Ozljeda endometrija za povećanje mogućnosti trudnoće nakon spolnog odnosa ili medicinski potpomognute oplodnje postupkom inseminacije
Abstract
Background
Intentional endometrial injury is currently being proposed as a technique to improve the probability of pregnancy in women undergoing assisted reproductive technologies (ART) such as in vitro fertilisation (IVF). Endometrial injury is often performed by pipelle biopsy or a similar technique, and is a common, simple, gynaecological procedure that has an established safety profile. However, it is also known to be associated with a moderate degree of discomfort/pain and requires an additional pelvic examination. The effectiveness of this procedure outside of ART, in women or couples attempting to conceive via sexual intercourse or with low complexity fertility treatments such as intrauterine insemination (IUI) and ovulation induction (OI), remains unclear.
Objectives
To evaluate the effectiveness and safety of intentional endometrial injury in subfertile women and couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI).
Search methods
We searched the Cochrane Gyanecology and Fertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS, DARE, ISI Web of Knowledge and ClinicalTrials.gov; as well as reference lists of relevant reviews and included studies. We performed the searches from inception to 31 October 2015.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated any kind of intentional endometrial injury in women planning to undergo IUI or attempting to conceive spontaneously (with or without OI) compared to no intervention, a mock intervention or intentional endometrial injury performed at a different time or to a higher/lower degree.
Data collection and analysis
Two review authors independently selected trials, extracted data and assessed trial quality using GRADE methodology. The primary outcomes were live birth/ongoing pregnancy and pain experienced during the procedure. Secondary outcomes were clinical pregnancy, miscarriage, ectopic pregnancy, multiple pregnancy and bleeding secondary to the procedure. We combined data to calculate pooled risk ratios (RRs) and 95% confidence intervals (CIs). Statistical heterogeneity was assessed using the I2 statistic.
Main results
Nine trials, which included a total of 1512 women, met the inclusion criteria of this Cochrane review. Most of these studies included women with unexplained infertility. In seven studies the women were undergoing IUI and in two studies the women were trying to conceive from sexual intercourse. Eight trials compared intentional endometrial injury with no injury/placebo procedure; of these two trials also compared intentional endometrial injury in the cycle prior to IUI with intentional endometrial injury in the IUI cycle. One trial compared higher vs. lower degree of intentional endometrial injury.
Intentional endometrial injury vs. either no intervention or a sham procedure
We are uncertain whether endometrial injury improves live birth/ongoing pregnancy as the quality of the evidence has been assessed as very low (risk ratio (RR) 2.22, 95% confidence interval (CI) 1.56 to 3.15; six RCTs, 950 participants; I² statistic = 0%, very low quality evidence). When we restricted the analysis to only studies at low risk of bias the effect was imprecise and the evidence remained of very low quality (RR 2.64, 95% CI 1.03 to 6.82; one RCT, 105 participants; very low quality evidence). Endometrial injury may improve clinical pregnancy rates however the evidence is of low quality (RR 1.98, 95% CI 1.51 to 2.58; eight RCTs, 1180 participants; I² statistic = 0%, low quality evidence).
The average pain experienced by participants undergoing endometrial injury was 6/10 on a zero‐10 visual analogue scale (VAS)(standard deviation = 1.5). However, only one study reported this outcome.
Higher vs. lower degree of intentional endometrial injury
When we compared hysteroscopy with endometrial injury to hysteroscopy alone, there was no evidence of a difference in ongoing pregnancy rate (RR 1.29, 95% CI 0.71 to 2.35; one RCT, 332 participants; low quality evidence) or clinical pregnancy rate (RR 1.15, 95% CI 0.66 to 2.01; one RCT, 332 participants, low quality evidence). This study did not report the primary outcome of pain during the procedure.
Timing of intentional endometrial injury
When endometrial injury was performed in the cycle prior to IUI compared to the same cycle as the IUI, there was no evidence of a difference in ongoing pregnancy rate (RR 0.65, 95% CI 0.37 to 1.16, one RCT, 176 participants; very low quality evidence) or clinical pregnancy rate (RR 0.82, 95% CI 0.50 to 1.36; two RCTs, 276 participants; very low quality evidence). Neither of these studies reported the primary outcome of pain during the procedure.
In all three comparisons there was no evidence of an effect on miscarriage, ectopic pregnancy or multiple pregnancy. No studies reported bleeding secondary to the procedure.
Authors' conclusions
It is uncertain whether endometrial injury improves the probability of pregnancy and live birth/ongoing pregnancy in women undergoing IUI or attempting to conceive via sexual intercourse. The pooled results should be interpreted with caution as we graded the quality of the evidence as either low or very low. The main reasons we downgraded the quality of the evidence were most included studies were at a high risk of bias and had an overall low level of precision. Further well‐conducted RCTs that recruit large numbers of participants and minimise internal bias are required to confirm or refute these findings.
PICO
Laički sažetak
Namjerno oštećenje sluznice maternice prije spolnog odnosa ili ubrizgavanja sperme u maternicu za poboljšanje stope trudnoća kod parova
Istraživačko pitanje
U ovom Cochrane sustavnom pregledu literature analiziran je učinak namjernog ozljeđivanja sluznice maternice (kao što je uzimanje biopsije ili uzorka) za postizanje trudnoće kod žena koje pokušavaju začeti putem spolnog odnosa ili ubrizgavanjem sperme u maternicu (intrauterina inseminacija, IUO).
Dosadašnje spoznaje
Za uspješnu trudnoću, spermiji i jajašca se trebaju spojiti i oploditi kako bi stvorili embrijo koji se treba usaditi (implantirat)i u sluznicu maternice. Pretpostavlja se da bi namjerno blago ozljeđivanje i oštećivanje sluznice (površinski sloj maternice koji se naziva endometrij) povećalo vjerojatnost implantacije. Ovaj postupak se može obaviti uzimanjem uzorka tkiva iz sluznice s malom plastičnom savitljivom cjevčicom.
Obilježja uključenih istraživanja
Devet randomiziranih kliničkih pokusa zadovoljilo je kriterije uključenja u ovaj sustavni pregled literature. U tim je studijama sudjelovalo ukupno 1512 žena. U sedam studija žene su pokušale zatrudniti putem IUO, a spolnim odnosom u dvije skupine. Većina žena imala je neobjašnjen razlog smanjene plodnosti što je potvrđeno nakon što rutinske pretrage nisu dale jasno objašnjenje zašto parovi nisu mogli imati dijete. U osam pokusa uspoređivala se namjerna ozljeda endometrija sa neozljeđivanjem/placebom; a od njih se u dva također uspoređivala namjerna ozljeda, ali u ciklusu prije IUO sa onom za vrijeme ciklusa. Jedno istraživanje uspoređivalo je viši s nižim stupnjem namjerne ozljede maternice. Dokazi se temelje na literaturi dostupnoj do 31. listopada 2015.
Ključni rezultati
Rezultati uključenih studija prikazuju pozitivan učinak ozljede endometrija za povećanje izgleda trudnoće, ali su studije imale brojna ograničenja. Stoga nije moguće reći sa sigurnošću može li ozljeda endometrija povećati vjerojatnost trudnoće. Autori nisu sigurni povećava li ozljeda vjerojatnost živorođenja ili trudnoće nakon 12 tjedana.
Postupak ozljeđivanja endometrija je uobičajen i poznato je da uzrokuje određen stupanj privremene boli ili neugodnosti. Samo je jedna studija prikazala jesu li žene iskusile bol tijekom postupka te je prosjek boli bio šest na ljestvici od jedan do deset. Izgleda da ozljeda endometrija ne uzrokuje pobačaj, vanmaterničnu ili višestruku trudnoću. Ni u jednoj studiji nije došlo do krvarenja nakon postupka.
Kvaliteta dokaza
Kvaliteta dokaza bila je niska ili vrlo niska. Općenito, studije uključene u ovaj sustavni pregled nisu bile dobro osmišljene te nisu uključivale dovoljan broj žena koji bi omogućili prikupljanje smislenih rezultata. Rezultatima stoga treba oprezno protumačiti te su potrebne dodatne studije da bi se potvrdili. Ostaje nejasno utječe li postupak ozljede endometrija na mogućnost začeća.
Authors' conclusions
Summary of findings
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with either: no intervention, or a sham procedure | Risk with Intentional endometrial injury | ||||
| Live birth or ongoing pregnancy | 87 per 1000 | 194 per 1000 | RR 2.22 | 950 | ⊕⊝⊝⊝ |
| Live birth or ongoing pregnancy ‐ sensitivity | 98 per 1000 | 259 per 1000 | RR 2.64 | 105 | ⊕⊝⊝⊝ |
| Pain during the procedure | Pain was not recorded in the control group | Pain was only recorded in the intervention group with an average of 6/10, standard deviation (SD) = 1.5 | — | (1 RCT) | — |
| Clinical pregnancy | 122 per 1000 | 241 per 1,000 | RR 1.98 | 1180 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels for risk of bias as many of the included studies are associated with a high risk of bias. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with lower degree of intentional endometrial injury | Risk with Higher | ||||
| Live birth or ongoing pregnancy | 102 per 1000 | 132 per 1000 | RR 1.29 | 332 | ⊕⊕⊝⊝ |
| Pain during the procedure | — | — | — | (0 study) | — |
| Clinical pregnancy | 120 per 1000 | 139 per 1000 | RR 1.15 | 332 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for indirectness as there was only 1 included study. Therefore the result was applicable only to cases of hysteroscopy plus injury vs hysteroscopy alone, and not other cases of higher vs. lower injury. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with injury in preceding cycle | Risk with injury in IUI cycle | ||||
| Live birth or ongoing pregnancy | 267 per 1000 | 173 per 1000 | RR 0.65 | 176 | ⊕⊝⊝⊝ |
| Pain during the procedure | — | — | — | (0 RCTs) | — |
| Clinical pregnancy | 329 per 1000 | 269 per 1000 | RR 0.82 | 276 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for risk of bias as both studies were at high risk of bias. | |||||
Background
Description of the condition
Subfertile couples are often defined as those who fail to achieve clinical pregnancy after 12 or more months of regular unprotected sexual intercourse (ASRM 2013; Zegers‐Hochschild 2009). It is estimated that up to 15% of couples will experience this condition (Thoma 2013), and that only 50% of these couples will conceive spontaneously in the next three years (Gnoth 2005). There are many causes of subfertility, including obstruction of the uterine tubes, endometriosis, ovulatory disorders and poor semen quality (Stern 2013). Whenever uterine tubes are functional and semen quality is satisfactory, pregnancy may be achieved naturally or by using simple methods, such as ovulation induction (OI) and intrauterine insemination (IUI) (van Rumste 2014).
Description of the intervention
Endometrial injury is defined as intentional damage to the endometrium performed with the objective of improving the reproductive outcomes of women or couples desiring pregnancy. The most common intervention is endometrial scratching performed using a pipelle (small flexible plastic tube) (Nastri 2012). Endometrial injury is a simple, low‐cost procedure which can be performed on an outpatient basis without anaesthetic, by endometrial pipelle biopsy or Novak curette.
How the intervention might work
Embryo implantation is the initial interaction between the embryo and the endometrium, and is a key step in the process required to achieve a successful pregnancy, and therefore live birth. Implantation involves complex signalling and synchronisation between the endometrium and the implanting embryo, but the exact mechanism of this process remains unclear (Edwards 2006; Lessey 2011; Philips 2013; Siristatidis 2014). Many studies have reported an increased probability of pregnancy in women who have undergone procedures involving instrumentation within the uterus, such as hysteroscopy or hysterosalpingogram (El‐Toukhy 2008; Mohiyiddeen 2015; Pundir 2014; Yun 2004). More recently, studies have demonstrated an increase in pregnancy rates in women who underwent an endometrial pipelle biopsy prior to an in vitro fertilisation (IVF) cycle (Nastri 2012). Endometrial injury resulting from these procedures is thought to help improve reproductive outcomes by increasing endometrial receptivity for an implanting embryo.
Although many theories have been proposed (Siristatidis 2014), there are two major overlapping hypotheses that may explain a beneficial reproductive effect for women trying to conceive naturally, or with either or both IUI and OI.
-
Endometrial injury induces decidualisation: transformation of the endometrium in preparation for the implantation of an embryo. Decidualisation naturally occurs under the influence of progesterone and involves the modification of endometrial stromal cells, uterine glands and vessels, as well as the population of uterine immune cells, in order to aid the implantation process (Barash 2003).
-
The healing response involves the release of a number of inflammatory factors, such as cytokines and growth factors. These molecules can improve vascularisation (Nastri 2013a) and are believed to help facilitate embryo implantation (Dekel 2014; Gnainsky 2010).
Regardless of the underlying mechanism, the apparent increased probability of pregnancy following endometrial injury in IVF cycles suggests that this procedure might also be beneficial in women who are trying to conceive naturally, or who are undergoing IUI and/or OI (Nastri 2012).
Why it is important to do this review
Many subfertile couples seek fertility treatments to help them conceive. IVF is the leading fertility treatment. However, it is a complex and expensive therapy which provides only a moderate chance of pregnancy of approximately 30% per cycle (Ferraretti 2013; Vélez 2014). While this intervention appears favourable in women undergoing IVF (Nastri 2012), the effectiveness and safety remains unclear in women or couples trying to conceive naturally, or with either or both IUI and OI. If endometrial injury improves reproductive outcomes in these situations, it would provide a cost‐effective treatment alternative for some couples before they consider undergoing IVF. This review will summarise the available evidence for this procedure in subfertile women or couples who are trying to get pregnant either with sexual intercourse or intrauterine insemination (IUI), with or without ovulation induction (OI).
Objectives
To assess the effectiveness and safety of intentional endometrial injury performed in women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI).
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with quasi‐randomisation, such as allocation based on alternate days or patient hospital numbers).
Crossover trials were eligible, but we would have only included data from the first phase in meta‐analyses, as the crossover is not a valid design in the context of fertility trials.
Types of participants
Subfertile women or couples who are trying to get pregnant either with sexual intercourse or intrauterine insemination (IUI), with or without ovulation induction (OI). We excluded women or couples undergoing assisted reproductive technology (ART) (e.g. in vitro fertilisation (IVF)) as this group of participants is the topic of another Cochrane review (Nastri 2015).
Types of interventions
Any intervention that caused intentional damage to the endometrium, performed with the objective of improving the reproductive outcomes of women desiring pregnancy. Intentional endometrial injury may be achieved by procedures such as endometrial pipelle biopsy or biopsy performed with a Novak curette. We excluded studies that evaluated interventions causing unintentional endometrial damage compared with control. Examples of unintentional endometrial injury are hysteroscopy, hysterosalpingography, endometrial sound, mock embryo transfer and cervical dilation.
Types of outcome measures
Primary outcomes
-
Live birth/ongoing pregnancy per woman randomised. Our definition for live birth was the delivery of live foetus(es) after 20 weeks of gestation. The delivery of singletons, twins or other multiple pregnancies counted as one live birth. If studies did not report live birth, where possible, we pooled ongoing pregnancy data (defined as pregnancies with live foetuses surpassing 12 weeks of pregnancy) with live birth data from other studies and this was subject to sensitivity analyses.
-
Pain experienced during the procedure (we preferred the 10 cm visual analogue scale and 11‐point Likert scale).
Secondary outcomes
-
Clinical pregnancy per woman randomised. As per the definition of each trial, or evidence of an intrauterine gestational sac on ultrasound or other definitive signs of pregnancy, and including ectopic pregnancy (Zegers‐Hochschild 2009).
-
Miscarriage per clinical pregnancy.
-
Multiple pregnancy per clinical pregnancy.
-
Ectopic pregnancy per clinical pregnancy.
-
Bleeding secondary to the procedure.
If studies did not report one of the above review outcomes, we contacted the study authors to determine whether the study authors recorded but did not report any of the above outcomes. If the study authors confirmed that the trial did not record any of the review outcomes, then we excluded the study.
Search methods for identification of studies
We searched for RCTs by using a search strategy developed in consultation with the Information Specialist for the Cochrane Gynaecology and Fertility Group. We did not apply any language restrictions or restrictions by publication status (i.e. unpublished studies were eligible).
Electronic searches
We searched the following electronic databases, trial registers and websites from inception to 31 October 2015: the Cochrane Gynaecology and Fertility Specialised Register of Controlled Trials, the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, PsycINFO and CINAHL. The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The EMBASE, PsycINFO and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://www.sign.ac.uk/methodology/filters.html#random) (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9).
Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials (with the search terms "endometrial injury", "endometrial scratching" and "endometrial biopsy"):
-
http://www.clinicaltrials.gov (a service of the US National Institutes of Health);
-
the World Health Organization International Trials Registry Platform (WHO ICTRP) search portal http://www.who.int/trialsearch/Default.aspx.
-
-
DARE (Database of Abstracts of Reviews of Effects) on the Cochrane Library http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html (for reference lists from relevant non‐Cochrane reviews).
-
The Web of Knowledge http://wokinfo.com/ (source of trials and conference abstracts).
-
LILACS database http://regional.bvsalud.org/php/index.php?lang=en (for trials from the Portuguese‐ and Spanish‐speaking world).
Searching other resources
We handsearched reference lists of relevant articles retrieved by the search. We contacted experts in the field (e.g. authors of included studies) for information on additional trials, including unpublished or in‐progress trials.
Data collection and analysis
Selection of studies
Firstly, two review authors (SL and MM or GT) independently screened the titles and abstracts of all articles retrieved from all searches according to the review inclusion criteria. The two review authors excluded any clearly irrelevant studies. We obtained the full‐text versions of all remaining potentially eligible studies, which two review authors then independently assessed for inclusion. We excluded articles that did not meet the review inclusion criteria. In instances where study eligibility was unclear, we contacted the study authors for clarification. The two review authors resolved any disagreements by discussion in the first instance, followed by consultation with a third review author (WPM) if required.
Data extraction and management
Two review authors (SL and MM or GT) independently extracted data from eligible studies using a data extraction form that we had pilot tested. They resolved any disagreements by discussion or by consultation with a third review author (WPM). Data extracted included study characteristics and outcome data. We corresponded with study investigators for further data on methods or results, or both, as required.
Assessment of risk of bias in included studies
Two review authors (SL and MM or GT) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool for the following bias domains: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting and other bias (see Appendix 10 for the rationale we used in assessing risk of bias). We resolved any disagreements by discussion or by consulting a third review author. We supported all judgements by excerpts from the study or comments from the review authors. We presented conclusions in 'Risk of bias' tables (see the 'Characteristics of included studies' section), which we incorporated into the interpretation of review findings by means of sensitivity analyses (see below). We took care to search for within‐trial selective reporting, such as trials that failed to report adverse outcomes. Where possible, we used published protocols or trial registration information for included studies to investigate selective reporting (i.e. a comparison of outcomes listed in the study protocol with outcomes reported in papers).
Measures of treatment effect
For dichotomous data (e.g. live birth), we used the numbers of events in the control and intervention groups of each study to calculate the Mantel‐Haenszel risk ratios (RRs). For continuous outcomes (e.g. pain) we calculated the mean difference. We presented 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
We used the number of randomised women as the denominator for live birth, clinical pregnancy, pain and bleeding. We used the number of clinical pregnancies as the denominator for miscarriage, multiple pregnancy and ectopic pregnancy as these outcomes can only occur in women who achieve a clinical pregnancy.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the study investigators. When we were unable to obtain missing data, we performed imputation of individual values as described below.
-
We assumed that live births and pregnancies had not occurred in participants without a reported outcome.
For other outcomes, we only analysed the available data. We subjected any imputation undertaken to sensitivity analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I² statistic, where we took an I² statistic value greater than 50% to indicate substantial heterogeneity. We planned to investigate the causes of any observed heterogeneity with prespecified subgroup analyses.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies, including in trial registries, and by being alert to data duplication. If there were 10 or more included studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
One review author entered the data and performed the statistical analysis in Review Manager (RevMan) (RevMan 2014). When a study reported ongoing pregnancy but did not report live birth, we pooled ongoing pregnancy data with live birth data from other included studies. Where this occurred, we also performed sensitivity analyses. We discussed data that we could not pool in a narrative format in the text. When we could confidently rule out significant clinical and statistical heterogeneity, we combined data from primary studies in a meta‐analysis with Revman (RevMan 2014). We used the Mantel‐Haenzel random‐effects model for the following comparisons.
-
Intentional endometrial injury vs. either no intervention or a sham procedure.
-
Higher vs. lower degree of intentional endometrial injury (e.g. two interventions vs. one intervention; Novak curette vs. pipelle).
-
Different timing of intentional endometrial injury (e.g. follicular phase vs. luteal phase).
We combined data using a random‐effects model, as we considered that the method and instruments used to cause endometrial injury were likely to differ across the trials in each analysis, and that most participants had unexplained infertility which is thought to be a heterogeneous condition. We displayed an increase in the risk of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. miscarriage), graphically in the meta‐analyses to the right of the centre‐line and displayed a decrease in the risk of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses only if substantial heterogeneity existed (I² statistic value of greater than 50%) and enough data were available.
-
Type of conception (e.g. IUI, OI, timed intercourse, regular intercourse); as benefit from endometrial injury may vary depending on the type of conception.
-
Cause of subfertility (e.g. unexplained subfertility, polycystic ovarian syndrome, endometriosis etc.); as benefit from endometrial injury may vary depending on the cause of subfertility.
-
Timing of endometrial injury (e.g. follicular phase, luteal phase); as benefit from endometrial injury may vary depending on the phase of the menstrual cycle in which the injury is performed.
-
Length of study period (e.g. only one attempted conception cycle, between one to three cycles, more than three cycles); to account for a higher probability of pregnancy with longer study durations, and to investigate the potential duration of benefit following endometrial injury.
-
Severity of injury (e.g. two interventions vs. one intervention; Novak curette vs. pipelle).
Sensitivity analysis
We conducted sensitivity analyses on the primary outcomes to determine whether the conclusions were robust to arbitrary decisions we made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if the following occurred.
-
We restricted eligibility to studies considered to be at low risk of bias.
-
We did not perform any imputation for live birth.
-
We did not pool ongoing pregnancy data with live birth data.
-
We had used a fixed‐effect model.
-
The summary effect measure was odds ratio rather than relative risk.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using the GRADEpro Guideline Development Tool (GDT) software (available from www.gradepro.org), as per standard Cochrane methods. This table evaluated the overall quality of the body of evidence for the primary review outcomes (live birth and pain during procedure) and clinical pregnancy using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (study limitations i.e. risk of bias, consistency of effect, imprecision, indirectness and publication bias). We justified, documented and incorporated judgements about evidence quality (high, moderate, low or very low) into reporting of results for each outcome. All review authors had input into the GRADE assessments of the quality of the evidence in the 'Summary of findings' tables.
Results
Description of studies
Results of the search
We performed the searches in October 2015. They retrieved 707 articles after removal of duplicates and we identified four additional studies through handsearches (see the study flow diagram in Figure 1). Five studies were ongoing and without available results (ACTRN12614000657628; ACTRN12614000656639; NCT02140398; NCT02345837; NCT02492451; see the 'Characteristics of ongoing studies' section). We excluded five studies for the following reasons: it was unclear whether or not they were truly randomised (Castellacci 2012; Dadras 2012); the study recorded only biochemical pregnancy, which is not a review outcome, and we confirmed this after we contacted trial authors (NCT02084914); performed unintentional rather than intentional injury (Seyam 2015); the study was discontinued after recruitment of only a small number of participants (information from author correspondence) (NCT01111799) (see the 'Characteristics of excluded studies' section). Nine studies met the inclusion criteria of this Cochrane review. One study was only available as an abstract (Mahey 2015), and another study was an unpublished master's thesis (Al‐Tamemi 2014) (see the 'Characteristics of included studies' section).

Study flow diagram.
Included studies
Study design and setting
We included nine parallel‐design randomised controlled trials (RCTs) in the review. Seven included studies had two arms (Al‐Tamemi 2014; El‐Khayat 2015; Gibreel 2013; Maged 2016; Mahey 2015; Parsanezhad 2013; Zarei 2014) and two included studies had three arms (Abdelhamid 2013; Wadhwa 2015). Eight studies were undertaken in fertility clinics in the Middle East: United Arab Emirates (UAE) (one), Iran (three) and Egypt (four); and two in India. The same research group conducted two studies (Parsanezhad 2013; Zarei 2014).
Participants
Together the nine studies included 1512 women; 821 participants in the intervention groups and 691 in the control groups. All nine studies included couples with unexplained infertility; five studies also included couples with mild male factor (Abdelhamid 2013; Al‐Tamemi 2014; El‐Khayat 2015; Wadhwa 2015; Zarei 2014); one also included women with ovulatory factor (Abdelhamid 2013), one included women with mild endometriosis (Zarei 2014) and one included women with unilateral tubal factor (Wadhwa 2015). All participants had a duration of subfertility of at least one year. The age of the included participants ranged from 18 to 40 years. In general the studies included women with an elevated body mass index (BMI), which averaged 30 or higher in several studies (Gibreel 2013; Maged 2016). The average duration of subfertility ranged between 3.4 years (El‐Khayat 2015) and six years (Gibreel 2013).
Interventions
Three studies used a pipelle device to cause the endometrial injury (Al‐Tamemi 2014; Gibreel 2013; Parsanezhad 2013). Other devices included a Tao brush (Abdelhamid 2013), a Novak curette (Zarei 2014), a neonatal feeding tube (Maged 2016), a cannula (Mahey 2015; Wadhwa 2015) and grasping teeth with forceps (El‐Khayat 2015)
Six studies compared a single endometrial injury with no endometrial injury (Abdelhamid 2013; Al‐Tamemi 2014; Maged 2016; Mahey 2015; Wadhwa 2015; Zarei 2014). Two studies used a sham procedure in the control group: one study used a mock pipelle biopsy, in which the pipelle was not inserted past the internal os of the cervix (Parsanezhad 2013); and one study used a uterine sound procedure in the control group (Gibreel 2013). Although unintended, both of these sham procedures are considered to potentially cause some degree of endometrial injury (Nastri 2013b). One study compared hysteroscopy and intentional injury with hysteroscopy only (El‐Khayat 2015).
Three studies performed endometrial injury in the follicular phase of the cycle preceding the first attempted conception cycle (Abdelhamid 2013; El‐Khayat 2015; Zarei 2014); three studies performed it in the luteal phase of the preceding cycle (Al‐Tamemi 2014; Gibreel 2013; Wadhwa 2015); three studies performed it in the follicular phase of the attempted conception cycle (Abdelhamid 2013; Mahey 2015; Wadhwa 2015) and two studies conducted it at the time of ovulation in the attempted conception cycle (Maged 2016; Parsanezhad 2013). In the two three‐arm studies, participants in one intervention group underwent endometrial injury in the cycle that preceded the stimulation cycle, while participants in the second intervention group underwent endometrial injury in the same cycle as the intrauterine insemination (IUI) cycle (Abdelhamid 2013;Wadhwa 2015).
The type of conception varied between studies. In eight studies participants were undergoing stimulated cycles (with clomiphene citrate or letrozole and gonadotrophin), followed by IUI (Abdelhamid 2013; Al‐Tamemi 2014; El‐Khayat 2015; Maged 2016; Mahey 2015; Wadhwa 2015; Zarei 2014) or regularly timed intercourse (Parsanezhad 2013), whereas in one study participants had spontaneous menstrual cycles followed by intercourse at their convenience (Gibreel 2013). The number of attempted conception cycles also varied between one (Abdelhamid 2013; Al‐Tamemi 2014; El‐Khayat 2015), three (Maged 2016; Mahey 2015; Parsanezhad 2013; Zarei 2014) and six (Gibreel 2013). One study intended that participants completed three consecutive IUI cycles, but the number of participants that attended for all three cycles differed between the study groups. In order to eliminate any bias associated with an unbalanced comparison, the study authors provided the data for the first cycle only (Wadhwa 2015).
Outcomes
-
Seven trials provided live birth data/ongoing pregnancy data.
-
One trial reported pain experienced during the procedure.
-
All nine included trials reported clinical pregnancy rate.
-
Seven trials reported multiple pregnancy rate.
-
Seven trials reported miscarriage/abortion rate, or we were able to calculate this based on other data provided (defined as the spontaneous loss of a clinical pregnancy).
-
Two trials reported ectopic pregnancy rate.
-
None of the included trials reported bleeding secondary to the procedure.
Excluded studies
We excluded five studies from the review for the following reasons: established as not being a RCT (Castellacci 2012); unclear whether truly randomised (Dadras 2012); reported only biochemical pregnancy rate and we confirmed this by author correspondence (NCT02084914); the injury in the control group was unintentional rather than intentional (Seyam 2015); and due to study discontinuation with no available data (NCT01111799).
Risk of bias in included studies
We assessed the risk of bias for each included trial (see the 'Characteristics of included studies' section). We summarised the results in the 'Risk of bias' summary (see Figure 2).

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' category for each included study.
Allocation
Sequence generation
Seven studies had a low risk of selection bias related to sequence generation as the studies used computer‐generated random numbers (Abdelhamid 2013; Al‐Tamemi 2014; El‐Khayat 2015; Gibreel 2013; Maged 2016; Mahey 2015; Wadhwa 2015). Two studies did not adequately describe the method used and we judged them at unclear risk of this bias even after we contacted the study authors (Parsanezhad 2013; Zarei 2014). Following author correspondence, the authors of Wadhwa 2015 stated that 24 participants were not randomised but allocated to the intervention groups to replace participant drop‐outs. They were able to provide data for only women who were randomly allocated to the study, and therefore we judged the study as at low risk.
Allocation concealment
Three studies were at low risk of allocation concealment as the studies used sequentially numbered, opaque, sealed envelopes (Abdelhamid 2013; El‐Khayat 2015; Gibreel 2013). One study used block randomisation with blocks of two (Parsanezhad 2013), which we therefore judged to be at high risk of bias, as every second allocation would be known in advance. Two studies used envelopes that were not numbered and we therefore judged them as at high risk (Maged 2016; Wadhwa 2015). Mahey 2015 described the randomisation as being read off a list of allocations and we therefore rated it as at high risk. Two studies failed to describe their methods of allocation concealment and we judged them as at an unclear risk of bias (Al‐Tamemi 2014; Zarei 2014).
Blinding
Performance bias: blinding of participants
Six studies compared a single endometrial injury with no endometrial injury, and therefore participants were not blinded to their study allocation and we rated these studies as at high risk of bias (Abdelhamid 2013; Al‐Tamemi 2014; Maged 2016; Mahey 2015; Wadhwa 2015; Zarei 2014). Two studies used a sham procedure in the control group: one study used a mock pipelle biopsy, in which the pipelle was not inserted past the internal os of the cervix and it is unclear whether this procedure would have truly blinded the study participants (Parsanezhad 2013). Two other studies used control procedures which were likely to blind participants to their allocation, but the trial authors did not assess this formally. One study used a uterine sound procedure in the control group (Gibreel 2013), and the other study compared hysteroscopy and intentional injury with hysteroscopy only (El‐Khayat 2015).
In two studies all participants were expected to complete three consecutive IUI cycles (Wadhwa 2015; Zarei 2014). Likely as a result of lack of blinding, many participants did not proceed to the second and third cycles and a greater number of cycles took place in the intervention groups, which created an unbalanced comparison. Therefore we graded one of these studies as at a high risk of bias (Zarei 2014). The authors of the other study provided the data for only the first IUI cycle that all participants underwent, which would reduce the potential for bias resulting from an unbalanced comparison. However, we still rated this study as at high risk as there was still the potential for bias due to lack of blinding (Wadhwa 2015).
Performance bias: blinding of personnel
We rated all included studies as at high risk of bias regarding blinding of personnel as none of the included studies blinded the trial personnel to participant allocation.
Detection bias
We rated all included studies as at low risk of detection bias as knowledge of participant allocation is unlikely to influence assessment of live birth or pregnancy outcomes.
Incomplete outcome data
Two studies had no missing outcome data and we graded them as at low risk of bias (Abdelhamid 2013; Maged 2016). We graded another five studies as at low risk of bias as the numbers of participant drop‐outs were not significant and were similar across study groups (El‐Khayat 2015; Gibreel 2013; Mahey 2015; Parsanezhad 2013; Zarei 2014). Two included studies reported the reasons for withdrawals/exclusions (Gibreel 2013; Parsanezhad 2013). We only rated one study as at unclear risk of bias as the attrition rate was approaching 10% and the study did not report the reasons for participant withdrawal (Al‐Tamemi 2014).
Selective reporting
Two studies were prospectively registered and the protocols were available; all outcomes were reported (El‐Khayat 2015; Gibreel 2013).The other studies were either registered retrospectively (Maged 2016; Mahey 2015; Parsanezhad 2013; Wadhwa 2015; Zarei 2014), or were not registered (Abdelhamid 2013; Al‐Tamemi 2014). We graded seven studies as at low risk of bias as the studies either reported all expected outcomes, which were available following author correspondence, or the study authors confirmed they had not collected them. Author correspondence was not possible for two studies and therefore we rate these as at unclear risk of this bias (Al‐Tamemi 2014; Zarei 2014).
Other potential sources of bias
We judged one study as at unclear risk of bias for this domain as information was insufficient for an evaluation and author correspondence was not possible (Al‐Tamemi 2014). We found no potential sources of within‐study bias in the other included studies.
Effects of interventions
See: Summary of findings for the main comparison Intentional endometrial injury vs. either no intervention or a sham procedure; Summary of findings 2 Higher vs. lower degree of intentional endometrial injury; Summary of findings 3 Different timing of intentional endometrial injury
We have presented the results below in the following three comparisons.
-
Eight studies compared intentional endometrial injury vs. either no intervention or a sham procedure.
-
One study compared higher vs. lower degree of intentional endometrial injury.
-
Two studies compared different timings of intentional endometrial injury.
See our 'Summary of findings' tables for the main comparison (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3).
1. Intentional endometrial injury vs. either no intervention or a sham procedure
We included eight studies in this comparison.
Primary outcomes
1.1 Live birth/ongoing pregnancy
None of the studies reported live birth, but we obtained this information for two studies after we contacted the authors. In one study the authors confirmed that all ongoing pregnancies proceeded to live birth (Parsanezhad 2013), and another study supplied the live birth data from an interim analysis (Mahey 2015). Three studies reported ongoing pregnancy (Maged 2016; Wadhwa 2015; Zarei 2014), or we obtained this information following author correspondence in two instances (Gibreel 2013; Mahey 2015). The pooled result appears favourable for the outcome of live birth/ongoing pregnancy, but the evidence was of very low quality (risk ratio (RR) 2.22, 95% confidence interval (CI) 1.56 to 3.15; six RCTs, 950 participants, I² statistic = 0%; very low quality evidence; Analysis 1.1; Figure 3).
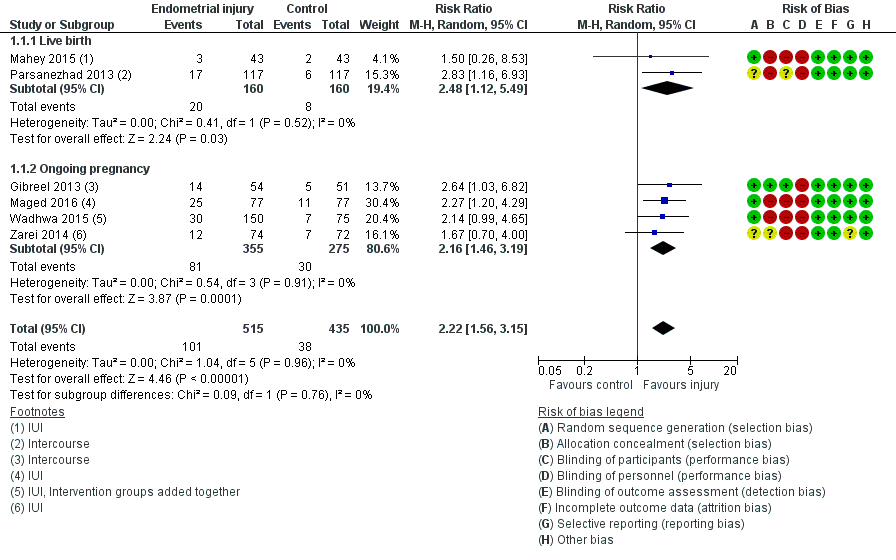
Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.1 Live birth or ongoing pregnancy: sensitivity analysis excluding studies at high or unclear risk of allocation concealment.
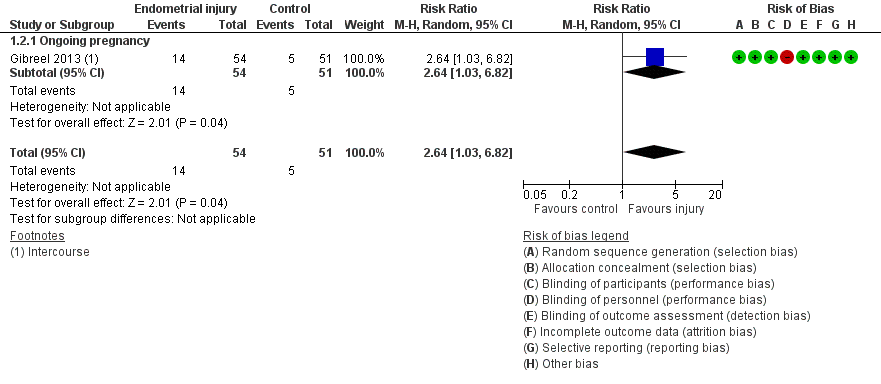
Due to the high risk of bias associated with many of the studies, we conducted a sensitivity analyses by exclusion of studies at high or unclear risk for allocation concealment. This analysis yielded only one study with greatly increased imprecision (RR 2.64, 95% CI 1.03 to 6.82; one RCT, 105 participants, very low quality evidence; Analysis 1.2; Figure 4).
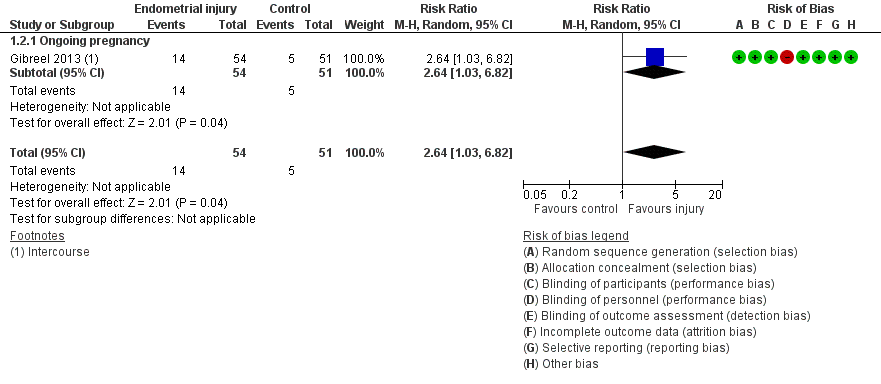
Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.2 Live birth or ongoing pregnancy: sensitivity analysis.
Additional sensitivity analyses (using a fixed‐effect model or odds ratio, restricting eligibility to studies that reported live birth) did not affect the significance of the findings.
1.2 Pain during the procedure
Pain recorded on a VAS 0‐10 scale was available from one included study, Mahey 2015, after we contacted the study authors. In this study the researchers used a device called Karman’s cannula No. 4 and reported the pain in the intervention group as an average of 6/10, with a standard deviation (SD) = 1.5. However, as there was no placebo procedure in the control group, we were unable to use this data in a meta‐analysis.
Secondary outcomes
1.3 Clinical pregnancy
All eight trials reported clinical pregnancy rate. The evidence suggests that endometrial injury is associated with a higher clinical pregnancy rate compared to no intervention/a sham procedure, but the evidence is of low quality (RR 1.98, 95% CI 1.51 to 2.58; eight RCTs, 1180 participants; I² statistic = 0%, low quality evidence; Analysis 1.3).
1.4 Miscarriage per clinical pregnancy
Six studies reported miscarriage rate. There was no evidence of a difference in miscarriage rate per clinical pregnancy (RR 0.73, 95% CI 0.38 to 1.39; six RCTs, 174 participants; I² statistic = 0%; Analysis 1.4).
Notably, miscarriages reported in one study referred to loss of pregnancies before confirmation of a clinical pregnancy (Gibreel 2013). Following author correspondence, the study authors confirmed that all clinical pregnancies proceeded to ongoing pregnancy.
1.5 Multiple pregnancy per clinical pregnancy
Six studies reported multiple pregnancy rate. There was no evidence of a difference in multiple pregnancy rate between endometrial injury and no intervention/a sham procedure (RR 0.93, 95% CI 0.31 to 2.78; six RCTs, 261 participants; I² statistic = 0%; Analysis 1.5).
1.6 Ectopic pregnancy
Two studies reported ectopic pregnancy rate. There was no evidence of a difference in ectopic pregnancy rate between endometrial injury and no intervention/a sham procedure (RR 0.54, 95% CI 0.09 to 3.46; two RCTs, 57 participants; I² statistic = 0%; Analysis 1.6)
1.7 Bleeding secondary to the procedure
None of the included studies reported bleeding secondary to the procedure.
2. Higher vs. lower degree of intentional endometrial injury
The study included in this comparison compared hysteroscopy with endometrial injury to hysteroscopy alone in women attempting to conceive from IUI (El‐Khayat 2015).
Primary outcomes
2.1 Live birth or ongoing pregnancy
There was no evidence of a difference in ongoing pregnancy rate when hysteroscopy with endometrial injury was compared to hysteroscopy alone (RR 1.29, 95% CI 0.71 to 2.35; one RCT, 332 participants, low quality evidence; Analysis 2.1; Figure 5).
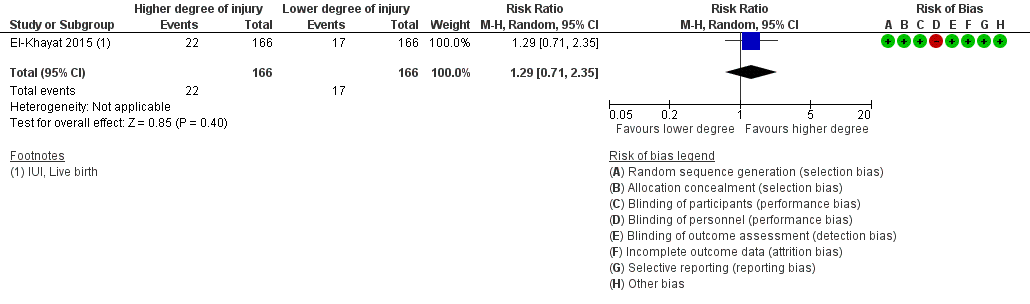
Forest plot of comparison: 2 Higher vs. lower degree of intentional endometrial injury, outcome: 2.1 Live birth or ongoing pregnancy.
2.2 Pain during the procedure
This study did not report pain during the procedure.
Secondary outcomes
2.3 Clinical pregnancy
There was no evidence of a difference in clinical pregnancy rate when hysteroscopy with endometrial injury was compared to hysteroscopy alone (RR 1.15, 95% CI 0.66 to 2.01; one RCT, 332 participants, low quality evidence; Analysis 2.2).
2.4 Miscarriage per clinical pregnancy
There was no evidence of a difference in miscarriage rate per clinical pregnancy when hysteroscopy with endometrial injury was compared to hysteroscopy alone (RR 0.29, 95% CI 0.03 to 2.57; one RCT, 43 participants; Analysis 2.3).
2.5 Multiple pregnancy per clinical pregnancy
There was no evidence of a difference in multiple pregnancy rate per clinical pregnancy when hysteroscopy with endometrial injury was compared to hysteroscopy alone (RR 0.87, 95% CI 0.20 to 3.83; one RCT, 43 participants; Analysis 2.4).
2.6 Ectopic pregnancy
This study did not report ectopic pregnancy.
2.7 Bleeding secondary to the procedure
This study did not report bleeding secondary to the procedure.
3. Timing of intentional endometrial injury
We included two groups per study from two three‐arm studies in this comparison. One study compared endometrial injury in the follicular phase of the cycle prior to IUI with endometrial injury in the follicular phase of the IUI cycle (Abdelhamid 2013). In this study all participants underwent one cycle of IUI. The second study compared endometrial injury in the luteal phase of the cycle prior to IUI with endometrial injury in the follicular phase of the cycle prior to IUI (Wadhwa 2015).
Primary outcomes
3.1 Live birth or ongoing pregnancy
There was no evidence of a difference in ongoing pregnancy rate when endometrial injury was performed in the cycle prior to an IUI cycle compared to the follicular phase of the cycle in which IUI takes place (RR 0.65, 95% CI 0.37 to 1.16; one RCT, 176 participants; very low quality evidence; Analysis 3.1; Figure 6).

Forest plot of comparison: 3 Timing of intentional endometrial injury, outcome: 3.1 Live birth or ongoing pregnancy.
3.2 Pain during the procedure
This study did not report on pain during the procedure.
Secondary outcomes
3.3 Clinical pregnancy
There was no evidence of a difference in clinical pregnancy rate when endometrial injury was performed in the cycle prior to an IUI cycle compared to the follicular phase of the cycle in which IUI takes place (RR 0.82, 95% CI 0.50 to 1.36; two RCTs, 276 participants, very low quality evidence; Analysis 3.2).
3.4 Miscarriage per clinical pregnancy
There was no evidence of a difference in miscarriage rate per clinical pregnancy when endometrial injury was performed in the cycle prior to an IUI cycle compared to the follicular phase of the cycle in which IUI takes place (RR 0.82, 95% CI 0.17 to 4.03; one RCT, 45 participants; Analysis 3.3).
3.5 Multiple pregnancy per clinical pregnancy
There was no evidence of a difference in multiple pregnancy rate per clinical pregnancy when endometrial injury was performed in the cycle prior to an IUI cycle compared to the follicular phase of the cycle in which IUI takes place (RR 0.82, 95% CI 0.17 to 4.04; two RCTs, 82 participants; Analysis 3.4).
3.6 Ectopic pregnancy
Neither study reported ectopic pregnancy.
3.7 Bleeding secondary to the procedure
Neither study reported bleeding secondary to the procedure.
Other analyses
In accordance with our protocol, Lensen 2014, we did not conduct any subgroup analyses due to the absence of heterogeneity in all comparisons. There were too few studies to construct the planned funnel plot in order to measure the potential for reporting biases.
Discussion
Summary of main results
The aim of this Cochrane review was to assess the evidence regarding the effectiveness and safety of intentional endometrial injury performed in women or couples attempting to conceive through sexual intercourse or intrauterine insemination (IUI). Although endometrial injury in women undergoing assisted reproductive technology (ART) has been the topic of many studies and reviews (Almog 2010; El‐Toukhy 2012; Li 2009; Nastri 2015; Potdar 2012), the procedure has not been reviewed in women or couples who are attempting to conceive without ART, for example with IUI or sexual intercourse.
Comparison of intentional endometrial injury with no intervention or a sham procedure
The overall analysis of women trying to get pregnant from IUI or sexual intercourse suggests that endometrial injury is associated with a greater probability of live birth/ongoing pregnancy and clinical pregnancy compared to either no intervention or a sham procedure. However, these results should be interpreted with a high degree of caution as the included studies have low numbers of participants, few events and are associated with a high risk of bias. As such, we graded the evidence as low or very low quality. When we restricted the analysis only to studies with adequate methods of allocation concealment, the pooled effect became only marginally significant. There was no evidence to suggest that endometrial injury is associated with an altered probability of miscarriage, ectopic pregnancy or multiple pregnancy.
No studies reported bleeding secondary to the procedure. One study provided data on the second primary outcome of pain during the procedure, as an average of 6/10, with a standard deviation (SD) = 1.5 (Mahey 2015). Although reporting of these adverse events was limited in the included studies, endometrial pipelle biopsy is a routine gynaecological procedure commonly used to obtain a sample of the endometrium when indicated. The procedure is safe and usually well‐tolerated, but some short‐term bleeding or spotting following the procedure is common. Pain during a pipelle sampling procedure ranges between 3.21 and 7.7 (on a scale of 0‐10), and significantly more pain is experienced when a tenaculum is used during the procedure (Kucukgoz Gulec 2014; Leclair 2011; Nastri 2013b; Stovall 1991).
Comparison of higher degrees of intentional endometrial injury with lower degrees of intentional endometrial injury
We included one study in this comparison. There was no evidence to suggest that endometrial injury at the time of hysteroscopy compared to hysteroscopy alone is associated with an increased chance of pregnancy. However, we judged this evidence as low quality as only a single trial examined this and the event rate remains low. Furthermore, there is no evidence to suggest that endometrial injury at the time of hysteroscopy compared to hysteroscopy alone is associated with an altered probability of miscarriage, ectopic pregnancy or multiple pregnancy.
Comparison of timing of intentional endometrial injury
We included two studies in this comparison. There was no evidence of a difference in pregnancy rates when endometrial injury was performed in the cycle before IUI (either luteal or follicular phase) compared to when the injury was performed in the follicular phase of the IUI cycle. However, the quality of the evidence was very low given the high risk of bias associated with the studies, and the small number of included participants and consequent level of imprecision.
Furthermore, in the first comparison there was no heterogeneity between the included studies even though the timing of the endometrial injury in each study varied between the follicular phase of the cycle preceding the first attempted conception cycle, and the follicular phase of the first attempted conception cycle. This may further suggest that the timing of the endometrial injury does not influence the probability of conception. However, it should also be kept in mind that a endometrial injury undertaken during the luteal phase of a menstrual cycle has the potential to disturb a very early pregnancy.
See the 'Summary of findings' tables for a complete overview (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3).
Overall completeness and applicability of evidence
Overall, the included studies were relevant to the review questions and were generally applicable to subfertile women attempting to conceive with IUI or sexual intercourse, with or without ovulation induction (OI). Only two studies in the main comparison provided the preferred outcome of live birth (Mahey 2015; Parsanezhad 2013), and we pooled this live birth data with ongoing pregnancy data from the other included studies in that comparison.
All studies included participants with unexplained subfertility, and five studies also included mild male factor (Abdelhamid 2013; Al‐Tamemi 2014; El‐Khayat 2015; Wadhwa 2015; Zarei 2014), one study included mild endometriosis (Zarei 2014), one study included ovulatory factor (Abdelhamid 2013) and another included unilateral tubal factor (Wadhwa 2015). Unexplained infertility is a diagnosis of exclusion, in that no obvious cause to explain the delay in conception can be found. Unexplained infertility is therefore a potentially heterogeneous condition, and the biological factors responsible for the experienced infertility may be variable, such as mild endometriosis, poor quality oocytes or sperm function, and non‐receptive endometrium. It is possible that this procedure may therefore benefit some women with unexplained infertility and not others.
The participants may be viewed to generally represent those attending an infertility clinic. However, the average body mass index (BMI) in the included studies was higher than might be expected, which is an important consideration given the known correlation between BMI and fertility (Gesink Law 2007). Furthermore, the duration of subfertility experienced by participants was generally quite long, as one study included women with an average duration of six years subfertility (Gibreel 2013) and the lowest average duration was 3.4 years (El‐Khayat 2015).
The type of conception differed between studies. In seven studies women attempted to conceive through IUI (Abdelhamid 2013; Al‐Tamemi 2014; El‐Khayat 2015; Maged 2016; Mahey 2015; Wadhwa 2015; Zarei 2014), and in the other two studies with intercourse (Gibreel 2013; Parsanezhad 2013). Due to a lack of observed heterogeneity between these studies and the assumption that the mechanism underlying any observed effect of endometrial injury on implantation would not differ between women undergoing IUI or having sexual intercourse, the results of these studies may be extrapolated to couples trying to conceive with either IUI or intercourse. However, in five of the included studies the participants were additionally on OI medication, which has been shown to exert effects at the level of the endometrium (Casper 2006) and is also not generally recommended in women with unexplained subfertility trying to conceive from IUI or intercourse (NICE 2013). In this way, the results may not be applicable to couples with unexplained subfertility who are trying to conceive naturally, i.e. without OI medication.
Three studies used the most common sampling device, the pipelle curette (Al‐Tamemi 2014; Gibreel 2013; Parsanezhad 2013). However, the other included studies used a wide variety of instruments including a Novak curette (Zarei 2014), a Tao brush (Abdelhamid 2013), grasping teeth with forceps (El‐Khayat 2015), a neonatal feeding tube (Maged 2016), and a cannula (Mahey 2015; Wadhwa 2015). Although these devices may cause slightly different levels of endometrial damage, they may all be considered to cause a minor local injury, as compared to a dilation and curettage procedure which would cause a more extensive injury.
Despite the general applicability of the included studies, only one study published the most clinically relevant and patient‐oriented outcome of live birth, and we were able to obtain data on live birth from another two studies after author correspondence. In the absence of live birth data, the outcome of ongoing pregnancy was used, as less than 5% of ongoing pregnancies will end in still birth on average (Say 2006). It has been argued that ongoing pregnancy is a preferred outcome of effectiveness compared to live birth in fertility trials (Braakhekke 2014). However, it remains possible that the results may have differed if all studies had followed up pregnancies until live birth.
There is some evidence to suggest that the inflammatory response generated from endometrial injury lasts within the endometrium for three months (Gnainsky 2010). The number of potential conception cycles in most included studies was three, but the number ranged from one to six. Due to the lack of heterogeneity between studies reported here, we are unable to comment on the potential duration of effect from endometrial scratching. Owing to the recent nature of this intervention and a lack of proven efficacy, current recommendations for the management of unexplained subfertility (the subfertile condition that was the focus of the included studies) do not mention endometrial injury (ASRM 2006; NICE 2013). Current evidence and recommendations suggest in vitro fertilisation (IVF) may be the most effective treatment in this population (NICE 2013; Pandian 2015).
If further well‐designed and conducted studies can confirm a beneficial effect of endometrial injury in couples trying to conceive from sexual intercourse or IUI, it may provide a cost‐effective fertility treatment for some couples before they consider more expensive and invasive methods such as IVF.
Quality of the evidence
Nine studies, which included 1512 women in total, met the inclusion criteria of this Cochrane review. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we rated the overall quality of the evidence as either low or very low for all outcomes in all comparisons (see the 'Summary of findings' tables: summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). The reasons for downgrading the evidence were risk of bias, imprecision and indirectness, as we have described below.
Risk of bias
The methodological quality of the included studies was variable and we noted a number of potentially very serious risks of bias. Therefore we downgraded the evidence by two levels in comparisons one and three. Some of the most serious risks included a lack of adequate allocation concealment, which is considered to be the most important risk of bias after adequate randomisation (Schulz 2002). For example, one study used block randomisation with blocks of two, which would result in every second allocation being known in advance and therefore would not be concealed (Parsanezhad 2013); and another study randomised patients from a list of allocations, which indicates there was no allocation concealment (Mahey 2015). Two studies did not supply the method of allocation concealment (Al‐Tamemi 2014; Zarei 2014). In two studies, lack of blinding resulted in a large number of participants committing protocol violations by failing to complete the three study cycles, which led to a severely unbalanced number of opportunities to conceive in each study arm (Wadhwa 2015; Zarei 2014).
One study was only available as an abstract (Mahey 2015) and another study was only available as an unpublished thesis which was photocopied from the University's library (Al‐Tamemi 2014) and were therefore not thoroughly peer reviewed. One of the abstracts was of an interim analysis, and on further correspondence the study authors provided a more recent interim analysis for use in this review (Mahey 2015). Conducting multiple interim analyses is considered a high risk of bias as the conduct of the study may be affected by interim results. For example, multiple 'looks' at interim analysis introduces a greater potential for finding false‐positive effects and may result in a biased early termination of a trial for an apparently beneficial effect (Zelen 2003).
It is generally considered desirable to blind participants in randomised controlled trials (RCTs), especially in cases where participants are more easily able to introduce performance bias; such as in fertility trials where sexual intercourse is required for conception. However, a sham procedure has the following disadvantages: the requirement for an uncomfortable and invasive procedure and associated time required to travel to and attend the appointment for the patient; use of doctor's time in performing the procedure; and use of resources such as pipelle, speculum and tenaculum. Furthermore, many patients feel deceived with the use of a placebo‐controlled trial and this can engender distrust between the doctor and the patient, and the potential for negative impacts on the trial, such as withdrawals/loss to follow‐up. Two included studies implemented a sham procedure; one study that involved no manipulation of the internal cervical os (Parsanezhad 2013); and the second study that administered a uterine sound (Gibreel 2013). Although not formally tested, it is uncertain whether these procedures would have sufficiently blinded participants to their allocation. However, as these sham procedures are themselves likely to cause some degree of endometrial damage, they are perhaps not adequate controls in this sense. This introduces a dilemma as, short of sedating participants at the time of the procedure/sham procedure/no treatment, it may not be possible to use a sham procedure that adequately blinds participants without causing some damage, and thus being an intervention in itself. In another study, the intervention group underwent hysteroscopy with endometrial injury and the control group underwent hysteroscopy only (El‐Khayat 2015).
Imprecision
In all comparisons we downgraded the evidence for imprecision for the primary outcome of live birth/ongoing pregnancy due to the small number of included studies and consequently wide confidence intervals (CIs). As a rule of thumb, if the total number of events is less than 300 then the result may be viewed as imprecise; as this outcome had 152 events we downgraded it for imprecision.
Indirectness
In comparisons two and three, which included only one (Abdelhamid 2013) and two studies respectively (Abdelhamid 2013; Wadhwa 2015), we also downgraded the evidence for indirectness. These studies included women with unexplained infertility undergoing IUI with OI with gonadotrophins and therefore it may not be appropriate to generalise the results of this study to women trying to conceive naturally (without IUI or OI). The endometrial injury was performed using a Tao brush (Abdelhamid 2013) or endometrial cannula (Wadhwa 2015), but the more common device was the pipelle curette (Nastri 2013b).
Potential biases in the review process
We conducted a comprehensive search with the help of an experienced Trials Search Co‐ordinator, as well as extensive manual searching, in an effort to retrieve all eligible studies. While we found three additional studies by handsearching (Al‐Tamemi 2014; Mahey 2015; Wadhwa 2015), it is possible that we may not have identified unpublished studies. We were unable to construct a funnel plot as fewer than 10 studies were available in any comparison, and therefore we were unable to estimate the existence of publication bias.
Although it was not possible to measure the potential for publication bias in this Cochrane review, there may be reason to suspect it. Only two of the nine included studies were registered prospectively. Five studies were registered retrospectively, and two studies were not registered at all. This review reports positive results from several small studies with no or only retrospective registration, which therefore signals a potential for publication bias. However, we did not downgrade the evidence for this.
This review intended to include studies that investigated the effect of intentional endometrial injury. We excluded interventions that may cause incidental endometrial injury, such as hysteroscopy or hysterosalpingogram. Two included studies employed a sham procedure in the control group which was not intended to cause any endometrial injury, but which may inadvertently have done so (Gibreel 2013; Parsanezhad 2013). We decided to include these studies in the first comparison (Endometrial injury vs no intervention or sham procedure) rather than the second (Higher vs lower degree of endometrial injury) given that the researchers did not intend for the mock procedure to cause any injury. On the other hand, we included the study that compared hysteroscopy and injury with hysteroscopy alone in the second comparison (Higher vs. lower degree of intentional endometrial injury) as we viewed hysteroscopy as an intervention rather than placebo procedure (El‐Khayat 2015).
Although we contacted authors for additional information, we could not obtain all of the requested information, which may have introduced bias due to the inclusion of trials with insufficient information. Furthermore, there remains the potential for study authors to provide inaccurate information and to provide overly positive answers.
Agreements and disagreements with other studies or reviews
Other studies and reviews in women undergoing ART have also shown an increased probability of pregnancy and live birth following intentional endometrial injury (Almog 2010; El‐Toukhy 2012; Li 2009; Nastri 2015; Potdar 2012). However, one recent adequately‐powered study demonstrated no effect (Yeung 2014). To our knowledge there are no other reviews on endometrial injury in women trying to conceive from intercourse or IUI.

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' category for each included study.

Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.1 Live birth or ongoing pregnancy: sensitivity analysis excluding studies at high or unclear risk of allocation concealment.
Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.2 Live birth or ongoing pregnancy: sensitivity analysis.

Forest plot of comparison: 2 Higher vs. lower degree of intentional endometrial injury, outcome: 2.1 Live birth or ongoing pregnancy.

Forest plot of comparison: 3 Timing of intentional endometrial injury, outcome: 3.1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 2 Live birth or ongoing pregnancy: sensitivity analysis.
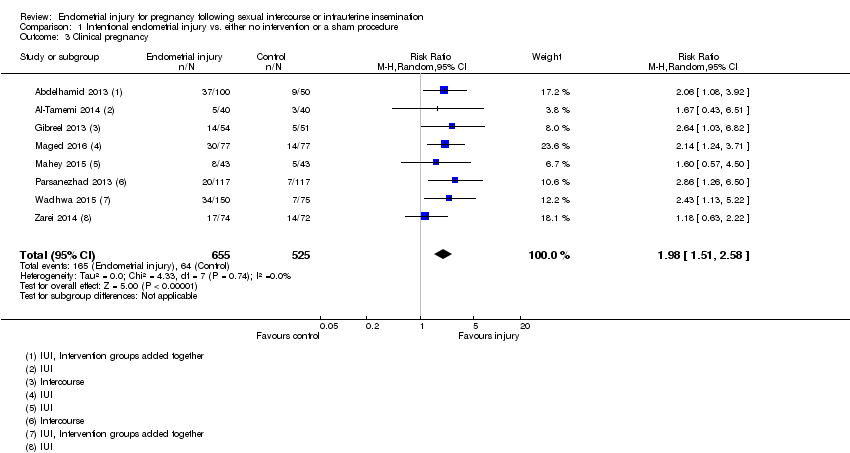
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 3 Clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 4 Miscarriage per clinical pregnancy.
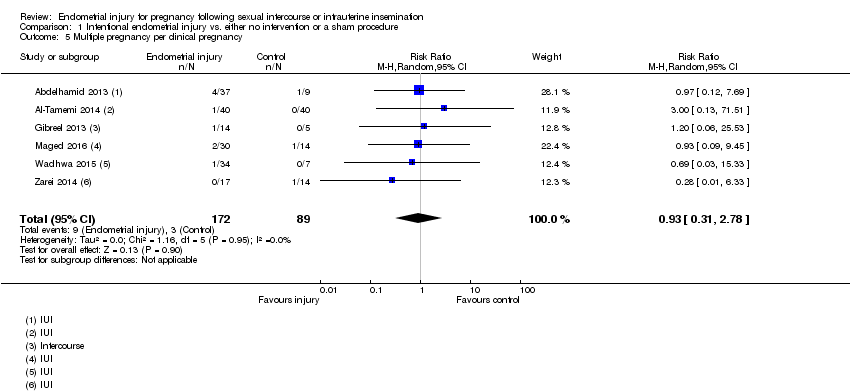
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 5 Multiple pregnancy per clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 6 Ectopic pregnancy per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 2 Clinical pregnancy.
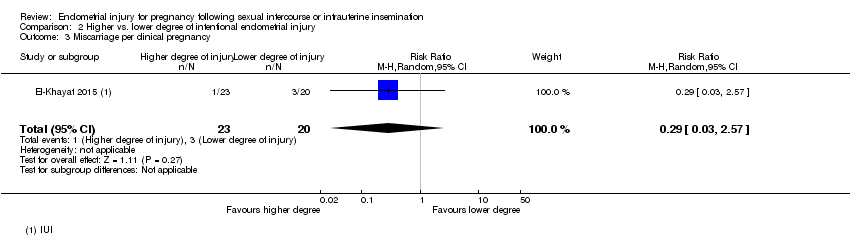
Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.
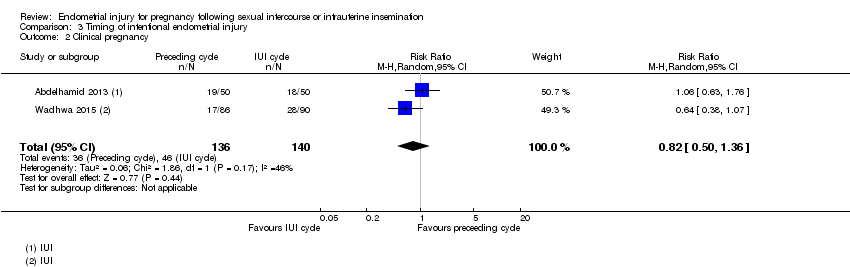
Comparison 3 Timing of intentional endometrial injury, Outcome 2 Clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.
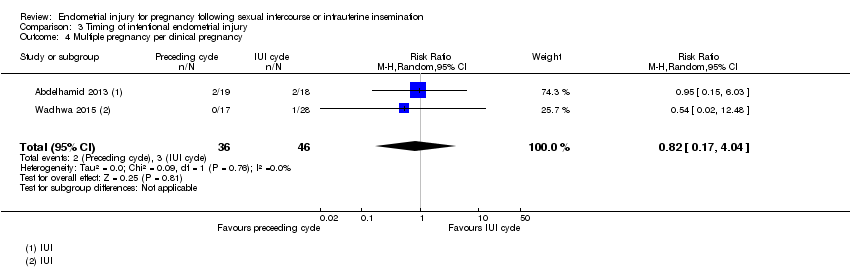
Comparison 3 Timing of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with either: no intervention, or a sham procedure | Risk with Intentional endometrial injury | ||||
| Live birth or ongoing pregnancy | 87 per 1000 | 194 per 1000 | RR 2.22 | 950 | ⊕⊝⊝⊝ |
| Live birth or ongoing pregnancy ‐ sensitivity | 98 per 1000 | 259 per 1000 | RR 2.64 | 105 | ⊕⊝⊝⊝ |
| Pain during the procedure | Pain was not recorded in the control group | Pain was only recorded in the intervention group with an average of 6/10, standard deviation (SD) = 1.5 | — | (1 RCT) | — |
| Clinical pregnancy | 122 per 1000 | 241 per 1,000 | RR 1.98 | 1180 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels for risk of bias as many of the included studies are associated with a high risk of bias. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with lower degree of intentional endometrial injury | Risk with Higher | ||||
| Live birth or ongoing pregnancy | 102 per 1000 | 132 per 1000 | RR 1.29 | 332 | ⊕⊕⊝⊝ |
| Pain during the procedure | — | — | — | (0 study) | — |
| Clinical pregnancy | 120 per 1000 | 139 per 1000 | RR 1.15 | 332 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for indirectness as there was only 1 included study. Therefore the result was applicable only to cases of hysteroscopy plus injury vs hysteroscopy alone, and not other cases of higher vs. lower injury. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with injury in preceding cycle | Risk with injury in IUI cycle | ||||
| Live birth or ongoing pregnancy | 267 per 1000 | 173 per 1000 | RR 0.65 | 176 | ⊕⊝⊝⊝ |
| Pain during the procedure | — | — | — | (0 RCTs) | — |
| Clinical pregnancy | 329 per 1000 | 269 per 1000 | RR 0.82 | 276 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for risk of bias as both studies were at high risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 6 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.56, 3.15] |
| 1.1 Live birth | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.12, 5.49] |
| 1.2 Ongoing pregnancy | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.46, 3.19] |
| 2 Live birth or ongoing pregnancy: sensitivity analysis Show forest plot | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 2.1 Ongoing pregnancy | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 3 Clinical pregnancy Show forest plot | 8 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.51, 2.58] |
| 4 Miscarriage per clinical pregnancy Show forest plot | 6 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.39] |
| 5 Multiple pregnancy per clinical pregnancy Show forest plot | 6 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.78] |
| 6 Ectopic pregnancy per clinical pregnancy Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.35] |
| 2 Clinical pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.66, 2.01] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.57] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.16] |
| 2 Clinical pregnancy Show forest plot | 2 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.36] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.03] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.04] |

