Intervenciones con tecnología móvil y por computadora para el autocuidado de la enfermedad pulmonar obstructiva crónica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011425.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 mayo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Catherine McCabe and Margaret McCann independently screened titles and abstracts for inclusion and retrieved and screened full‐text study reports/publications when relevant. Anne Marie Brady and Catherine McCabe independently extracted study characteristics from included studies, and Catherine McCabe transferred data into the Review Manager (Review Manager 2012) file and conducted analyses. All review authors contributed to the discussion of study findings.
Sources of support
Internal sources
-
Head of School, Professor Catherine Comiskey, School of Nursing and Midwifery, Trinity College Dublin, Ireland.
External sources
-
The review authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
Catherine McCabe and Anne‐Marie Brady received a commercialisation feasibility grant from Enterprise Ireland to conduct a market survey on the possibility of commercialisation of an assistive navigational software platform to enable self‐management in COPD. The marketing exercise was completed, and no plans are in place to develop this further. Catherine McCabe and Anne‐Marie Brady were members of a research team, funded by Intel Ireland Ltd and the Technology Research for Independent Living Centre, exploring the use of mobile and fixed technology to provide motivating educational material (videos for peer learning) to people living with chronic illnesses (e.g. COPD) to bring about behavioural change for sustained self‐management and improved quality of life. The funding supported a post‐doc researcher who produced several relevant short videos on topics that included exercise and social activity. This project was completed several years ago, and a related publication was produced at that time. See also Published notes.
Margaret McCann: none known.
Acknowledgements
The review authors would like to thank Liz Stovold (Cochrane Airways Group) for assistance in developing the search strategy and conducting the search. Julia Walters was the Editor for this review and commented critically on the review, and Emma Jackson (Editorial Assistant, Cochrane Airways Group) assisted in obtaining all relevant abstracts. We would like to thank also translators Samantha Holmes and Ubai Alsharif for their assistance.
Julia Walters was the Contact Editor and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 23 | Computer and mobile technology interventions for self‐management in chronic obstructive pulmonary disease | Review | Catherine McCabe, Margaret McCann, Anne Marie Brady | |
| 2014 Dec 10 | Computer and mobile technology interventions for self management in chronic obstructive pulmonary disease | Protocol | Catherine McCabe, Margaret McCann, Anne‐Marie Brady | |
Differences between protocol and review
Although it was not originally a prespecified outcome of interest for this review, we included reporting of adverse events because it is recommended by MECIR standards.
In our protocol, we indicated that we would include six outcomes in the 'Summary of findings' table (hospital admissions, acute exacerbations, health‐related quality of life (HRQoL), self‐efficacy, functional capacity, and anxiety and depression). However, as the included studies reported on only three primary outcomes ‐ HRQoL, hospitalisations and acute exacerbations ‐ we included the outcome measure, sustained behaviour change (smoking and physical activity), as these were reported as secondary outcomes in the included studies.
Notes
The authors have confirmed that the Technology Research for Independent Living (TRIL) Centre comprised University College Dublin, Trinity College Dublin, Intel and GE Healthcare and was also supported financially by the Industrial Development Authority. Authors Catherine McCabe and Anne‐Marie Brady, as part of a wider team, applied to the TRIL Centre for funding, which was allocated on a rolling basis, rather than for a specific call. A Board made up of representative from the members of the Centre determined funding recipients; not all applications were successful. Review co‐author, Margaret McCann, was not part of the TRIL‐funded team. The review itself was published more than 3 years after the TRIL‐funded project was completed and there was no financial relationship between the review team and industry at the time of producing the review. Furthermore, this review is currently being superseded with a new review for which the author team is fully compliant with current COI policy.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Disease Progression;
- Exercise;
- Hospitalization [statistics & numerical data];
- *Microcomputers;
- *Mobile Applications;
- Patient Education as Topic;
- Pulmonary Disease, Chronic Obstructive [*therapy];
- Quality of Life;
- Self Care [*methods];
- *Smartphone;
- Smoking Cessation [statistics & numerical data];
- Therapy, Computer-Assisted [*methods];
- Time Factors;
Medical Subject Headings Check Words
Female; Humans; Male; Middle Aged;
PICO
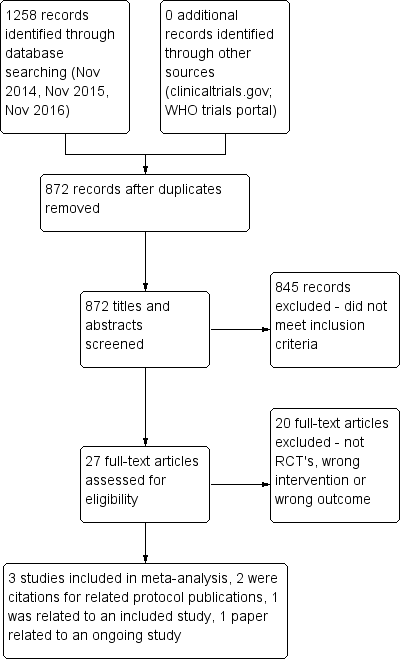
PRISMA flow diagram.
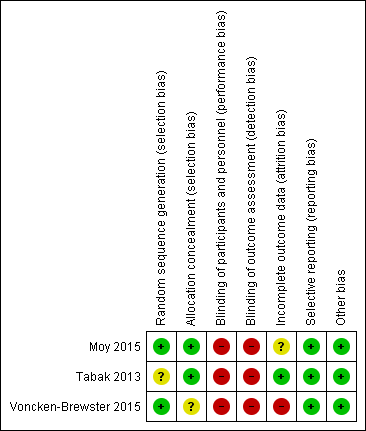
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Smart technology versus face‐to‐face/digital and/or written support, outcome: 1.1 Health related quality of life (CCQ and SGRQ) up to six months.

Forest plot of comparison: 1 Smart technology versus face‐to‐face/digital and/or written support, outcome: 1.2 Health related quality of life (CCQ only) up to six months

Forest plot of comparison: 1 Smart technology versus face‐to‐face/digital and/or written support, outcome: 1.3 Daily step count up to four months.

Forest plot of comparison: 1 Smart technology versus face‐to‐face/digital and/or written support, outcome: 1.5 Daily step count sub group 2 (at 4 weeks).

Comparison 1 Smart technology versus face‐to‐face/digital and/or written support, Outcome 1 Health‐related quality of life (CCQ and SGRQ) up to 6 months.
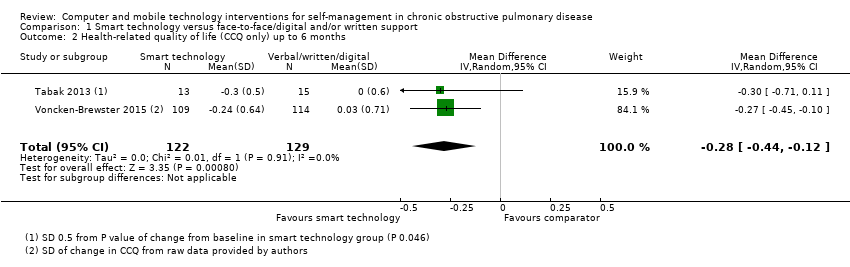
Comparison 1 Smart technology versus face‐to‐face/digital and/or written support, Outcome 2 Health‐related quality of life (CCQ only) up to 6 months.

Comparison 1 Smart technology versus face‐to‐face/digital and/or written support, Outcome 3 Daily step count up to 4 months.

Comparison 1 Smart technology versus face‐to‐face/digital and/or written support, Outcome 4 Daily step count (all time points).
| Smart technology compared with face‐to‐face/digital and/or written support for self‐management in chronic obstructive pulmonary disease | ||||||
| Participant or population: adults with a clinical diagnosis of COPD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with face‐to‐face/digital and/or written support | Risk with smart technology | |||||
| Hospital admission | ‐ | ‐ | ‐ | 239 (1 RCT; Moy 2015 at 12 months) | ⊕⊕ Lowa | Hospital admission not reported at 4 months. At 12 months. smart technology did not significantly impact the number of hospital admissions |
| Acute exacerbations requiring general practitioner (GP) visit and/or additional treatment | ‐ | ‐ | ‐ | 239 (1 RCT; Moy 2015 at 12 months) | ⊕⊕ Lowa | Acute exacerbations were not reported at 4 months. At 12 months, smart technology did not significantly impact the number of acute exacerbations |
| Health‐related quality of life (HRQoL) | Mean HRQoL ranged across control groups from 0.08 to 1.686 | SMD in HRQoL in the intervention group was 0.22 lower (0.44 to 0.03 lower) | ‐ | 472 (3 RCTs) | ⊕⊕ Lowa | Lower scores on both SGRQ and CCQ indicate better HRQoL. The SGRQ scale ranges from 0 to 100, and a change in score of 4 units is regarded as the minimum clinically important difference (MCID). The SMD in the lower score indicates better HRQoL with smart technology |
| Daily step count | Mean daily step count was 3200 to 4617 steps | Mean daily step count in the intervention group improved by 864 steps (369.66 to 1358.46 higher) | ‐ | 230 (2 RCTs; Moy 2015 at 4 months and Tabak 2013 at 4 weeks) | ⊕⊕ Lowa | The follow‐up period differed between studies, from 4 weeks to 4 months. Smart technology significantly improved physical activity as seen in daily step counts |
| Self‐efficacy | ‐ | ‐ | 0 | ‐ | This outcome was not measured in any of the included studies | |
| Behaviour change: smoking cessation | ‐ | ‐ | 284 (1 RCT) | ⊕⊕⊕ | Results showed no significant effect on smoking cessation | |
| Functional capacity (6‐minute walking test or similar) | ‐ | ‐ | 0 | ‐ | None of the included studies measured this outcome | |
| Anxiety and depression | ‐ | ‐ | 0 | ‐ | None of the included studies measured this outcome | |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aCI is wide owing to the small number of studies and the small sample sizes, which may impact precision of estimates bCI is wide owing to the single study and the small sample size, which may impact precision of estimates | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life (CCQ and SGRQ) up to 6 months Show forest plot | 3 | 472 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.03] |
| 2 Health‐related quality of life (CCQ only) up to 6 months Show forest plot | 2 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.44, ‐0.12] |
| 3 Daily step count up to 4 months Show forest plot | 2 | 230 | Mean Difference (IV, Random, 95% CI) | 864.06 [369.66, 1358.46] |
| 4 Daily step count (all time points) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Daily step count at 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Daily step count at 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Daily step count at 12 months (after 8‐month 'maintenance' phase) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

