Ozljeda endometrija za povećanje mogućnosti trudnoće nakon spolnog odnosa ili medicinski potpomognute oplodnje postupkom inseminacije
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
PROCITE platform
From inception until 29.10.15
Keywords CONTAINS "Intrauterine Insemination" or "IUI" or "artificial insemination" or "expectant management" or "intercourse" or "coitus" or Title CONTAINS "Intrauterine Insemination" or "IUI" or "artificial insemination" or "expectant management" or "intercourse" or "coitus" AND Keywords CONTAINS "endometrial biopsy"or "endometrial injury"or"endometrial trauma"or "mock embryo transfer" or "endometrial sampling"or "endometrial local injury"or "endometrial priming" or Title CONTAINS "endometrial biopsy"or "endometrial injury"or"endometrial trauma"or "mock embryo transfer" or "endometrial sampling"or "endometrial local injury"or "endometrial priming" (70)
Appendix 2. CENTRAL search strategy
Ovid platform
From inception until 29.10.15
1 exp insemination, artificial/ or exp insemination, artificial, heterologous/ or exp insemination, artificial, homologous/ (296)
2 artificial insemination.tw. (98)
3 intrauterine insemination.tw. (500)
4 IUI.tw. (398)
5 intercourse.tw. (1195)
6 ovulation induction.tw. (544)
7 coitus.tw. (95)
8 exp Infertility/ (1668)
9 subfertil$.tw. (203)
10 pregnanc$.tw. (13832)
11 or/1‐10 (16030)
12 (endometri$ adj3 sampl$).tw. (181)
13 (endometri$ adj3 biops$).tw. (480)
14 (endometri$ adj3 scratch$).tw. (6)
15 (endometri$ adj3 injur$).tw. (27)
16 pipelle.tw. (64)
17 local injury.tw. (23)
18 (endometri$ adj5 trauma$).tw. (4)
19 (endometri$ adj5 harm$).tw. (1)
20 (endometri$ adj5 damag$).tw. (8)
21 (endometri$ adj5 inflammation).tw. (5)
22 (endometri$ adj5 wound$).tw. (61)
23 (endometri$ adj5 lesion$).tw. (70)
24 (endometri$ adj5 insult$).tw. (0)
25 (mock adj3 transfer$).tw. (5)
26 (endometri$ adj3 stimul$).tw. (105)
27 (endometri$ adj3 prim$).tw. (67)
28 or/12‐27 (876)
29 11 and 28 (145)
Appendix 3. MEDLINE search strategy
Ovid platform
From inception until 29.10.15
1 exp insemination, artificial/ or exp insemination, artificial, heterologous/ or exp insemination, artificial, homologous/ (10413)
2 artificial insemination.tw. (5293)
3 intrauterine insemination.tw. (1919)
4 IUI.tw. (1329)
5 intercourse.tw. (16057)
6 ovulation induction.tw. (3083)
7 coitus.tw. (2590)
8 exp Infertility/ (56134)
9 subfertil$.tw. (3927)
10 pregnanc$.tw. (330376)
11 or/1‐10 (398545)
12 (endometri$ adj3 sampl$).tw. (2417)
13 (endometri$ adj3 biops$).tw. (3772)
14 (endometri$ adj3 scratch$).tw. (23)
15 (endometri$ adj3 injur$).tw. (103)
16 pipelle.tw. (209)
17 local injury.tw. (374)
18 (endometri$ adj5 trauma$).tw. (90)
19 (endometri$ adj5 harm$).tw. (26)
20 (endometri$ adj5 damag$).tw. (189)
21 (endometri$ adj5 inflammation).tw. (329)
22 (endometri$ adj5 wound$).tw. (174)
23 (endometri$ adj5 lesion$).tw. (2781)
24 (endometri$ adj5 insult$).tw. (4)
25 (mock adj3 transfer$).tw. (42)
26 (endometri$ adj3 stimul$).tw. (790)
27 (endometri$ adj3 prim$).tw. (1618)
28 or/12‐27 (11373)
29 11 and 28 (2103)
30 randomized controlled trial.pt. (415041)
31 controlled clinical trial.pt. (91985)
32 randomized.ab. (336476)
33 randomised.ab. (68617)
34 placebo.tw. (173735)
35 clinical trials as topic.sh. (179555)
36 randomly.ab. (242878)
37 trial.ti. (148303)
38 (crossover or cross‐over or cross over).tw. (66278)
39 or/30‐38 (1051606)
40 exp animals/ not humans.sh. (4137311)
41 39 not 40 (969497)
42 29 and 41 (217)
Appendix 4. EMBASE search strategy
OVID platform
From inception until 29.10.15
1 exp artificial insemination/ (13034)
2 artificial insemination.tw. (5054)
3 intrauterine insemination.tw. (2703)
4 IUI.tw. (2290)
5 intercourse.tw. (19013)
6 ovulation induction.tw. (4146)
7 coitus.tw. (2495)
8 exp Infertility/ (97346)
9 subfertil$.tw. (4961)
10 pregnanc$.tw. (393427)
11 (endometri$ adj3 sampl$).tw. (3271)
12 (endometri$ adj3 biops$).tw. (4964)
13 (endometri$ adj3 scratch$).tw. (47)
14 (endometri$ adj3 injur$).tw. (167)
15 pipelle.tw. (398)
16 local injury.tw. (461)
17 (endometri$ adj5 trauma$).tw. (102)
18 (endometri$ adj5 harm$).tw. (51)
19 (endometri$ adj5 damag$).tw. (260)
20 (endometri$ adj5 inflammation).tw. (433)
21 (endometri$ adj5 wound$).tw. (220)
22 (endometri$ adj5 lesion$).tw. (3941)
23 (endometri$ adj5 insult$).tw. (6)
24 (mock adj3 transfer$).tw. (70)
25 (endometri$ adj3 stimul$).tw. (993)
26 (endometri$ adj3 prim$).tw. (2108)
27 or/1‐10 (496718)
28 or/11‐26 (15089)
29 27 and 28 (3285)
30 Clinical Trial/ (852134)
31 Randomized Controlled Trial/ (386850)
32 exp randomization/ (68548)
33 Single Blind Procedure/ (21172)
34 Double Blind Procedure/ (124394)
35 Crossover Procedure/ (44827)
36 Placebo/ (264947)
37 Randomi?ed controlled trial$.tw. (125572)
38 Rct.tw. (18572)
39 random allocation.tw. (1460)
40 randomly allocated.tw. (23491)
41 allocated randomly.tw. (2066)
42 (allocated adj2 random).tw. (739)
43 Single blind$.tw. (16493)
44 Double blind$.tw. (155737)
45 ((treble or triple) adj blind$).tw. (496)
46 placebo$.tw. (222385)
47 prospective study/ (311493)
48 or/30‐47 (1514482)
49 case study/ (34294)
50 case report.tw. (292773)
51 abstract report/ or letter/ (941827)
52 or/49‐51 (1262329)
53 48 not 52 (1474450)
54 29 and 53 (446)
Appendix 5. PsycINFO search strategy
OVID Platform
From inception until 29.10.15
1 exp Reproductive Technology/ (1519)
2 artificial insemination.tw. (235)
3 intrauterine insemination.tw. (19)
4 IUI.tw. (26)
5 intercourse.tw. (8063)
6 ovulation induction.tw. (19)
7 coitus.tw. (778)
8 exp Infertility/ (1837)
9 subfertil$.tw. (69)
10 pregnanc$.tw. (30684)
11 or/1‐10 (40989)
12 (endometri$ adj3 sampl$).tw. (4)
13 (endometri$ adj3 biops$).tw. (15)
14 (endometri$ adj3 scratch$).tw. (0)
15 (endometri$ adj3 injur$).tw. (1)
16 pipelle.tw. (0)
17 local injury.tw. (25)
18 (endometri$ adj5 trauma$).tw. (2)
19 (endometri$ adj5 harm$).tw. (0)
20 (endometri$ adj5 damag$).tw. (3)
21 (endometri$ adj5 inflammation).tw. (1)
22 (endometri$ adj5 wound$).tw. (0)
23 (endometri$ adj5 lesion$).tw. (13)
24 (endometri$ adj5 insult$).tw. (0)
25 (mock adj3 transfer$).tw. (0)
26 (endometri$ adj3 stimul$).tw. (4)
27 (endometri$ adj3 prim$).tw. (6)
28 or/12‐27 (69)
29 11 and 28 (7)
30 random.tw. (45128)
31 control.tw. (349729)
32 double‐blind.tw. (19333)
33 clinical trials/ (9139)
34 placebo/ (4286)
35 exp Treatment/ (633786)
36 or/30‐35 (972934)
37 29 and 36 (3)
Appendix 6. CINAHL search strategy
EBSCO Platform
From inception until 29.10.15
| # | Query | Results |
| S43 | S28 AND S42 | 62 |
| S42 | S29 OR S30 or S31 or S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 893,293 |
| S41 | TX allocat* random* | 3,912 |
| S40 | (MH "Quantitative Studies") | 12,084 |
| S39 | (MH "Placebos") | 8,756 |
| S38 | TX placebo* | 31,642 |
| S37 | TX random* allocat* | 3,912 |
| S36 | (MH "Random Assignment") | 37,333 |
| S35 | TX randomi* control* trial* | 73,395 |
| S34 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 717,399 |
| S33 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 105 |
| S32 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 0 |
| S31 | TX clinic* n1 trial* | 163,636 |
| S30 | PT Clinical trial | 76,052 |
| S29 | (MH "Clinical Trials+") | 175,404 |
| S28 | S11 AND S27 | 113 |
| S27 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 | 756 |
| S26 | TX(mock N3 transfer*) | 3 |
| S25 | TX(endometri* N5 insult*) | 90 |
| S24 | TX(endometri* N5 insult*) | 0 |
| S23 | TX(endometri* N5 lesion*) | 110 |
| S22 | TX(endometri* N5 wound*) | 24 |
| S21 | TX(endometri* N5 inflammation) | 16 |
| S20 | TX(endometri* N5 damag*) | 5 |
| S19 | TX(endometri* N5 harm*) | 1 |
| S18 | TX (endometri* N5 trauma*) | 2 |
| S17 | TX (local N3 injury) | 186 |
| S16 | TX pipelle | 26 |
| S15 | TX(endometri* N3 injur*) | 17 |
| S14 | TX(endometri* N3 scratch*) | 4 |
| S13 | TX(endometri* N3 biops*) | 238 |
| S12 | TX(endometri* N3 sampl*) | 116 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 136,488 |
| S10 | TX pregnanc* | 127,954 |
| S9 | TX Infertil* | 6,916 |
| S8 | TX subfertil* | 396 |
| S7 | (MM "Infertility") | 3,462 |
| S6 | TX coitus | 1,634 |
| S5 | TX intercourse | 3,551 |
| S4 | TX IUI | 71 |
| S3 | TX intrauterine insemination | 135 |
| S2 | TX artificial insemination | 436 |
| S1 | (MM "Insemination, Artificial") | 229 |
Appendix 7. LILACS search strategy
Web platform
From inception until 29.10.15
(tw:(endometrial injury)) OR (tw:(endometrial sampling)) OR (tw:(endometrial trauma)) OR (tw:(endometrial biopsy)) OR (tw:(pipelle)) AND (tw:(intercourse)) OR (tw:(coitus)) OR (tw:(intrauterine insemination)) OR (tw:(iui)) (0)
Appendix 8. DARE search strategy
From inception until 29.10.15
1 artificial insemination.tw. (2)
2 intrauterine insemination.tw. (26)
3 iui.tw. (5)
4 intercourse.tw. (59)
5 coitus.tw. (9)
6 ovulation induction.tw. (85)
7 subfertil$.tw. (34)
8 infertil$.tw. (191)
9 pregnan$.tw. (1754)
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (1852)
11 (endometri$ adj3 sampl$).tw. (6)
12 (endometri$ adj3 injur$).tw. (2)
13 pipelle.tw. (4)
14 local injury.tw. (1)
15 (endometri$ adj3 trauma$).tw. (0)
16 (endometri$ adj3 harm$).tw. (0)
17 (endometri$ adj3 inflammation).tw. (0)
18 (endometri$ adj3 biops$).tw. (11)
19 (endometri$ adj3 scratch$).tw. (0)
20 (endometri$ adj3 stimul$).tw. (0)
21 (endometri$ adj3 prim$).tw. (9)
22 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (25)
23 10 and 22 (2)
Appendix 9. ISI Web of Knowledge search strategy
Web Platform
From inception until 29.10.15
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
TOPIC: ("artificial insemination") OR TOPIC: ("intrauterine insemination") OR TOPIC: (iui) OR TOPIC: (intercourse) OR TOPIC: (coitus) OR TOPIC: (infertil&) OR TOPIC: (subfertil$) OR TOPIC: (pregnan$) AND (TOPIC: ((endometri$ and sampl$)) OR TOPIC: ((endometri$ adj3 biops$)) AND TOPIC: ((endometri$ adj3 biops$)) OR TOPIC: ((endometri$ adj3 injur$)) OR TOPIC: (pipelle) OR TOPIC: ((endometri$ adj3 trauma$).) OR TOPIC: ((endometri$ adj3 damag$)) OR TOPIC: ((endometri$ adj3 wound$))) (4)
Appendix 10. 'Risk of bias' assessments
We considered the following methods of random sequence generation adequate.
-
Referring to a random number table.
-
Using a computer random number generator.
-
Coin tossing.
-
Shuffling cards or envelopes.
-
Throwing dice.
-
Drawing of lots.
We considered the following methods of allocation concealment adequate.
-
Central allocation (including telephone, Internet‐based and pharmacy‐controlled randomisation).
-
Sequentially numbered, opaque, sealed envelopes.
Blinding of personnel was not considered important in this review.‐ change back to old text
We considered blinding of personnel important as personnel may treat their patients differently with knowledge of their allocation. We deemed blinding of personnel adequate if the study authors described taking any measures to blind their staff to participant allocation
We considered blinding of participants to be important as knowledge of allocation may lead to changes in behaviour, such as intercourse patterns, and therefore introduce performance bias. We deemed blinding of participants adequate if the study authors described any of the following.
-
Use of a sham procedure.
-
Blinding of women is assessed.
We considered blinding of outcome assessors important only for the subjective outcome of pain. We deemed blinding adequate for this outcome if the study authors described any of the following.
-
Blinding of participants and personnel involved in asking/recording reported pain.
-
Unblinding of participants and personnel involved in asking/recording reported pain (at the end of the study).

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' category for each included study.

Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.1 Live birth or ongoing pregnancy: sensitivity analysis excluding studies at high or unclear risk of allocation concealment.
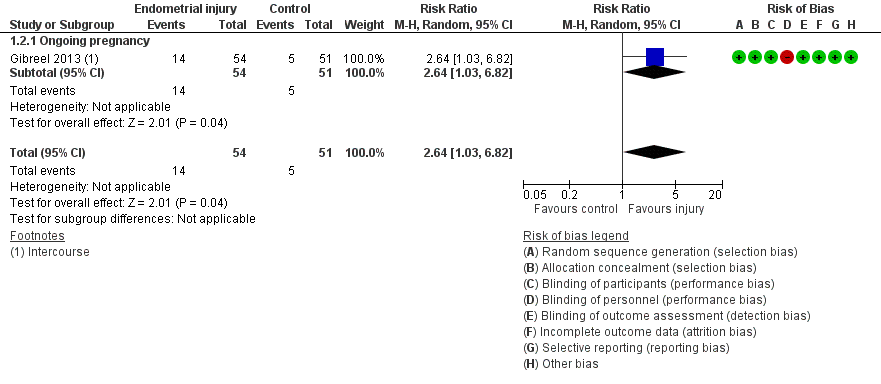
Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.2 Live birth or ongoing pregnancy: sensitivity analysis.

Forest plot of comparison: 2 Higher vs. lower degree of intentional endometrial injury, outcome: 2.1 Live birth or ongoing pregnancy.

Forest plot of comparison: 3 Timing of intentional endometrial injury, outcome: 3.1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 2 Live birth or ongoing pregnancy: sensitivity analysis.
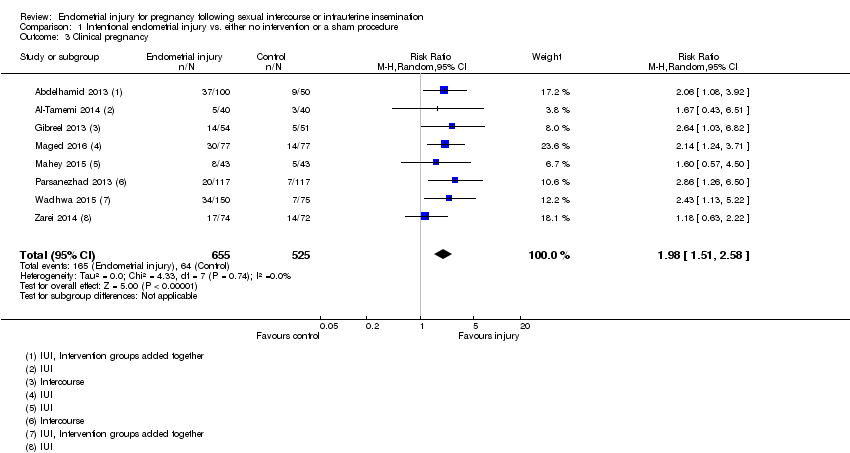
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 3 Clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 4 Miscarriage per clinical pregnancy.
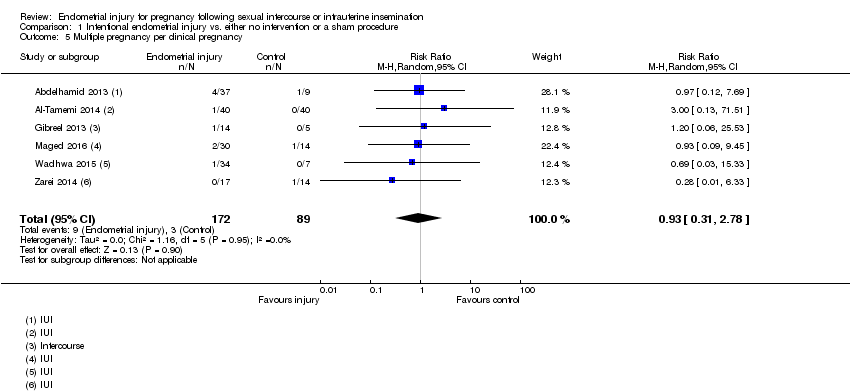
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 5 Multiple pregnancy per clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 6 Ectopic pregnancy per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 2 Clinical pregnancy.
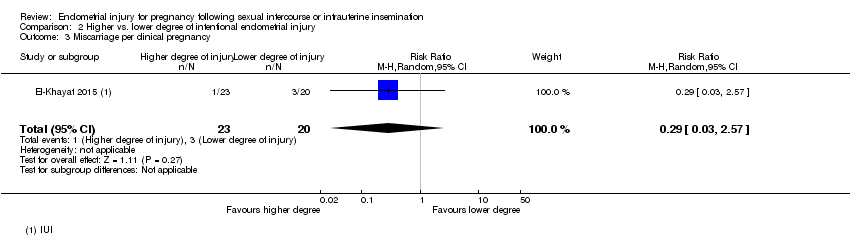
Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.
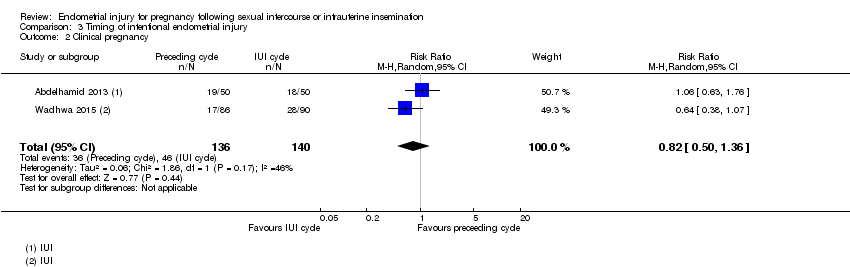
Comparison 3 Timing of intentional endometrial injury, Outcome 2 Clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.
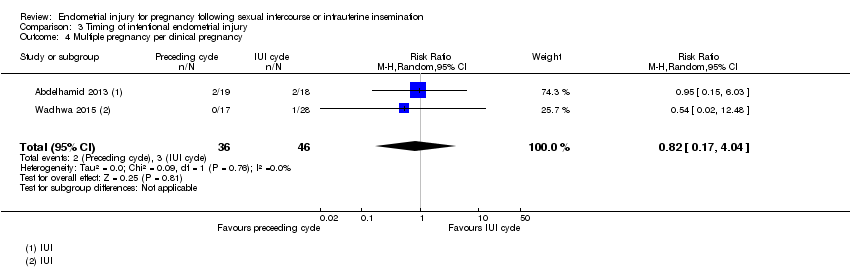
Comparison 3 Timing of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with either: no intervention, or a sham procedure | Risk with Intentional endometrial injury | ||||
| Live birth or ongoing pregnancy | 87 per 1000 | 194 per 1000 | RR 2.22 | 950 | ⊕⊝⊝⊝ |
| Live birth or ongoing pregnancy ‐ sensitivity | 98 per 1000 | 259 per 1000 | RR 2.64 | 105 | ⊕⊝⊝⊝ |
| Pain during the procedure | Pain was not recorded in the control group | Pain was only recorded in the intervention group with an average of 6/10, standard deviation (SD) = 1.5 | — | (1 RCT) | — |
| Clinical pregnancy | 122 per 1000 | 241 per 1,000 | RR 1.98 | 1180 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels for risk of bias as many of the included studies are associated with a high risk of bias. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with lower degree of intentional endometrial injury | Risk with Higher | ||||
| Live birth or ongoing pregnancy | 102 per 1000 | 132 per 1000 | RR 1.29 | 332 | ⊕⊕⊝⊝ |
| Pain during the procedure | — | — | — | (0 study) | — |
| Clinical pregnancy | 120 per 1000 | 139 per 1000 | RR 1.15 | 332 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for indirectness as there was only 1 included study. Therefore the result was applicable only to cases of hysteroscopy plus injury vs hysteroscopy alone, and not other cases of higher vs. lower injury. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with injury in preceding cycle | Risk with injury in IUI cycle | ||||
| Live birth or ongoing pregnancy | 267 per 1000 | 173 per 1000 | RR 0.65 | 176 | ⊕⊝⊝⊝ |
| Pain during the procedure | — | — | — | (0 RCTs) | — |
| Clinical pregnancy | 329 per 1000 | 269 per 1000 | RR 0.82 | 276 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for risk of bias as both studies were at high risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 6 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.56, 3.15] |
| 1.1 Live birth | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.12, 5.49] |
| 1.2 Ongoing pregnancy | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.46, 3.19] |
| 2 Live birth or ongoing pregnancy: sensitivity analysis Show forest plot | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 2.1 Ongoing pregnancy | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 3 Clinical pregnancy Show forest plot | 8 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.51, 2.58] |
| 4 Miscarriage per clinical pregnancy Show forest plot | 6 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.39] |
| 5 Multiple pregnancy per clinical pregnancy Show forest plot | 6 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.78] |
| 6 Ectopic pregnancy per clinical pregnancy Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.35] |
| 2 Clinical pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.66, 2.01] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.57] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.16] |
| 2 Clinical pregnancy Show forest plot | 2 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.36] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.03] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.04] |

