training versus No upper limb training, Outcome 11 Activities of Daily Living. | ||||
| 11.1 Endurance Training | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.19, 2.08] |
| 11.2 Resistance Training | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.69, 1.52] |
| 12 Healthcare Utilisation Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐3.07, 1.35] |
| Analysis 1.12  Comparison 1 Upper limb training versus No upper limb training, Outcome 12 Healthcare Utilisation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
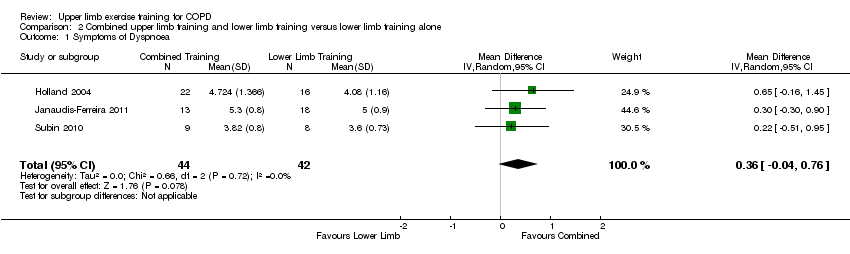
| 1 Symptoms of Dyspnoea Show forest plot | 3 | 86 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.04, 0.76] |
| Analysis 2.1  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 1 Symptoms of Dyspnoea. | ||||
| 2 Health‐Related Quality of Life Show forest plot | 3 | 95 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.40, 0.43] |
| Analysis 2.2  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 2 Health‐Related Quality of Life. | ||||
| 3 Peak Upper Limb Exercise Capacity: Supported Show forest plot | 3 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.55, 0.44] |
| Analysis 2.3  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 3 Peak Upper Limb Exercise Capacity: Supported. | ||||
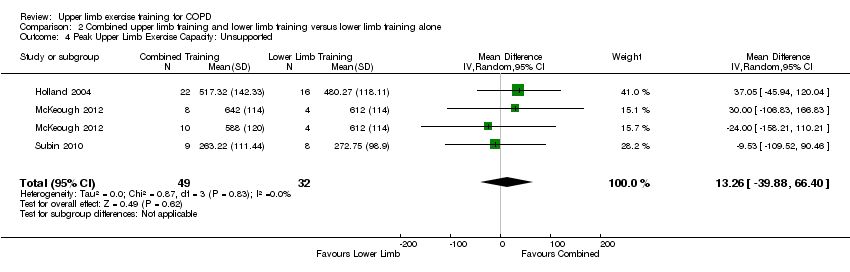
| 4 Peak Upper Limb Exercise Capacity: Unsupported Show forest plot | 3 | 81 | Mean Difference (IV, Random, 95% CI) | 13.26 [‐39.88, 66.40] |
| Analysis 2.4  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 4 Peak Upper Limb Exercise Capacity: Unsupported. | ||||
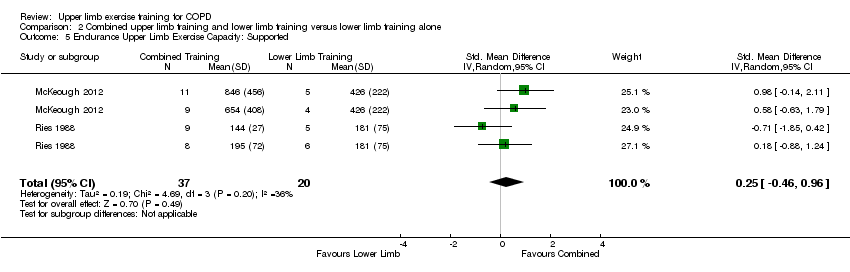
| 5 Endurance Upper Limb Exercise Capacity: Supported Show forest plot | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.46, 0.96] |
| Analysis 2.5  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 5 Endurance Upper Limb Exercise Capacity: Supported. | ||||
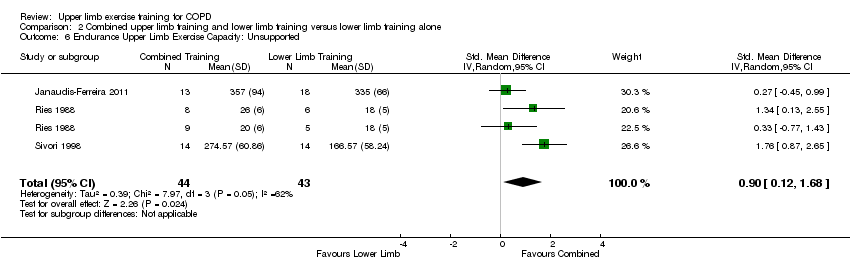
| 6 Endurance Upper Limb Exercise Capacity: Unsupported Show forest plot | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.90 [0.12, 1.68] |
| Analysis 2.6  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 6 Endurance Upper Limb Exercise Capacity: Unsupported. | ||||
| 7 Upper Limb Strength Show forest plot | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.70, 0.73] |
| Analysis 2.7  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 7 Upper Limb Strength. | ||||
| 8 Respiratory Muscle Strength Show forest plot | 3 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐8.99, 8.07] |
| Analysis 2.8  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 8 Respiratory Muscle Strength. | ||||
| 9 Activities of Daily Living Show forest plot | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.12, 1.47] |
| Analysis 2.9  Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 9 Activities of Daily Living. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐Related Quality of Life Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐5.00 [‐20.85, 6.85] |
| Analysis 3.1  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 1 Health‐Related Quality of Life. | ||||
| 2 Peak Upper Limb Exercise Capcity: Supported Show forest plot | 2 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.29, 1.02] |
| Analysis 3.2  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 2 Peak Upper Limb Exercise Capcity: Supported. | ||||
| 3 Peak Upper Limb Exercise Capacity: Unsupported Show forest plot | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐54.0 [‐162.50, 54.50] |
| Analysis 3.3  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 3 Peak Upper Limb Exercise Capacity: Unsupported. | ||||
| 4 Endurance Upper Limb Exercise Capacity: Supported Show forest plot | 2 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.03, 1.31] |
| Analysis 3.4  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 4 Endurance Upper Limb Exercise Capacity: Supported. | ||||
| 5 Endurance Upper Limb Exercise Capacity: Unsupported Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 6.00 [0.29, 11.71] |
| Analysis 3.5  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 5 Endurance Upper Limb Exercise Capacity: Unsupported. | ||||
| 6 Respiratory Muscle Strength Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐28.21, ‐1.79] |
| Analysis 3.6  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 6 Respiratory Muscle Strength. | ||||
| 7 Activities of Daily Living Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 27.0 [‐148.71, 202.71] |
| Analysis 3.7  Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 7 Activities of Daily Living. | ||||
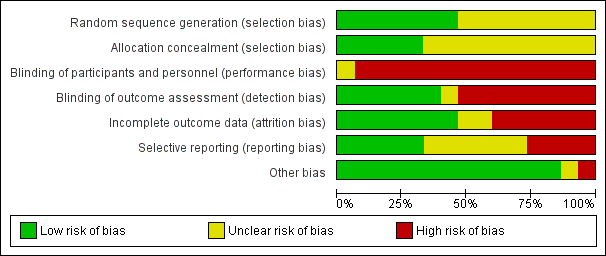
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Upper limb training only versus control, outcome: 1.1 Symptoms of Dyspnoea.

Forest plot of comparison: 1 Upper limb training only versus control, outcome: 1.2 Health‐Related Quality of Life.

Forest plot of comparison: 1 Upper limb training versus No upper limb training, outcome: 1.6 Endurance Upper Limb Exercise Capacity: Unsupported.
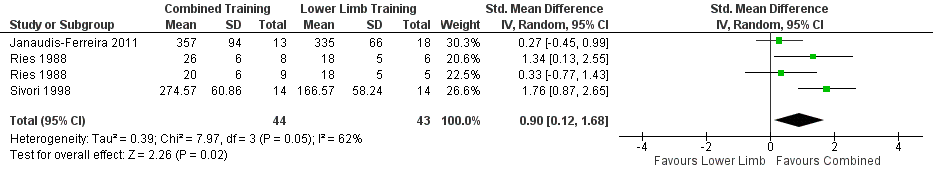
Forest plot of comparison: 2 Combined upper limb training and lower limb training versus lower limb training alone, outcome: 2.6 Endurance Upper Limb Exercise Capacity: Unsupported.

Comparison 1 Upper limb training versus No upper limb training, Outcome 1 Symptoms of Dyspnoea.
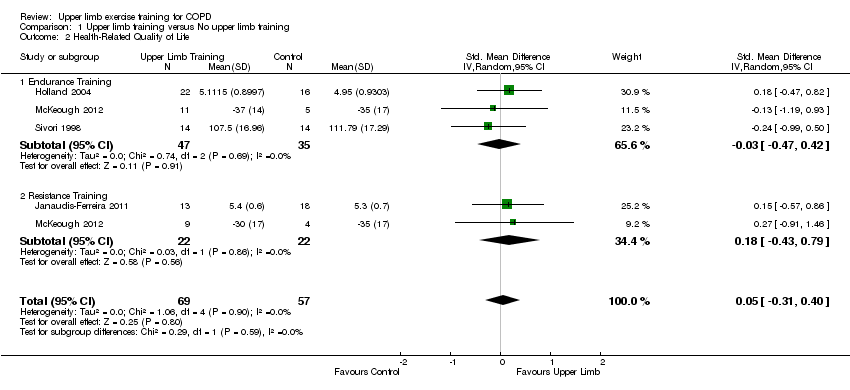
Comparison 1 Upper limb training versus No upper limb training, Outcome 2 Health‐Related Quality of Life.
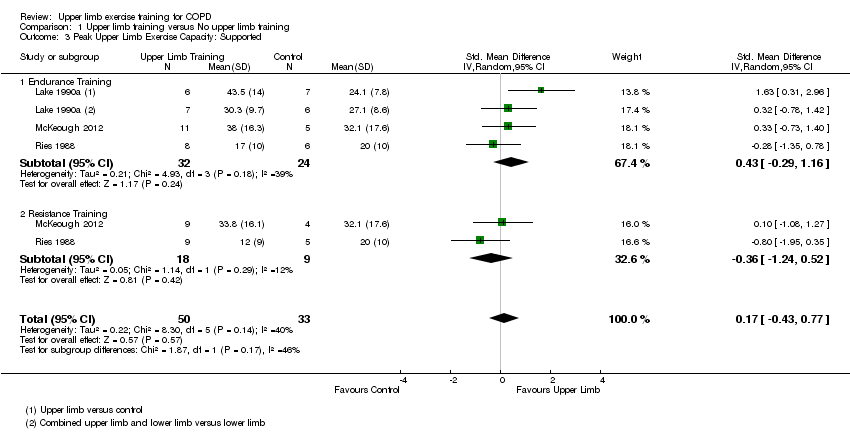
Comparison 1 Upper limb training versus No upper limb training, Outcome 3 Peak Upper Limb Exercise Capacity: Supported.

Comparison 1 Upper limb training versus No upper limb training, Outcome 4 Peak Upper Limb Exercise Capacity: Unsupported.

Comparison 1 Upper limb training versus No upper limb training, Outcome 5 Endurance Upper Limb Exercise Capacity: Supported.

Comparison 1 Upper limb training versus No upper limb training, Outcome 6 Endurance Upper Limb Exercise Capacity: Unsupported.

Comparison 1 Upper limb training versus No upper limb training, Outcome 7 Upper Limb Strength.

Comparison 1 Upper limb training versus No upper limb training, Outcome 8 Respiratory Muscle Strength.

Comparison 1 Upper limb training versus No upper limb training, Outcome 9 Physical Activity Level: Subjective.

Comparison 1 Upper limb training versus No upper limb training, Outcome 10 Physical Activity Level: Objective.

Comparison 1 Upper limb training versus No upper limb training, Outcome 11 Activities of Daily Living.

Comparison 1 Upper limb training versus No upper limb training, Outcome 12 Healthcare Utilisation.
Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 1 Symptoms of Dyspnoea.

Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 2 Health‐Related Quality of Life.

Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 3 Peak Upper Limb Exercise Capacity: Supported.
Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 4 Peak Upper Limb Exercise Capacity: Unsupported.
Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 5 Endurance Upper Limb Exercise Capacity: Supported.
Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 6 Endurance Upper Limb Exercise Capacity: Unsupported.

Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 7 Upper Limb Strength.

Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 8 Respiratory Muscle Strength.
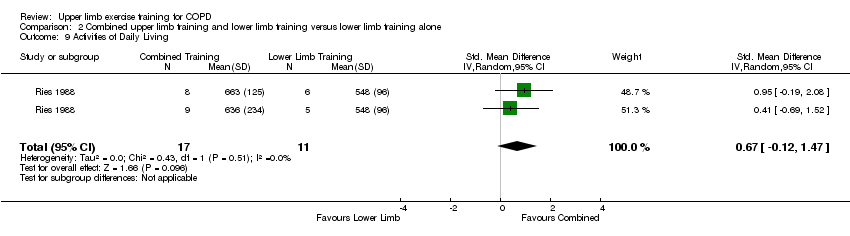
Comparison 2 Combined upper limb training and lower limb training versus lower limb training alone, Outcome 9 Activities of Daily Living.

Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 1 Health‐Related Quality of Life.
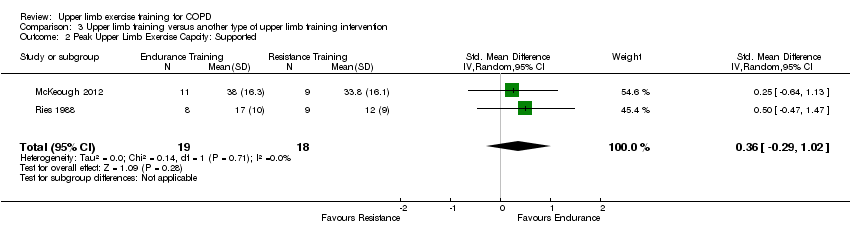
Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 2 Peak Upper Limb Exercise Capcity: Supported.

Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 3 Peak Upper Limb Exercise Capacity: Unsupported.
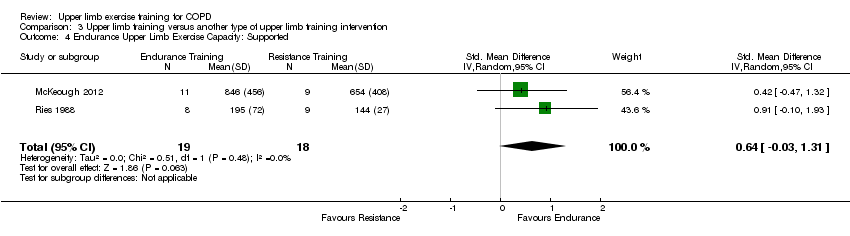
Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 4 Endurance Upper Limb Exercise Capacity: Supported.
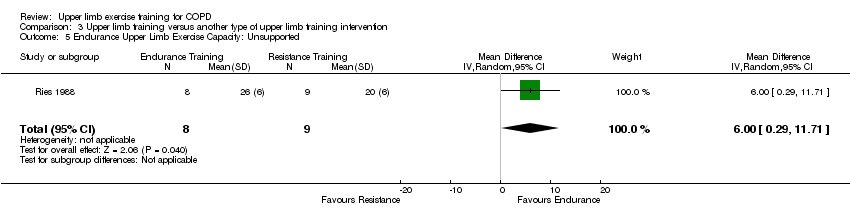
Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 5 Endurance Upper Limb Exercise Capacity: Unsupported.
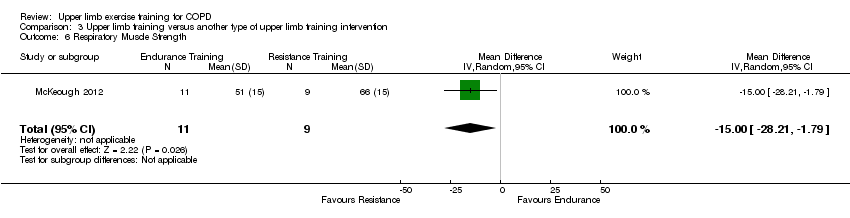
Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 6 Respiratory Muscle Strength.

Comparison 3 Upper limb training versus another type of upper limb training intervention, Outcome 7 Activities of Daily Living.
| Comparison 1: Upper limb training vs No upper limb training for people with COPD | ||||||
| Patient or population: Stable COPD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with No upper limb training | Risk with Upper limb training | |||||
| Symptoms of dyspnoea | The mean symptoms of dyspnoea was 4.2 points. | The mean symptoms of dyspnoea in the intervention group was 0.37 points higher (0.02 to 0.72 points). | 129 | ⊕⊕⊕o | Higher value post‐intervention is favourable indicating improvement in dyspnoea. The MID for dyspnoea component of the chronic respiratory disease questionnaire is 0.5. | |
| Health‐Related Quality of Life assessed with: Chronic Respiratory Disease Questionnaire Total Score. Follow up: end of rehabilitation (range 6 weeks to 8 weeks) | The mean health‐related quality of life was 5.3 points. | The mean health‐related quality of life in the intervention group was 0.05 points higher (0.3 points lower to 0.36 points higher). | 126 | ⊕⊕⊕o | Higher value post‐intervention is favourable indicating improvement in quality of life. Control group risk determined from studies using the chronic respiratory disease questionnaire. Intervention group risk determined by back transforming the SMD to the CRQ scale. MID of the chronic respiratory disease questionnaire is 0.5. | |
| Peak Upper Limb Exercise Capacity (Supported) assessed with an incremental arm crank test. Follow up: end of rehabilitation (8 weeks) | The mean peak upper limb exercise capacity was 26 watts | The mean peak upper limb exercise capacity in the intervention group was 2.1 watts higher (8 watts lower to 12 watts higher) | 70 | ⊕⊕oo lowa,b | Control group risk determined from studies using peak power output in watts. Intervention group risk determined by back transforming the SMD to watts. | |
| Peak Upper Limb Exercise Capacity (Unsupported) assessed with the incremental unsupported arm test. Follow up: end of rehabilitation (range 4 weeks to 8 weeks) | The mean peak upper limb exercise capacity was 483 seconds. | The mean peak upper limb exercise capacity in the intervention group was 21 seconds higher (20.5 seconds lower to 63 seconds higher). | 112 | ⊕⊕⊕o | ||
| Endurance Upper Limb Exercise Capacity (Supported) assessed with an arm crank test. Follow up: end of rehabilitation (8 weeks) | The mean endurance upper limb exercise capacity was 426 seconds. | The mean endurance upper limb exercise capacity in the intervention group was 56 seconds higher (102 seconds lower to 213 seconds higher). | 57 | ⊕⊕oo lowa,c | Control group risk determined from an arm crank test at 80% peak work and represents time of the test in seconds. Intervention group risk determined by back transforming the SMD to time in seconds. | |
| Endurance Upper Limb Exercise Capacity (Unsupported) assessed by total number of rings moved in 6 minutes. Follow up: end of rehabilitation (range 6 weeks to 8 weeks) | The mean upper limb exercise capacity was 225 rings moved in 6 minutes. | The mean upper limb exercise capacity in the intervention group was 42 more rings moved (12 rings more to 71 rings more moved). | 142 | ⊕⊕oo lowa,d | Control group risk determined from a test that counts the number of rings moved in 6 minutes. Intervention group risk determined by back transforming the SMD to the number of rings moved. | |
| Upper Limb Strength assessed with dynamometry during shoulder flexion in kg. Follow up: end of rehabilitation (range 4 weeks to 16 weeks) | The mean upper limb strength was 21.4 kg | The mean upper limb strength in the intervention group was 1.4 kg higher (2 kg lighter to 5 kg higher) | 43 | ⊕⊕oo lowa,e | Control group risk determined from an arm dynamometry test of shoulder flexion in kg. Intervention group risk determined by back transforming the SMD to kg. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Meta‐anlysis was limited to few studies with small sample sizes and wide confidence intervals (imprecision −1) b Meta‐analysis limited by missing information on sequence generation and allocation concealment (2 studies), no blinding of outcome assessment (2 studies), incomplete data (2 studies) (risk of bias −1) c Meta‐analysis limited by missing information on sequence generation and allocation concealment (1 study), no blinding of outcome assessment (1 study), incomplete data (2 studies) (risk of bias −1) d Meta‐analysis limited by missing information on sequence generation and allocation concealment (4 studies), no blinding of outcome assessment (4 studies), incomplete data (2 studies) (risk of bias −1) e Meta‐analysis limited by missing information on sequence generation and allocation concealment (1 study), and no blinding of outcome assessment (1 study) (risk of bias −1) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms of Dyspnoea Show forest plot | 4 | 129 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.02, 0.72] |
| 1.1 Endurance Training | 2 | 55 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.13, 0.95] |
| 1.2 Resistance Training | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.11, 0.80] |
| 2 Health‐Related Quality of Life Show forest plot | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.31, 0.40] |
| 2.1 Endurance Training | 3 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.47, 0.42] |
| 2.2 Resistance Training | 2 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.43, 0.79] |
| 3 Peak Upper Limb Exercise Capacity: Supported Show forest plot | 3 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.43, 0.77] |
| 3.1 Endurance Training | 3 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.29, 1.16] |
| 3.2 Resistance Training | 2 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.24, 0.52] |
| 4 Peak Upper Limb Exercise Capacity: Unsupported Show forest plot | 4 | 112 | Mean Difference (IV, Random, 95% CI) | 21.23 [‐20.45, 62.92] |
| 4.1 Endurance Training | 3 | 69 | Mean Difference (IV, Random, 95% CI) | 10.29 [‐47.37, 67.95] |
| 4.2 Resistance Training | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 33.22 [‐27.12, 93.57] |
| 5 Endurance Upper Limb Exercise Capacity: Supported Show forest plot | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.46, 0.96] |
| 5.1 Endurance Training | 2 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.23, 1.35] |
| 5.2 Resistance Training | 2 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.35, 1.18] |
| 6 Endurance Upper Limb Exercise Capacity: Unsupported Show forest plot | 6 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.19, 1.13] |
| 6.1 Endurance Training | 4 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.99 [0.32, 1.66] |
| 6.2 Resistance Training | 3 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.31, 0.76] |
| 7 Upper Limb Strength Show forest plot | 2 | 43 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.39, 0.89] |
| 7.1 Resistance Training | 2 | 43 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.39, 0.89] |
| 8 Respiratory Muscle Strength Show forest plot | 5 | 148 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐8.35, 4.94] |
| 8.1 Endurance Training | 4 | 92 | Mean Difference (IV, Random, 95% CI) | ‐3.41 [‐11.02, 4.20] |
| 8.2 Resistance Training | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐9.87, 17.46] |
| 9 Physical Activity Level: Subjective Show forest plot | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.30, 0.30] |
| 10 Physical Activity Level: Objective Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.68, 2.68] |
| 11 Activities of Daily Living Show forest plot | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.12, 1.47] |
| 11.1 Endurance Training | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.19, 2.08] |
| 11.2 Resistance Training | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.69, 1.52] |
| 12 Healthcare Utilisation Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐3.07, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms of Dyspnoea Show forest plot | 3 | 86 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.04, 0.76] |
| 2 Health‐Related Quality of Life Show forest plot | 3 | 95 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.40, 0.43] |
| 3 Peak Upper Limb Exercise Capacity: Supported Show forest plot | 3 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.55, 0.44] |
| 4 Peak Upper Limb Exercise Capacity: Unsupported Show forest plot | 3 | 81 | Mean Difference (IV, Random, 95% CI) | 13.26 [‐39.88, 66.40] |
| 5 Endurance Upper Limb Exercise Capacity: Supported Show forest plot | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.46, 0.96] |
| 6 Endurance Upper Limb Exercise Capacity: Unsupported Show forest plot | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.90 [0.12, 1.68] |
| 7 Upper Limb Strength Show forest plot | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.70, 0.73] |
| 8 Respiratory Muscle Strength Show forest plot | 3 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐8.99, 8.07] |
| 9 Activities of Daily Living Show forest plot | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.12, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐Related Quality of Life Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐5.00 [‐20.85, 6.85] |
| 2 Peak Upper Limb Exercise Capcity: Supported Show forest plot | 2 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.29, 1.02] |
| 3 Peak Upper Limb Exercise Capacity: Unsupported Show forest plot | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐54.0 [‐162.50, 54.50] |
| 4 Endurance Upper Limb Exercise Capacity: Supported Show forest plot | 2 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.03, 1.31] |
| 5 Endurance Upper Limb Exercise Capacity: Unsupported Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 6.00 [0.29, 11.71] |
| 6 Respiratory Muscle Strength Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐28.21, ‐1.79] |
| 7 Activities of Daily Living Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 27.0 [‐148.71, 202.71] |



