Monoterapia con fármacos antiepilépticos para la epilepsia: un metanálisis en red de los datos de los participantes individuales
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blinded, parallel‐group trial conducted in Finland 2 treatment arms: OXC and PHT | |
| Participants | Adult participants with newly diagnosed epilepsy and "normal intellectual capacity" with a minimum of 2 seizures in the last 2 years or 1 seizure and an epileptiform EEG Number randomised: OXC = 19, PHT = 18 Number completed and included in analysis: OXC = 14, PHT = 15 (see Notes) 11 male participants (38%) out of 29 included participants 21 participants with partial epilepsy (72%) out of 29 included participants Mean age of included participants (SD): OXC = 33.6 (14) years, PHT = 32.7 (12.5) years | |
| Interventions | Monotherapy with OXC or PHT 4‐ to 8‐week titration period followed by a maintenance phase of 12 months. Doses achieved not stated Range of follow‐up not stated | |
| Outcomes | Neuropsychological assessment and cognitive functioning in 3 major areas at baseline, 6 months' and 12 months' follow‐up: Verbal learning and memory Sustained attention Simple psychomotor speed | |
| Notes | Participants experiencing inadequate seizure control, adverse events or those who were non‐compliant were withdrawn from the trial and excluded from analysis (5 from OXC group and 3 from PHT group). Results presented only for 29 participants (OXC = 14 and PHT = 15) completing the trial Outcomes chosen for this review were not reported; contact could not be made with trial author to provide IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned" to treatment; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | "The study followed a double blind design" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The study followed a double blind design"; no further information provided about whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | ITT approach not taken: results reported only for 29 participants (OXC = 14 and PHT = 15) who completed 12‐month follow‐up. 8 participants experiencing inadequate seizure control, adverse events or those who were non‐compliant (OXC = 5 and PHT = 3) were excluded from analysis and results |
| Selective reporting (reporting bias) | Low risk | No protocol available and outcomes chosen for this review not reported. Neuropsychological and cognitive outcomes well reported and treatment withdrawal rates reported |
| Other bias | Low risk | None identified |
| Methods | Single‐centre, double‐blind RCT of participants recruited from clinical referral to a multidisciplinary child development centre at a children's hospital in Dhaka, Bangladesh 2 treatment arms: CBZ and PHB | |
| Participants | 108 children aged 2‐15 years with 2 or more generalised tonic‐clonic, partial, or secondarily generalised seizures in the previous year Number randomised: CBZ = 54, PHB = 54 61 boys (56%) 59 participants with partial epilepsy (55%) 26 participants had previous AED treatment (24%) Mean age (range): 6 years (1‐15 years) | |
| Interventions | Monotherapy with CBZ (immediate release) or PHB Starting daily dose: CBZ = 1.5 mg/kg/d, PHB = 5 mg/kg/d, maximum daily dose: CBZ = 4 mg/kg/d, PHB = 16 mg/kg/d Trial duration: 12 months, range of follow‐up: 0‐22 months | |
| Outcomes | Seizure control: seizure freedom during the last quarter of the 12‐month follow‐up Time to first seizure after randomisation Time to treatment withdrawal due to adverse events Change in behaviour from baseline according to age‐appropriate questionnaire Incidence of behavioural side‐effects | |
| Notes | We received IPD for all randomised participants from the trial author. We received reasons for withdrawal of allocated treatment as well as the date of the last follow‐up visit, but withdrawal of allocated treatment did not always coincide with the date of the last follow‐up visit (i.e. several participants had the allocated treatment substituted for the other trial drug and continued to be followed up). Dates of withdrawal of allocated treatment could not be provided; therefore, we could not calculate 'Time to withdrawal of allocated treatment'. We received the date of first seizure after randomisation, but dates of other seizures in the follow‐up time could not be provided; therefore, we calculated 'Time to first seizure' for all participants, but we could not calculate the time to 6‐ and 12‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned to treatment"; the method of randomisation was not stated and not provided by the trial authors |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by sealed envelopes prepared on a different site to the site of recruitment of participants |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, a psychologist, and a therapist were blinded throughout the trial. The treating physician was unblinded for practical and ethical reasons |
| Blinding of outcome assessment (detection bias) | Unclear risk | A researcher performing outcome assessment was blinded throughout the trial but unblinded for analysis. It was unclear if this could have influenced the results |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were reported. We analysed all randomised participants from the IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | We calculated 1 outcome for this review from the IPD provided (see footnote 2). We could not calculate other outcomes for this review as the appropriate data were not recorded/not available. All cognitive outcomes from the trial were well reported |
| Other bias | High risk | There were inconsistencies between rates of seizure recurrence between the data provided and the published paper, which the trial authors could not resolve |
| Methods | Randomised, double‐blind, parallel‐group, non‐inferiority trial, conducted in 120 centres in Asia, Australia, and Europe 2 treatment arms: CBZ and ZNS | |
| Participants | Participants aged 18‐75 years with newly diagnosed epilepsy, at least 2 partial seizures (with or without secondary generalisation) or generalised tonic‐clonic seizures without clear focal origin in the previous 12 months and at least 1 seizure in the previous 3 months, and had not previously received AEDs or had been treated with 1 AED for no more than 2 weeks. Number randomised: CBZ = 301, ZNS = 282 347 (60%) male participants 100% partial epilepsy Mean age (range): 36 (18‐75 years) | |
| Interventions | Monotherapy with CBZ or ZNS Titration over 4 weeks to a target dose of CBZ = 600 mg/d and ZNS = 300 mg/d Range of follow‐up: 0‐29 months | |
| Outcomes | Proportion of participants who achieved seizure freedom for 26 weeks or more (maintenance period) in the per‐protocol population Incidence of treatment‐emergent results Time to 26‐week (6‐month) remission Time to 52‐week (12‐month) remission Proportion of participants with no seizures for at least 52 weeks Time to withdrawal because of absence of efficacy or adverse events | |
| Notes | IPD provided for all outcomes of this review by trial sponsor Eisai | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation scheme was generated centrally by computer programme, which produced a randomisation list with a pseudo‐random number generator |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by the use of a telephone interactive voice‐response system to dispense the allocated treatment |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, investigators, and sponsor personnel administering medication, assessing outcomes, and analysing data were masked to the allocation. Masking was maintained by use of matching placebo tablets. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, investigators, and sponsor personnel administering medication, assessing outcomes, and analysing data were masked to the allocation. Masking was maintained by use of matching placebo tablets. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Six‐month, systematic, simple randomised trial of children referred to a child neurology clinic (the author was from Guilan University of Medical Sciences, Iran, so it was likely that the trial was also conducted there) 2 treatment arms: CBZ and PHB | |
| Participants | Children aged 2‐12 years with partial seizures with secondary generalisation Number randomised: CBZ = 36, PHB = 35 36 boys (53%) 100% of participants with partial epilepsy Percentage newly diagnosed was not stated Age range: 2‐12 years | |
| Interventions | Monotherapy with CBZ or PHB Doses started or achieved not stated Trial duration: 6 months, range of follow‐up not stated | |
| Outcomes | Proportion seizure‐free Response rate and rate of side‐effects Seizure frequency and seizure duration | |
| Notes | The trial was reported in abstract form only with very limited information. Outcomes chosen for this review were not reported; IPD were not available, trial author could not be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as a 'systematic simple randomised study'; no further information was provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | No attrition rates were reported; it was unclear if all participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol available; the trial was available in abstract format only. Outcomes for this review were not available |
| Other bias | Low risk | None identified |
| Methods | Multicentre, double‐blind, parallel‐group trial conducted in centres in Argentina, Brazil, Mexico, South Africa 2 treatment arms: OXC and PHT | |
| Participants | Participants aged 16‐65 years with newly diagnosed epilepsy with partial or generalised tonic clonic seizures A minimum of 2 seizures, separated by at least 48 h, within 6 months preceding trial entry No previous AED, except emergency treatment of seizures for a maximum of 3 weeks prior to trial entry Number randomised: total = 287, OXC = 143, PHT = 144 174 male participants (61%); 182 participants with partial epilepsy (63%) Mean age (range) = 26 (15‐91) years | |
| Interventions | Monotherapy with OXC or PHT 8‐week titration period started with 300 mg OXC or 100 mg PHT, increased bi‐weekly, based on clinical response After 8 weeks participants were to be on a three‐times‐a‐day regimen with daily doses of 450 mg‐2400 mg OXC or 150 mg‐800 mg PHT Continued during 48‐week maintenance with adjustment according to clinical response A third long‐term, open‐label extension phase followed the maintenance period. Double‐blind results only were reported Range of follow‐up = 0‐19 months | |
| Outcomes | The proportion of seizure‐free participants who had at least one seizure during the maintenance period Time to premature discontinuation due to adverse experiences Rate of premature discontinuations for any reason Overall assessments of efficacy and tolerability and therapeutic effect Individual adverse experiences Laboratory values Seizure frequency during maintenance | |
| Notes | IPD provided for all outcomes of this review from trial sponsor Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment groups randomised in 1:1 ratio across centres via computer‐generated randomisation numbers over balanced blocks of size 6 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved with sequentially‐numbered packages that were identical and contained identical tablets (information provided by trial statistician) |
| Blinding of participants and personnel (performance bias) | Low risk | Trial conducted in 2 phases: 56‐week, double‐blind phase followed by long‐term, open‐label extension. Double‐blind phase results reported only. Blind achieved with divisible OXC and PHT tablets identical in appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported in both treatment phases, participants withdrawing from treatment were no longer followed up so seizure outcomes had to be censored at time of withdrawal and therefore analyses for remission and seizure outcomes could not adopt an ITT approach |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel group, multicentre trial conducted in the USA. 2 treatment arms: LTG and VPS | |
| Participants | Participants > 12 years with newly diagnosed or previously diagnosed epilepsy of any seizure type, not currently using an AED Number randomised: LTG = 66, VPS = 69, ITT population: LTG = 65, VPS = 68 (2 participants withdrew before drug escalation phase) 60 male participants (44%) 82 participants with partial epilepsy (60%) Proportion newly diagnosed not stated Mean age (range): 32 (12‐76) years | |
| Interventions | Monotherapy with LTG or VPS Dose‐escalation phase of 8 weeks to target doses of LTG = 200 mg/d and VPS = 20 mg/kg/d Trial duration: 32 weeks | |
| Outcomes | Weight change The proportion of participants seizure‐free during the entire trial Incidence of the most common drug‐related adverse events Time to withdrawal from the trial | |
| Notes | IPD provided for remote analysis by trial sponsor Glaxo Smith Kline for time to treatment withdrawal, time to first seizure and time to six‐month remission. IPD had to be treated as aggregate data in network meta‐analysis due to remote access to data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme was used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Results presented to investigator in a "blinded" fashion |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 8 centres in the UK. 2 treatment arms: LTG and CBZ | |
| Participants | Adults and children > 13 years with newly diagnosed epilepsy. None had received previous AED treatment. Number randomised: LTG = 70, CBZ = 66 56 male participants (41%) 82 with partial epilepsy (60%); Mean age (range): 34 (14‐71) years | |
| Interventions | Monotherapy with LTG or CBZ 4‐week escalation phase leading to LTG = 150 mg/d, CBZ = 600 mg/d Range of follow‐up = 0‐14 months | |
| Outcomes | Time to first seizure after 6 weeks of treatment Time to withdrawal Proportion of randomised participants remaining seizure‐free during the last 40 and 24 weeks of trial Percentages of participants who reported adverse events | |
| Notes | IPD provided by trial sponsor Glaxo Smith Kline for time to treatment withdrawal, time to first seizure and time to six‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence (information provided by drug manufacturer). Stratification by seizure type |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed, opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind achieved using LTG tablets formulated to be identical in appearance to CBZ tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 8 centres in the UK 2 treatment arms: LTG and CBZ | |
| Participants | Adults and children > 13 years with newly diagnosed epilepsy. None had received previous AED treatment. Number randomised: LTG = 61, CBZ = 63 56 male participants (45%) 62 participants with partial epilepsy (50%) Mean age (range): 30 (14‐81) years | |
| Interventions | Monotherapy with LTG or CBZ 4‐week escalation phase leading to LTG = 150 mg/d, CBZ = 600 mg/d Range of follow‐up = 0‐13 months | |
| Outcomes | Time to first seizure after 6 weeks of treatment Time to withdrawal Proportion of randomised participants remaining seizure‐free during the last 40 and 24 weeks of trial Percentages of participants who reported adverse events | |
| Notes | IPD provided by trial sponsor Glaxo Smith Kline for time to treatment withdrawal, time to first seizure and time to six‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence (information provided by drug manufacturer). Stratification by seizure type |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed, opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind achieved using LTG tablets formulated to be identical in appearance to CBZ tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, double‐blind, parallel‐group trial conducted in the UK 2 treatment arms: LTG and CBZ randomised in a 2:1 ratio | |
| Participants | Adults > 65 years with newly diagnosed epilepsy with ≥ 2 seizures in the previous year with at least 1 seizure in the last 6 months. None had received previous AED treatment. Number randomised: LTG = 102, CBZ = 48 83 male participants (55%) 105 participants with partial epilepsy (70%) Mean age (range): 77 (65‐94) years | |
| Interventions | Monotherapy with LTG or CBZ 4‐week escalation phase leading to LTG = 100 mg/d, CBZ = 400 mg/d Range of follow‐up = 0‐13.5 months | |
| Outcomes | Time to first seizure after 6 weeks of treatment Time to withdrawal Percentage of participants reporting an adverse event Proportion of participants who were both seizure‐free in the last 16 weeks of the trial and did not discontinue treatment | |
| Notes | IPD provided by trial sponsor Glaxo Smith Kline for time to treatment withdrawal and time to first seizure (plus seizure‐freedom rates at 24 weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence (information provided by drug manufacturer). Participants randomised in a 2:1 ratio (LTG:CBZ) |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed, opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind achieved using LTG tablets formulated to be identical in appearance to CBZ tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, double‐blind trial conducted in 41 centres in Europe and Australia 2 treatment arms: GBP and LTG | |
| Participants | Participants > 16 years with at least 2 partial seizures with or without secondary generalisation or primary generalised tonic clonic seizures in the last 12 months. All participants were untreated in the previous 6 months or AED naive Number randomised: GBP = 158, LTG = 151. Evaluable population (exclusions due to protocol violations): GBP = 148, LTG = 143 152 male participants (52%) out of evaluable population 233 participants with partial epilepsy (80%) out of evaluable population Mean age of evaluable population (SD, range): GBP: 35.8 years (16.4, 13‐78), LTG: 37.9 (16.7, 16‐78) | |
| Interventions | Monotherapy with GBP or LTG Titration of 2 weeks for GBP to a dose range of 1200 mg/d‐3600 mg/d and titration of 6 weeks for LTG to a dose range of 100 mg/d‐300 mg/d Titration period followed by 24‐week maintenance period. Range of follow‐up not stated | |
| Outcomes | Time to exit Percentage of completers/time to withdrawal for any reason Time to first seizure Percentage who remained seizure‐free during the final 12 weeks of the 30‐week evaluation period Withdrawal rate due to adverse events | |
| Notes | IPD requested from trial sponsor Pfizer but data could not be provided due to time elapsed since the trial was completed. Additional information provided in a clinical study report. Aggregate data extracted for time to exit from the trial and time to first seizure extracted from the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with permuted blocks, stratified within each centre by seizure type. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Masking was achieved by double‐dummy dosing. A dose range was permitted within the trial to maintain the blind of two drugs with different titration rates (2 weeks for GBP and 6 weeks for LTG) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants included in an ITT analysis (even though demographics presented for 'evaluable population' only) |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted at 85 centres in 12 European countries and in South Africa 2 treatment arms: CBZ (controlled release) and LEV | |
| Participants | Adults (> 16 years) with 2 partial or generalised tonic–clonic seizures separated by at least 48 h in the previous year with at least one seizure in the last 3 months Number randomised: CBZ = 291, LEV = 288 319 male participants (55%) 466 participants with partial epilepsy (80%) Mean age (range): 39 (15‐82 years) | |
| Interventions | Monotherapy with CBZ or LEV Titration for 2 weeks to target dose of CBZ = 400 mg/d, LEV = 1000 mg/d Range of follow‐up = 0‐28 months | |
| Outcomes | Proportion of per‐protocol (PP) participants achieving at least 6 months of seizure freedom at the last evaluated dose One year seizure‐freedom rate 6‐month and 1‐year seizure‐freedom rate by dose level Time to trial withdrawal Incidence of adverse events | |
| Notes | IPD provided for all outcomes of this review by trial sponsor UCB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised following a central 1:1 randomisation scheme with a statistical block size of 2 and stratified by seizure category |
| Allocation concealment (selection bias) | Low risk | Participants were randomised using an interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | To ensure blinding, LEV and CBZ‐CR tablets were identically encapsulated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Single‐centre, randomised, parallel‐group trial of participants referred for assessment at Cork Regional Hospital, Ireland 3 treatment arms: CBZ, PHT, VPS | |
| Participants | Adults and children with a minimum of 2 untreated generalised or partial seizures in the 6 months preceding the trial Number randomised: PHT = 58, CBZ = 59, VPS = 64 95 male participants (52%) 79 participants (44%) with partial epilepsy Age range: 4‐75 years | |
| Interventions | Monotherapy with CBZ, PHT or VPS Mean daily dose achieved: PHT = 5.4 mg/kg, CBZ = 10.9 mg/kg, VPS = 15.6 mg/kg Duration of treatment (range in months): 14‐24 months | |
| Outcomes | Seizure control:
Side effects | |
| Notes | Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation based on two Latin squares without stratification. The first, second and third preference of drug for the participant appears to have been taken into account in the process. Unclear if assignment was completely random |
| Allocation concealment (selection bias) | High risk | An independent person (department secretary) selected the “drug of first preference” from randomisation list on a sequential basis. Allocation not adequately concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attirition rates reported. ITT approach taken, all randomised participants analysed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (seizure control) and secondary outcomes (side effects) reported sufficiently |
| Other bias | Low risk | None identified |
| Methods | Randomised trial of participants with epileptic seizures following stroke conducted in Italy 2 treatment arms: CBZ (controlled release) and LEV | |
| Participants | Participants with "vascular epilepsy", newly onset following stroke. Not stated if participants had been previously treated with AEDs Number randomised: CBZ = 17, LEV = 18 17 male participants (49%) Proportion of participants with partial epilepsy not stated Mean age: 70 (43‐90) years | |
| Interventions | Monotherapy with CBZ or LEV Dose achieved: CBZ: 400 mg/d‐1200 mg/d, LEV = 1000 mg/d‐3000 mg/d Trial duration and range of follow‐up not stated | |
| Outcomes | Seizure freedom Adverse events during the trial Discontinuations of the trial drug | |
| Notes | The trial was published in Italian; the characteristics and outcomes were translated. Outcomes chosen for this review were not reported; IPD were not available (author confirmed that the data had been lost) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised ('randomizzazione' in Italian); no further information was available |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, no formal statistical analysis performed so withdrawals did not influence results |
| Selective reporting (reporting bias) | Unclear risk | Methods brief, efficacy and tolerability reported in the results. Outcomes chosen for this review not reported. No protocol available so unclear which outcomes were planned a priori |
| Other bias | Low risk | None identified |
| Methods | Randomised, open‐label trial to evaluate event‐related potentials on the effect of CBZ and LEV cognitive functions, conducted in Italy 2 treatment arms, CBZ (controlled release) and LEV | |
| Participants | Participants with newly diagnosed partial epilepsy Number randomised: CBZ = 14, LEV = 13 14 male participants (52%) 100% of participants had partial epilepsy Mean age (years): CBZ = 38, LEV = 42, range not stated | |
| Interventions | Monotherapy with CBZ or LEV Fifteen‐day titration to CBZ = 800 mg/d and LEV = 100 mg/d Trial duration: 24 weeks (assessments at baseline and 12 weeks), range of follow‐up not stated | |
| Outcomes | Event‐related potential recordings Neuropsychological assessments | |
| Notes | The trial was published in Italian; the characteristics and outcomes were translated. Outcomes chosen for this review were not reported; IPD were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised ('randomizzazione' in Italian); no further information was available |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition rates reported (3 dropouts from the CBZ group, 11% of total participants). These participants are excluded from analysis; this is not an ITT approach |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in methods section well reported in results section. No seizure outcomes or adverse events reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | None identified |
| Methods | Randomised (partially), double‐blind, multicenter trial conducted at 25 sites in Europe, Australia, South Africa and Canada. 4 treatment arms: GBP (3 arms, 300 mg/d, 900 mg/d and 1800 mg/d) and CBZ. Dose of GBP was masked within the treatment arm but CBZ was given open‐label due to difficulties of blinding tablets and capsules and differing titration periods for the two drugs. | |
| Participants | Participants with newly diagnosed partial epilepsy, with at least 2 unprovoked partial or generalised tonic clonic seizures in the 6 months prior to trial entry, who were AED naive or had received fewer than 2 weeks of AED therapy, which had to be discontinued before trial entry. Participants with a seizure recurrence after at least 2 years of remission were also eligible. Number randomised: CBZ = 74, GBP = 218 157 male participants (54%) 100% participants with partial epilepsy Mean age (range): 35 (12‐86 years) | |
| Interventions | Monotherapy with GBP or CBZ Titration period of 7 d for GBP to target doses 300 mg/d, 900 mg/d or 1800 mg/d. Titration period of 21 d for CBZ to target dose 600 mg/d. Titration period followed by an evaluation period of 24 weeks and an optional open‐label period Range of follow‐up: 0‐77 months | |
| Outcomes | Time to exit Time to exit event plus withdrawals because of adverse events Completion rate (percentage of participants attending end‐of‐phase visit) Exit event rate (percentage of participants who experienced an exit event during the evaluation phase) Adverse event withdrawal rate (percentage of participants who withdrew because of adverse events during either titration or evaluation phases) Exit plus adverse event withdrawal rate (the sum of the exit rate plus the adverse event withdrawal rate) Incidence of adverse events | |
| Notes | IPD provided for all outcomes of this review by trial sponsor Pfizer. In primary analysis, three arms of GBP are pooled and compared to CBZ (see Data extraction and management) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation schedule was prepared separately for each trial centre in blocks of four and eight |
| Allocation concealment (selection bias) | Low risk | Trial medication was distributed centrally via a pharmacy |
| Blinding of participants and personnel (performance bias) | High risk | The trial was partially double‐blinded (the dose of GBP was blinded but GBP was not blinded compared to CBZ). Given that the main comparison made in this review is GBP compared to CBZ rather than comparisons between the doses of GBP, this trial is be treated as an open‐label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specifically stated |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, parallel‐group trial conducted in Taiwan 3 treatment arms: CBZ, PHB, VPS | |
| Participants | Children with 2 or more previously untreated unprovoked epileptic seizures Number randomised: CBZ = 26, PHB = 25, VPS = 25; number analysed: CBZ = 25, PHB = 23, VPS = 25 (see notes) 38 boys (52%) 38 participants with partial epilepsy (52%) Mean age (range) for participants analysed: CBZ = 10.8 (7‐15 years), PHB = 9.9 (7‐15 years), VPS = 9.9 (7‐15 years) | |
| Interventions | Monotherapy with CBZ, PHB or VPS Dose started or achieved not stated Trial duration: 12 months, range of follow‐up: not stated | |
| Outcomes | Cognitive/psychometric outcomes: IQ (WISC‐R scale) and developmental delay (Bender‐Gestalt test) Auditory event‐related potentials (neurophysiological outcome) Incidence of allergic reactions Seizure control | |
| Notes | 2 children from the PHB group and 1 child from the CBZ group withdrew from the trial because of allergic reactions. Published results were presented for children who completed the trial only. Outcomes chosen for this review were not reported; IPD were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated with "simple randomisation of block size 3" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | The cognitive assessor was "single blinded", implying that participants and personnel were unblinded, but no further information was provided |
| Blinding of outcome assessment (detection bias) | Low risk | The cognitive assessor was "single blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawal rates were reported; results were presented only for those who completed the trial (3/73 (4%) excluded from analysis). An ITT approach was not taken but unclear whether the exclusion of this small proportion of participants would influence results |
| Selective reporting (reporting bias) | Low risk | All cognitive, efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Randomised trial conducted in Republic of Korea 2 treatment arms: CBZ (controlled release) and LEV | |
| Participants | Participants with newly diagnosed partial epilepsy who had their first seizure between 6 and 1 month prior to entry into the trial and had not taken any AEDs previously. Number completing the trial: CBZ = 15, LEV = 16 (number randomised not stated) 22 male participants (71%) 100% of participants had partial epilepsy Mean age (SD, range): CBZ = 29.8 (9.31, 15‐49), LEV = 31.4 (15.3, 15‐66) years | |
| Interventions | Monotherapy with CBZ or LEV Treatment regimens were CBZ = 400 mg/d and LEV = 1000 mg/d Trial duration 4‐6 weeks, range of follow‐up not stated | |
| Outcomes | Change in overnight PSG scores (sleep latency, REM sleep latency, total sleep time, sleep efficiency, percentage of each sleep stage, arousal index, and Wake time After Sleep Onset) from baseline after 4‐6 weeks of treatment Change in sleep questionnaires (sleep diaries, the Pittsburg Sleep Quality Index, the Korean version of the Epworth Sleepiness Scale, Beck’s depression inventory‐2 and the Hospital Anxiety Scale) and National Hospital Seizure Severity Scale (NHS3) from baseline after 4‐6 weeks of treatment | |
| Notes | IPD could not be provided for the trial due to concerns over institutional review board approval (information provided by corresponding author). Outcomes chosen for this review were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Low risk | PSG scores were interpreted by a certified physician who was blinded to treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Number randomised not stated, results provided only for those who completed the trial |
| Selective reporting (reporting bias) | Low risk | All sleep, efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Multicentre, double‐blind, parallel‐group trial conducted in centres in Europe, Brazil and South Africa 2 treatment arms: OXC and VPS | |
| Participants | Participants aged 16‐65 years with newly diagnosed epilepsy with partial or generalised tonic clonic seizures A minimum of 2 seizures, separated by at least 48 h, within 6 months preceding trial entry No previous AED, except emergency treatment of seizures for a maximum of 3 weeks prior to trial entry Number randomised: OXC = 128, VPS = 121 127 male participants (51%) 154 participants with partial epilepsy (62%) Mean age (range): OXC: 32.45 (15‐65), VPS: 32.47 (15‐64) | |
| Interventions | Monotherapy with OXC or VPS Titration period of 8 weeks to target doses of 900 mg/d‐2400 mg/d of OXC or VPS Titration period followed by 48‐week maintenance period and the possibility of a long‐term open‐label extension of 1 year | |
| Outcomes | The proportion of seizure‐free participants who had at least 1 seizure during the maintenance period Time to premature discontinuation due to adverse experiences Rate of premature discontinuations for any reason Overall assessments of efficacy and tolerability and therapeutic effect Individual adverse experiences Seizure frequency during maintenance | |
| Notes | IPD requested from trial sponsor Novartis but data could not be provided due to time elapsed since the trial was completed. Aggregate data extracted from graph of time to premature discontinuation in the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised in a 1:1 ratio, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | The trial treatment with OXC or VPS was administered as non‐divisible film‐coated tablets of identical appearance containing 300 mg of active substance. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates reported, only those who reached the maintenance period were included in efficacy analyses. This is not an ITT approach |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. |
| Other bias | Low risk | None identified |
| Methods | Multicentre, open‐label randomised trial conducted in two centres in Italy 2 treatment arms: CBZ and LEV | |
| Participants | Participants > 18 years with late post‐stroke seizures (2 weeks to 3 years after stroke) seen in the Cerebrovascular Unit between September 2008 and March 2009. No previous AED treatments were allowed except for emergency treatments Number randomised: CBZ = 66, LEV = 62. Number completing the trial: CBZ = 54, LEV = 52 58 male participants (55%) of those completing the trial 74 participants with partial epilepsy (74%) of those completing the trial Mean age of those completing trial (SD): CBZ = 69.7 (13.2), LEV = 74.1 (11.3) | |
| Interventions | Monotherapy with CBZ or LEV 2‐week titration period to CBZ: 600 mg/d or LEV: 1000 mg/d Titration period followed by 52‐week maintenance period. Range of follow‐up not stated | |
| Outcomes | Frequency of seizures during the treatment period Rentention of treatment from the first intake Changes in cognitive measures and quality‐of‐life measures at the end of the treatment period:
Changes in EEG assessments at the end of the treatment period Tolerability of treatment | |
| Notes | Contact made with trial author who provided additional information for one of the trial centres but full IPD dataset unavailable. Aggregate data extracted from graph of time to seizure recurrence in the publication. Trial was terminated early due to financial reasons when 128 out of a target 630 participants had been recruited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation numbers were sequentially assigned across centres, and a computer‐generated randomisation scheme was used to provide balanced blocks of participants for each treatment group within each centre |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open label trial |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates reported, only those who completed the trial were included in efficacy analyses. This is not an ITT approach |
| Selective reporting (reporting bias) | Low risk | All efficacy, cognitive and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. |
| Other bias | High risk | Likely that trial is underpowered from the early termination with 20% of target sample size recruited |
| Methods | Randomised, double‐blind trial to assess short‐term therapy of CBZ and PHB on cognitive and memory function conducted in Italy Three treatment arms: CBZ, PHB, and placebo | |
| Participants | Participants with newly diagnosed and untreated temporal lobe epilepsy with no seizures in the previous month Number randomised: CBZ = 6, PHB = 6 1 man and 5 women in each group 100% partial (temporal lobe epilepsy), 100% newly diagnosed Mean age (SD): CBZ = 26.33 (9.73) years, PHB = 18.5 (2.56) years. Age range: 15‐45 years | |
| Interventions | Monotherapy with CBZ or PHB Dose started and achieved not stated Trial duration: 3 weeks; all participants completed in 3 weeks | |
| Outcomes | Changes in memory function from baseline after 3 weeks of treatment (verbal, visual, (visual‐verbal and visual‐non‐verbal), acoustic, tactile, and spatial). | |
| Notes | The trial was published in Italian; the characteristics and outcomes were translated. Outcomes chosen for this review were not reported; contact could not be made with trial author to provide IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised ('randomizzazione' in Italian); no further information was available |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Trial was described as double blind ('condizioni di doppia cecità' in Italian), we assume this refers to participants and personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed this short trial and contributed to analysis |
| Selective reporting (reporting bias) | Unclear risk | Cognitive and memory outcomes described in methods section well reported in results section. No seizure outcomes or adverse events reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | High risk | Very small participant numbers and very short‐term follow‐up. Unclear if this trial was adequately powered and of sufficient duration to detect differences |
| Methods | Parallel design, RCT conducted in the UK 2 treatment arms: PHT and VPS | |
| Participants | Participants > 60 years with newly onset seizures (1 or more generalised tonic‐clonic seizures or 2 or more partial seizures) Number randomised: PHT = 81, VPS = 85 71 male participants (43%) 80 participants with partial epilepsy (48%) Mean age (range): 78 (61‐95 years) | |
| Interventions | Monotherapy with PHT or VPS Starting doses: PHT: 200 mg/d, VPS: 400 mg/d Median daily dose achieved: PHT 247 mg (range 175‐275); VPS: 688 mg (range 400‐1000) Range of follow‐up: 0‐22 months | |
| Outcomes | Psychological tests (cognitive function, anxiety and depression) Adverse event frequency Seizure control | |
| Notes | Trial paper reports on a subset of 38 participants. Full individual participant dataset provided by trial authors and used for this review includes all 166 participants randomised in the trial. IPD provided for 3/4 outcomes of this review ('withdrawal from allocated treatment' not available). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised stratified minimisation programme, stratified for age group, gender and seizure type |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled allocation, prescription disclosed to general practitioner and consultant |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | The main investigator performing cognitive testing was blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported. ITT analysis undertaken with all randomised participants from IPD (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported in published report or provided in IPD (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | 36‐month randomised, comparative trial conducted in Poland 4 treatment arms: CBZ, PHB, PHT, VPS | |
| Participants | Adults with newly diagnosed epilepsy Number randomised: CBZ = 30, PHT = 30, PHB = 30, VPS = 30 100% of participants had partial epilepsy Age range: 18‐40 years Percentage male and range of follow‐up not mentioned (outcome recorded at 3 years) | |
| Interventions | Monotherapy with CBZ, PHT, PHB or VPS Starting doses CBZ = 400 mg/d, PHT = 200 mg/d, PHB = 100 mg/d, VPS: 600 mg/d Dose achieved not stated | |
| Outcomes | Proportion achieving 24‐month remission at 3 years and exclusions after randomisation due to adverse effects or no efficacy | |
| Notes | Abstract only. Outcomes chosen for this review were not reported, contact made with trial authors but IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial randomised but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | "Exclusion rates" reported for all treatment groups, no further information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, trial available in abstract format only. Outcomes for this review not available |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, double‐blind trial conducted in 20 centres across four European countries 2 treatment arms: CBZ and OXC | |
| Participants | Participants aged 15‐65 years with newly diagnosed and previously untreated epilepsy Number randomised: total of 235 but 41 excluded for protocol violations (number randomised by treatment group not stated). Number analysed: CBZ = 100, OXC = 94 96 male participants (49%) out of those analysed Proportion with partial epilepsy not stated Median age (range): 33 (14‐63) | |
| Interventions | Monotherapy with CBZ or OXC Starting daily dose CBZ: 200 mg OXC: 300 mg. Mean daily dose (range) achieved CBZ: 684 (300 mg‐1400 mg), OXC: 1040 (300 mg‐1800 mg) Titration period of 4‐8 weeks followed by a maintenance period of 48 weeks Mean (range) duration of follow‐up (maintenance period): 336 (10‐390) days | |
| Outcomes | Changes in seizure frequency between baseline and the end of each maintenance period Changes in EEG tracings between baseline and the end of each maintenance period Global evaluation of therapeutic efficacy and tolerability by the investigator at the end of each maintenance period Side effects observed by participants and investigators each visit Laboratory tests (while blood cell counts and liver function tests, blood pressure and pulse, drug trough serum levels) | |
| Notes | Trial authors could not be contacted to request IPD. Outcomes chosen for this review were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Trial was of double‐blind design |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate reported, up to 30% of randomised participants who did not complete the trial were excluded from analyses; this is not an ITT approach |
| Selective reporting (reporting bias) | Low risk | Efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, parallel‐group, open‐label paediatric trial conducted in 2 centres in the UK 4 treatment arms: CBZ, PHB, PHT, VPS | |
| Participants | Children with newly diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the trial) Number randomised: CBZ = 54, PHB = 10, PHT = 54, VPS = 49 86 boys (50%) 89 children with partial epilepsy (51%) Mean age (range): 10 (3‐16) years | |
| Interventions | Monotherapy with CBZ, PHT, PHB or VPS Median daily dose achieved: CBZ = 400 mg/d, PHT = 175 mg/d, PHB = not stated (see notes), VPS= 600 mg/d Range of follow‐up (months): 10‐164 | |
| Outcomes | Time to first seizure recurrence after start of therapy Time to 12‐month remission from all seizures Adverse effects and withdrawals due to adverse events | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD. 6 of the first 10 children assigned to PHB had unacceptable adverse effects, so no further children were assigned to PHB. The 10 children randomised to PHB were retained in analysis. IPD provided for all outcomes of this review by the Medical Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of concealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded, authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance.” Lack of masking could have led to early withdrawal of the PHB arm from the trial, which was likely to have influenced the overall results |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded, authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance.” Lack of masking could have led to early withdrawal of the PHB arm from the trial, which was likely to have influenced the overall results |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Prospective quasi‐randomised, open‐label trial conducted at a single hospital in Turkey 2 treatment arms: CBZ and OXC | |
| Participants | Children with partial epilepsy (not stated how many were newly diagnosed) Number randomised: CBZ = 26, OXC = 26 21 boys (40%) 100% of participants had partial epilepsy Mean age (range): 11 (4‐15 years) | |
| Interventions | Monotherapy with CBZ or OXC CBZ prescribed at 20‐25 mg/kg/d and OXC at 30‐50 mg/kg/d Range of follow‐up: 3.5 to 26 months | |
| Outcomes | Seizure recurrence Most common side effects Number of participants switching treatment | |
| Notes | IPD provided for all outcomes of this review by trial author. Trial publication available as abstract only, additional data provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation by alternately allocating participants to CBZ or OXC (information provided by trial authors) |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed (alternate allocation) |
| Blinding of participants and personnel (performance bias) | High risk | Open‐ label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐ label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Multicentre, randomised, open‐label trial conducted at 21 sites in seven European countries between December 2001 and December 2003 3 treatment arms: CBZ, OXC, VPS (randomised in a 1:2:1 ratio) | |
| Participants | Children and adolescents (aged 6‐17) with newly diagnosed partial seizures. Participants must have had at least 2 unprovoked partial seizures (simple and complex partial and partial evolving into secondarily generalised seizures) in the 3 months prior to study entry Number randomised: CBZ = 28, OXC = 55, VPS = 29 51 male participants (46%) 100% of participants had partial epilepsy Median age (range): 10 (6‐16) | |
| Interventions | Monotherapy with CBZ, OXC or VPS Dose achieved (mean (SD)): CBZ =14.4 (3.6) mg/kg/d, VPS = 20.7 (7.5) mg/kg/d Study duration: 6 months, Range of follow‐up not stated | |
| Outcomes | Cognitive testing: Computerized Visual Searching Task, assessing mental information processing speed and attention. Rey Auditory Verbal Learning Test and Raven’s Standard Progressive matrices for children: psychomotor speed, alertness, memory and learning, and non‐verbal intelligence. Percentage of participants remaining seizure‐free throughout treatment Most common adverse events Treatment satisfaction on a 4‐point scale from poor to very good | |
| Notes | IPD requested from trial sponsor Novartis but data could not be provided due to time elapsed since the trial was completed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive voice‐response system was used to automate the randomisation of participants to treatment groups within age strata |
| Allocation concealment (selection bias) | Low risk | An interactive voice‐response system was used to automate the randomisation of participants to treatment groups within age strata |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study (justified as primary and secondary cognitive outcomes were objective) |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study (justified as primary and secondary cognitive outcomes were objective) |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate reported. Most results reported only for the per‐protocol population who completed the study |
| Selective reporting (reporting bias) | Low risk | All cognitive, efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None detected |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in 7 hospitals in Republic of Korea 2 treatment arms: LTG and CBZ | |
| Participants | Children aged 6‐12 years with a new diagnosis of partial epilepsy and at least 2 seizures in the last 6 months. Number randomised: LTG = 43, CBZ = 41 48 male participants (57%) 100% of participants had partial epilepsy Not stated if any participants had received previous AED treatment Mean age (range): 9 (5‐13) years | |
| Interventions | Monotherapy with LTG or CBZ 8‐week escalation phase leading to LTG = 3‐6 mg/kg/d, CBZ = 10‐20 mg/kg/d Range of follow‐up: 0.5‐28 months | |
| Outcomes | Seizure‐free rate over 6 months (maintenance period) by treatment group Change in cognition (neuropsychological), behaviour and quality of life from screening to the end of the maintenance phase by treatment group Incidence of adverse events | |
| Notes | IPD provided by trial author for time to treatment withdrawal, time to first seizure and time to 6‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each centre received a separate and independent computer‐generated random code list |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, parallel‐group trial conducted among residents of the Nakuru district, a semi‐urban population of rural Kenya 2 treatment arms: CBZ and PHB | |
| Participants | Participants had a history of generalised tonic‐clonic seizures and at least 2 generalised tonic‐clonic seizures within the preceding year (with or without other seizure types) and untreated in the 3 months prior to the trial. 79 (26%) participants had been treated in the past with AEDs Number randomised: PHB = 150, CBZ = 152 173 male participants (57%) 115 of participants with partial epilepsy (38%) Mean age (range): 21 (6‐65 years) | |
| Interventions | Monotherapy with CBZ or PHB Starting doses: PHB: 6‐10 years: 30 mg/d, 11‐15 years: 45 mg/d, > 16 years: 60 mg/d CBZ: 6‐10 years of age: 400 mg/d, 11‐15 years of age: 500 mg/d, > 16 years of age: 600 mg/d Dose achieved not stated Range of follow‐up: participants followed up for up to 1 year | |
| Outcomes | Adverse effects Withdrawals from allocated treatment Seizure frequency (during second 6 months of trial) | |
| Notes | IPD were made available but not used because of inconsistencies and problems with the data provided (see Included studies for further details) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised with random number list |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via sealed, opaque envelopes (information provided by trial author) |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates reported, results presented only for participants completing 12 months' follow‐up (results not presented for 53 (17.5%) participants out of 302 who withdrew from treatment), approach is not ITT |
| Selective reporting (reporting bias) | Low risk | No protocol available, outcomes chosen for this review not reported. Seizure outcomes and adverse events well reported |
| Other bias | High risk | Inconsistencies with IPD and published results so IPD could not be used (see Included studies for further details) |
| Methods | Single‐centre, randomised, parallel‐group trial conducted in the UK 3 treatment arms: CBZ, PHT, VPS | |
| Participants | Children with at least 3 newly diagnosed generalised or partial seizures within a period of 6 months Number randomised: CBZ = 23, PHT = 20, VPS = 21 No information on epilepsy type or sex Age range: 5‐14 years | |
| Interventions | Monotherapy with CBZ, PHT or VPS Mean dose: CBZ = 17.9 mg/d, PHT = 6.1 mg/d, VPS: 25.3 mg/d Trial duration: 12 months, range of follow‐up not stated | |
| Outcomes | Cognitive assessments Summary of withdrawals from randomised drug | |
| Notes | Outcomes chosen for this review were not reported IPD not available, but could be constructed from the publication for the outcome 'time to withdrawal of allocated drug' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quota allocation by sex, age, seizure type and current treatment is an inadequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Personnel and participants (and parents) unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors single‐blinded for cognitive testing |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, results reported and analysed for all participants randomised and all who completed various stages of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | One of four outcomes for this review reported. Cognitive outcomes described in methods section well reported in results section. Adverse effects reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | None identified |
| Methods | Prospective, open‐label, randomised trial conducted in Germany 2 treatment arms: LTG and OXC | |
| Participants | Participants with untreated epilepsy, number newly diagnosed not stated Number randomised: LTG = 21, OXC = 27 26 male participants (54%) Proportion of participants with partial epilepsy not stated Age range: 15‐61 | |
| Interventions | Monotherapy with LTG or OXC Doses started or achieved not stated Range of follow‐up and trial duration not stated | |
| Outcomes | Seizure reduction Cognition, mood and health‐related quality of life | |
| Notes | Abstract only. Trial authors could not be contacted to request IPD Results refer to reduction of seizures to only "simple seizures" remaining so we assume that this population of participants has the eligible seizure type for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments were "randomly assigned", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only, attrition rate not stated. Insufficient information to make a judgement |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient information to make a judgement |
| Other bias | Low risk | None identified |
| Methods | Randomised, single‐centre, open‐label, parallel‐group trial conducted at Tel Aviv University and Medical Centre, Israel 2 treatment arms: LTG and CBZ | |
| Participants | Adults admitted to the neurological department with a first seizure event after an ischaemic stroke Number randomised: LTG = 32, CBZ = 32 46 male participants (72%) 100% of participants had partial epilepsy Unclear if any participants had received previous AED treatment Mean age (range): 67.5 (38‐90) years | |
| Interventions | Monotherapy with LTG or CBZ for 12 months Dose escalation phase (length not stated) leading to LTG 100 mg/d, CBZ 300 mg/d Range of follow‐up: not stated | |
| Outcomes | The appearance of a second seizure under treatment or by finishing the 12‐month follow‐up without seizures Tolerability: incidence of adverse events Withdrawals due to adverse events | |
| Notes | Contact made with trial author who was willing to provide IPD but data never received. Aggregate data extracted from graphs in the publication. Stated in the title of the paper that LTG and CBZ were monotherapy treatments but Table 1 of the paper refers to Total no. AED, unclear if all participants were receiving monotherapy treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in a 1:1 ratio, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rate reported, all randomised participants included in analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available. Seizure outcomes and adverse events well reported |
| Other bias | Unclear risk | Unclear if all participants were receiving monotherapy treatment |
| Methods | Multicentre, double‐blind, parallel‐group trial conducted in centres in Argentina and Brazil 2 treatment arms: OXC and PHT | |
| Participants | Participants aged > 5 years with newly diagnosed epilepsy with partial seizures or generalised tonic‐clonic seizures A minimum of 2 seizures, separated by at least 48 h, within 6 months preceding trial entry No previous AED, except emergency treatment of seizures for a maximum of 3 weeks prior to trial entry Number randomised: OXC = 997, PHT = 94 100 male participants (52%); 143 of participants had partial epilepsy (74%) Mean age (range): 18.5 (5‐53) years | |
| Interventions | Monotherapy with OXC or PHT 8‐week titration period started with 150 mg OXC or 50 mg PHT, increased bi‐weekly, based on clinical response to a regimen with daily doses of 450 mg‐2400 mg OXC or 150 mg‐800 mg PHT Continued during 48‐week maintenance with adjustment according to clinical response A third long‐term, open‐label extension phase followed the maintenance period. Double‐blind results only were reported Range of follow‐up: 1‐28 months | |
| Outcomes | The proportion of seizure‐free participants who had at least 1 seizure during the maintenance period Time to premature discontinuation due to adverse experiences Rate of premature discontinuations for any reason Overall assessments of efficacy and tolerability and therapeutic effect Individual adverse experiences Laboratory values Seizure frequency during maintenance | |
| Notes | IPD provided for all outcomes of this review by trial sponsor Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment groups randomised in 1:1 ratio across centres via computer‐generated randomisation numbers over balanced blocks of size 6 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved with sequentially‐numbered packages which were identical and contained identical tablets (information provided by trial statistician) |
| Blinding of participants and personnel (performance bias) | Low risk | Trial conducted in 2 phases: 56‐week, double‐blind phase followed by long‐term, open‐label extension. Double‐blind phase results reported only Blind achieved with divisible OXC and PHT tablets identical in appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported in both treatment phases, participants withdrawing from treatment were no longer followed up so seizure outcomes had to be censored at time of withdrawal and therefore analyses for remission and seizure outcomes could not adopt an ITT approach |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, parallel‐group, open‐label trial conducted in 2 centres in the UK 4 treatment arms: CBZ, PHB, PHT, VPS | |
| Participants | Adults with newly diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the trial) Number randomised: CBZ = 61, PHB = 58, PHT = 63, VPS = 61 117 male participants (48%) 102 participants with partial epilepsy (42%) Mean age (range): 32 (13‐77) years | |
| Interventions | Monotherapy with CBZ, PHB, PHT or VPS Median daily dose achieved: CBZ = 600 mg/d, PHB = 105 mg/d, PHT = 300 mg/d, VPS = 800 mg/d Range of follow‐up: 0‐166 months | |
| Outcomes | Time to first seizure recurrence after start of therapy Time to 12‐month remission from all seizures Adverse effects and withdrawals due to adverse events | |
| Notes | IPD provided for all outcomes of this review by the Medical Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of concealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded, authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate.” Lack of blinding may have influenced the withdrawal rate. |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded, authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate.” Lack of blinding may have influenced the withdrawal rate. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Multicenter, randomised, open‐label, non‐inferiority trial conducted across 7 centres in Republic of Korea 2 treatment arms: CBZ and LEV | |
| Participants | Children aged 4‐16 years with newly diagnosed focal epilepsy, no previous anti‐epileptic therapy and "above borderline" intelligence Number randomised: CBZ = 64, LEV = 57 (ITT population) 69 male participants (57%) 100% of participants with partial epilepsy Mean age (SD): CBZ = 8.05 (3.02), LEV = 9.28 (3.37) years | |
| Interventions | Monotherapy with CBZ or LEV 4‐week dose titration period to a minimal target dose of CBZ = 20/mg/kg/d or LEV = 40/mg/kg/d Trial duration: 52 weeks, range of follow‐up not stated | |
| Outcomes | Neuropsychological outcomes; change from baseline to 52 weeks in neurocognitive (Korean‐WISC‐III or Korean‐Wechsler Preschool and Primary Scale of Intelligence‐III), behavioural (Korean‐CBCL), and emotional (Children's Depression Inventory and Revised Children's Manifest Anxiety Scale) function assessments Mean percentage change in seizure frequency from baseline Seizure‐freedom rates Incidence of adverse events | |
| Notes | IPD could not be provided for the trial due to restrictions on data sharing from the Korean Food and Drug Administration (information provided by corresponding author). Outcomes chosen for this review were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised independently at each centre using a computerised random code assignment based on stratified permuted block randomisation that were designed separately and independently for each participating centre |
| Allocation concealment (selection bias) | Low risk | At each centre, allocation concealment was carried out by the pharmacy in order to blind those assessing outcomes from the trial medication |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial for participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Those assessing outcomes were blinded to trial medication (pharmacy allocation) |
| Incomplete outcome data (attrition bias) | High risk | 7 randomised participants did not take any trial medication so were not included in ITT population. Results for neuropsychological outcomes recorded only for those who completed the trial ‐ 81/121 participants (67%) |
| Selective reporting (reporting bias) | Low risk | All neuropsychological, efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Open‐label, multicentre, randomised trial. Authors based in Denmark and Finland 2 treatment arms: CBZ (slow release) and LTG | |
| Participants | Participants with newly onset partial and/or generalised tonic clonic seizures Number randomised: CBZ = 70, LTG = 73 No information provided about age and gender or previous AED use | |
| Interventions | Monotherapy with CBZ or LTG for 52 weeks Mean dosage during maintenance period: CBZ = 549 mg/d, LTG = 146 mg/d Range of follow‐up not stated | |
| Outcomes | Seizure freedom Cognitive assessments | |
| Notes | IPD requested from trial sponsor Glaxo Smith Kline but data could not be located. Abstract publication only available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments were "randomly assigned", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only, attrition rate not stated. Insufficient information to make a judgement |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient information to make a judgement |
| Other bias | Low risk | None identified |
| Methods | Randomised trial of outpatients of a hospital in Berlin, Germany 3 treatment arms: CBZ, LEV, VPS | |
| Participants | Newly diagnosed ("de novo") participants Number randomised: CBZ = 6, LEV = 6, VPS = 3 12 (80%) partial epilepsy No information on age or gender | |
| Interventions | Monotherapy with CBZ, LEV or VPS Doses started or achieved not stated Assessments performed at 6 and 12 weeks | |
| Outcomes | Cognitive performance Neuropsychological assessment | |
| Notes | Abstract only. Trial authors could not be contacted to request IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments were "randomly assigned", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only, attrition rate not stated. Insufficient information to make a judgement |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient information to make a judgement |
| Other bias | Low risk | None identified |
| Methods | Phase IV, open‐label, randomised, multicentre trial conducted in 21 centres in Republic of Korea 2 treatment arms: CBZ and LTG | |
| Participants | Participants were untreated epileptics who had at least 2 unprovoked seizures (partial or generalised tonic clonic) during the last 24 weeks before the study start, more than 24 h apart Number randomised: CBZ = 129, LTG = 264 (ITT population) 154 male participants (39%) 288 participants (73%) with partial epilepsy Mean age (SD): CBZ = 37.6 (15.8), LTG = 34.2 (16.3) years | |
| Interventions | Monotherapy with CBZ or LTG Permitted doses LTG: 100 mg/d–500 mg/d for LTG , CBZ: 400 mg/d–1200mg/d | |
| Outcomes | Retention rate at study end Terminal 24‐week seizure‐free rate and time interval from the end of dose titration phase to the first seizure | |
| Notes | Full text of the trial published in Korean. Abstract and clinical trial summary available in English. IPD request for this trial ongoing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate reported, not all participants included in analysis, which is not an ITT approach |
| Selective reporting (reporting bias) | Low risk | Results for all outcomes summarised for all listed outcomes |
| Other bias | Low risk | None identified |
| Methods | Randomised, open‐label trial conducted in 2 hospitals in Hong Kong 2 treatment arms: LTG and VPS | |
| Participants | Chinese patients with newly diagnosed, untreated epilepsy or a recurrence of seizures after a period of remission with AED therapy completely withdrawn for at least a year, aged 18‐55 years and not receiving AED therapy were recruited from the Prince of Wales Hospital and United Christian Hospital in Hong Kong. Number randomised: LTG = 37, VPS = 44 40 male participants (49%) 29 participants with partial epilepsy (36%) Mean age (range): 34 (16‐56 years) | |
| Interventions | Monotherapy with LTG or VPS Titration of 4 weeks to target dose of LTG = 100 mg/d and VPS = 800 mg/d Range of follow‐up: 0‐15 months | |
| Outcomes | Difference in mean fasting serum insulin concentration at 12 months between the 2 treatment groups Difference in mean changes from baseline at various time points in metabolic and endocrine measurements and BMI between the 2 treatment groups and by gender Frequency of common adverse events experienced by at least 10% of participants by treatment group | |
| Notes | IPD provided for all outcomes of this review by trial author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised stratified for sex and hospital, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in the Korea 2 treatment arms: LTG and CBZ | |
| Participants | Adults over the age of 16 with newly diagnosed partial epilepsy or untreated partial epilepsy for at least one year Number randomised: LTG = 57, CBZ = 53 57 male participants (52%) 95 participants with partial epilepsy (86%) Not stated how many participants had received previous AED treatment Mean age (range): 36 (16‐60) years | |
| Interventions | Monotherapy with LTG or CBZ 8‐week escalation phase leading to LTG = 200 mg/d, CBZ = 600 mg/d Range of follow‐up: 0‐16.5 months | |
| Outcomes | Change of neuropsychological and cognitive scores from baseline: general intellectual ability, learning and memory, attention and executive function (group‐by‐time interaction) Frequency of psychological and health‐related quality of life symptoms Proportion with seizure freedom during the maintenance period | |
| Notes | IPD provided by trial author for time to treatment withdrawal, time to first seizure and time to six‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size four) via a computer randomisation programme (information provided by trial author) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Prospective, open‐label randomised trial conducted in Serbia and Montenegro 2 treatment arms: LTG and VPS | |
| Participants | Participants with newly diagnosed, previously untreated epilepsy Number randomised: LTG = 35, VPS = 38 51 (70%) with partial epilepsy Median age (range): 34 (18‐76) years No information on gender | |
| Interventions | Monotherapy with LTG or VPS All participants to be followed up for at least 6 months | |
| Outcomes | Seizure freedom Retention on treatment | |
| Notes | Abstract of interim results only available. Contact was made with trial author who was unable to provide IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Unclear risk | Interim report, proportion of participants completing the trial period presented. Unclear if an ITT approach was taken to analysis |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient information to make a judgement |
| Other bias | Low risk | None identified |
| Methods | Multicentre, randomised, parallel‐group, double‐blinded trial over 10 centres in the USA with separate randomisation schemes used for each seizure type. 4 treatment arms: CBZ, PHB, PHT and primidone | |
| Participants | Adults with previously untreated or under‐treated simple or complex partial or secondary generalised tonic‐clonic seizures Number randomised: PHB: 155, PHT = 165, CBZ = 155 413 male participants (87%) 99.8% of participants with partial epilepsy Mean age (range): 41 (18‐82) years | |
| Interventions | Monotherapy with PHT or CBZ Median daily dose achieved: CBZ = 800 mg/d, PHB = 160 mg/d, PHT = 400 mg/d Range of follow‐up: 0‐78 months | |
| Outcomes | Participant retention/time to drug failure (length of time participant continued to take randomised drug) Composite scores of seizure frequency (seizure rates and total seizure control) and toxicity Incidence of side effects | |
| Notes | IPD provided for all outcomes of this review by the Department of Veterans Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised with stratification for seizure type. Method of randomisation not stated and not provided by authors |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (participants and personnel) achieved using an additional blank tablet |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Double‐blind, multicentre trial across 13 Veteran’s Affairs medical centres (USA) 2 treatment arms: CBZ and VPS | |
| Participants | Adults (18‐70 years) with previously untreated or under‐treated complex partial seizures, secondarily generalised tonic‐clonic seizures Number randomised: CBZ = 236, VPS = 244 445 male participants (93%) 100% of participants had partial epilepsy. Mean age (range): 47 (18‐83) years | |
| Interventions | Monotherapy with CBZ or VPS Mean daily dose achieved by month 12 CBZ = 722+‐ 230 mg/d, VPS = 2099 +‐824 mg/d Range of follow‐up: 0‐73 months | |
| Outcomes | Total number of seizures (of each type) during 12 months Number of seizures per month Percentage of participants with seizures completely controlled Time to first seizure Seizure rating score (severity of seizures) at 12 and 24 months Composite score (combined score for the control of seizures and incidence of adverse events) Incidence of systemic and neurologic adverse events (and severity) Time to treatment failure | |
| Notes | IPD provided for all outcomes of this review by the Department of Veterans Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to treatment using random permuted blocks with a different randomisation scheme for two seizure groups (complex partial and secondarily generalised tonic clonic) |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed via sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind (participants and personnel) achieved with additional matching placebo tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessment was blinded, no information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, single‐centre, parallel paediatric trial conducted in Los Angeles, USA 2 treatment arms: CBZ and PHB | |
| Participants | Children with newly diagnosed epilepsy Number randomised: PHB = 18, CBZ = 15 20 boys (61%) 100% of participants had partial epilepsy Mean age (range): PHB = 7.89 (2‐12 years), CBZ = 6.07 (2‐12 years) | |
| Interventions | Monotherapy with PHB or CBZ Doses started and achieved not stated Trial duration: 12 months Range of follow‐up: not reported | |
| Outcomes | Change in cognitive, intelligence (IQ), behavioural, and psychometric scores between baseline, 6 months, and 12 months Compliance, drug changes, and withdrawal rates Seizure control at 6 and 12 months (excellent/good/fair/poor) | |
| Notes | 33 participants were randomised to PHB (18) and CBZ (15) in this trial; 6 children were enrolled into a 6‐month pilot trial (PHB (4) CBZ (2)) prior to the randomised trial. The 6 children were included in 6‐month follow‐up psychometric data Outcomes for this review were not reported; IPD were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 33 children were "randomised using a scheme that balanced drug distribution by age and sex"; no further details were provided on the randomisation scheme. 6 non‐randomised children were also used in some analyses |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | The trial blinded participants (and parents); clinicians were unblinded for clinical follow‐up |
| Blinding of outcome assessment (detection bias) | High risk | The trial blinded participants (and parents); clinicians were unblinded for clinical follow‐up |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were reported; results were reported for all children who completed each stage of follow‐up |
| Selective reporting (reporting bias) | Low risk | Cognitive/behavioural outcomes, seizure control outcomes, and adverse events were all well reported. No protocol was available; outcomes for this review were not reported |
| Other bias | High risk | There was evidence that the trial may have been underpowered to detect differences (e.g. 55% power to find a 5‐point difference in IQ score). The behavioural questionnaire was not fully validated. Non‐randomised children from a pilot trial were included in the results for psychometric outcomes and medical outcomes |
| Methods | Prospective, randomised trial of participants newly referred to the pediatric clinic of Kitasato University School of Medicine, Japan 3 treatment arms: CBZ, PHT and VPS | |
| Participants | Children aged 1‐14 with previously untreated partial seizures and/or generalised tonic‐clonic seizures Number randomised: CBZ = 66, PHT = 51, VPS = 46 116 participants with partial epilepsy (71%) No information on age and gender | |
| Interventions | Monotherapy with PHT or CBZ Initial daily dose: CBZ = 13.0 +/‐ 1.6 mg/kg/d, PHT = 7.2 +/‐ 1.4 mg/kg/d, VPS = 22.9 +/‐ 4.9 mg/kg/d Range of follow‐up: 6‐66 months, mean follow‐up: 34 months in CBZ group, 37 in PHT group and 40 in VPS group | |
| Outcomes | Proportion of all randomised participants with seizure recurrence (by seizure type) Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | |
| Notes | Very limited information available, the trial was reported in a summary publication of 3 different studies (other 2 studies are not monotherapy designs). Outcomes chosen for this review were not reported, IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial was described as "randomised" but no further details were provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Ranges of follow‐up given for both treatment groups. Results reported "at the end of follow up," no withdrawals or exclusions mentioned, all participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Seizure recurrence outcomes described well reported. No adverse events reported; no protocol available so unclear if adverse events were planned a priori. Outcomes for this review not available |
| Other bias | Low risk | None identified |
| Methods | Double‐blind randomised trial performed in a single centre in Tehran, Iran 2 treatment arms: LEV and LTG | |
| Participants | Participants > 60 years who were referred to the neurologic clinic at Sina University Hospital, Iran in 2012. Participants must have had a diagnosis of epilepsy for at least 1 year and experienced a minimum of 1 unprovoked partial or generalised epileptic seizure over the last 6 months Number randomised: LEV = 50, LTG = 50 55 male participants (58%) out of 95 participants who completed the trial 67 participants with partial epilepsy (71%) out of 95 participants who completed the trial Mean age (SD, range): 72.4 (5.87, 63‐85) years for all randomised participants | |
| Interventions | Monotherapy with LEV or LTG LEV was initiated with 250 mg twice daily and was increased to 500 mg twice daily, LTG was initiated with 25 mg daily and was increased up to a maximum dose of 100 mg twice daily Trial duration: 20 weeks, range of follow‐up not stated | |
| Outcomes | Seizure recurrence Abnormal laboratory values Adverse events | |
| Notes | The trial was published in Persian; the characteristics and outcomes were translated. Outcomes chosen for this review were not reported; contact could not be made with trial author to provide IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based table was generated by balanced block randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | The participant received a drug with a specific code and did not know the name of the drug. The physician in charge of the participant follow‐up was unaware of the drug provided for the participant |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Only those who completed the trial were included in analyses, five participants excluded |
| Selective reporting (reporting bias) | Low risk | Seizure recurrence outcomes and adverse events were all well reported. No protocol was available; outcomes for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Phase 4, randomised, parallel‐design, open‐label trial in Republic of Korea 2 treatment arms: LEV and OXC | |
| Participants | Participants aged16‐80 years with newly diagnosed partial epilepsy Partcipants must have had at least 2 seizures separated by a minimum of 48 h and 1 in the 6 months prior to screening and no AEDs in the previous 6 months Number enrolled: LEV = 175, OXC = 178 190 male participants (54%) 100% of participants had partial epilepsy Mean age (SD): LEV = 39.5 (16.7), OXC = 42.7 (17.3) | |
| Interventions | Monotherapy with LEV or OXC Titration for 2 weeks up to a maximum of LEV = 1000 mg/d‐3000 mg/d, OXC = 900 mg/d‐24,000 mg/d Trial duration: 50 weeks, range of follow‐up not stated | |
| Outcomes | Percentage of participants with a treatment failure after 50 weeks Time to the first seizure defined as the time from the first dose of medication to the occurrence of the first seizure during the 48 weeks' treatment period Percentage of subjects who achieved seizure freedom for 24 consecutive weeks during the 48 weeks' treatment period at any time Percentage of subjects who achieved seizure freedom during the 48 weeks' treatment period | |
| Notes | Trial registered as NCT001498822 on ClincalTrials.gov and listed as completed and trial results published online but no published manuscript was available. Trial sponsored by UCB Korea, inquiries regarding this trial made to the sponsor. Data cannot be made available until a manuscript has been published; if IPD is provided at a future date, this trial will be included in analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate reported, not all participants included in analysis which is not an ITT approach |
| Selective reporting (reporting bias) | Low risk | Results for all outcomes reported online for listed outcomes |
| Other bias | Low risk | None identified |
| Methods | Phase 3, randomised, open‐label, parallel‐group trial conducted in China 2 treatment arms: CBZ and LEV | |
| Participants | Chinese participants > 16 years, recent onset partial seizures, at least 2 unprovoked seizures in the year preceding randomisation, of which at least 1 unprovoked seizure occurred in the 3 months preceding randomisation Number enrolled: CBZ = 215, LEV = 218 233 male participants (54%) 100% of participants had partial epilepsy Mean age (SD): CBZ = 33.3 (14.3), LEV = 37.8 (16.2) | |
| Interventions | Monotherapy with CBZ or LEV Titration of 3 weeks to CBZ = 400 mg/d, LEV = 1000 mg/d | |
| Outcomes | Proportion of subjects remaining seizure‐free during the 6‐month evaluation period Proportion of subjects retained in the trial for the duration of the period covering the up‐titration period, stabilization period, and evaluation period Time to first seizure or discontinuation due to an adverse event (AE)/lack of efficacy (LOE) during the evaluation period Time to first seizure during the evaluation period Time to first seizure during the period covering the up‐titration period, stabilisation period, and evaluation period from the first dose of trial drug | |
| Notes | Trial registered as NCT01954121 on ClincalTrials.gov and listed as completed and trial results published online but no published manuscript was available. Trial sponsored by UCB SA, inquiries regarding this trial made to the sponsor. Data cannot be made available until a manuscript has been published; if IPD is provided at a future date, this trial will be included in analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate reported, not all participants included in analysis which is not an ITT approach |
| Selective reporting (reporting bias) | High risk | Results reported online for only some of the outcomes, no statistical analysis reported for the Time to First Seizure outcomes. |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in Europe and Mexico 2 treatment arms: LTG and CBZ randomised in a 2:1 ratio | |
| Participants | Adults and children over the age of 2 years with newly diagnosed or currently untreated partial epilepsy with ≥ two seizures in the previous 6 months and with at least 1 seizure in the last 3 months Number randomised: LTG = 420, CBZ = 202 329 male participants (53%) 619 participants with partial epilepsy (99.5%) Not stated how many participants had received previous AED treatment Mean age (range): 27 (2‐84) years | |
| Interventions | Monotherapy with LTG or CBZ 6‐week escalation phase leading to minimum of LTG 2 mg/kg/d age range 2‐12 years, 200 mg/d age range 13‐64 years and 100 mg/d age > 65 years. CBZ aged 2‐12 years 5 mg/kg‐40 mg/kg, age > 12 years 100 mg/d‐1500 mg/d Range of follow‐up: 0‐245 days | |
| Outcomes | Proportion of participants seizure‐free during the last 16 weeks of treatment Efficacy success: proportion of participants who did not withdraw before the end of week 18 and were seizure‐free in the last 16 weeks of the trial Time to withdrawal from the trial (proportion of participants completing the trial) Proportion of participants experiencing adverse events Withdrawals due to adverse events | |
| Notes | IPD provided by trial sponsor Glaxo Smith Kline for time to treatment withdrawal and time to first seizure (plus seizure‐freedom rates at 24 weeks) Dates of seizures during the first 4 weeks not provided with IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. Participants randomised in a 2:1 ratio (LTG:CBZ), stratified by age group and country |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed, opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Protocol provided. Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Double‐blinded, parallel‐group, randomised trial conducted in a single centre in Nigeria. 3 treatment arms: CBZ, PHB, PHT | |
| Participants | Consecutive newly diagnosed participants aged ≥ 14 years presenting at the outpatient neurology clinic of the University Teaching Hopsital, Benin City, Nigeria with recurrent, untreated afebrile seizures Number randomised: PHT = 18, PHB = 18, CBZ = 19 34 male participants (62%) 10 participants with partial epilepsy (18%) Mean age (range): 27.5 years (14‐55 years) | |
| Interventions | Monotherapy with PHT or CBZ Median daily dose (range): CBZ = 600 mg (400 mg‐1200 mg), PHT = 200 mg (100 mg‐300 mg), PHB = 120 mg (60 mg‐180 mg) All participants followed up for 12 weeks | |
| Outcomes | Cognitive measures (reaction times, mental speed, memory, attention) | |
| Notes | IPD provided for all randomised participants by the trial author. Trial duration was 12 weeks; all participants completed the trial without withdrawing, therefore outcomes, time to withdrawal of allocated drug, time to six‐month remission and time to 12‐month remission could not be calculated. Time to first seizure calculated from IPD provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial randomised using simple randomisation. Each participant was asked to pick one from a table of numbers (1‐60), numbers corresponded to allocation of 1 of 3 drugs (information provided by trial author) |
| Allocation concealment (selection bias) | Low risk | Recruitment/randomisation of participants and allocations of treatments took place on different sites (information provided by trial author) |
| Blinding of participants and personnel (performance bias) | Low risk | Participants single‐blinded. Research assistant recruiting participants and counselling on medication adherence was not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators performing cognitive assessments were single‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants completed the trial. All randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. One outcome for this review calculated from IPD provided (see footnote 2). Other outcomes for this review not available due to short trial length. All cognitive outcomes from the trial well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, parallel‐group trial conducted in a rural district of West Bengal, India 2 treatment arms: PHB and PHT | |
| Participants | Children from a rural district of a developing country (India) who had experienced 2 or more unprovoked seizures within the 12 months preceding the trial and had been untreated in the 3 months preceding the trial Number randomised: PHB = 47 ; PHT = 47 47 boys (50%) 60 children had partial epilepsy (64%) Mean age (range): 11 (2‐18) years | |
| Interventions | Monotherapy with PHB or PHT Maintenance doses: PHT = 5 mg/kg/d, PHB = 3 mg/kg/d. Daily dose achieved not stated Range of follow‐up: 0.5‐13 months | |
| Outcomes | Time to first seizure Proportion seizure‐free in each trial quarter Proportion of adverse events including behavioural side effects | |
| Notes | IPD provided for remission and seizure outcomes of this review by the trial author. Withdrawal information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | First 10 participants randomised from a pre‐prepared balanced random number list, following participants randomised by minimisation with stratification by age group and presence of cerebral impairment |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Participants, parents and treating physicians unblinded for “practical and ethical reasons.” Withdrawal information from treatments not available, however lack of blinding may have influenced withdrawal rates |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors single‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, parallel‐group trial conducted in the context of existing community health care in a rural highland area of a developing country (Ecuador) 2 treatment arms: CBZ and PHB | |
| Participants | Participants with a history of at least 2 afebrile seizures and no previous AED treatment in the 4 weeks preceding the trial were eligible Number randomised: PHB = 97, CBZ = 95 67 male participants (35%) 133 participants with partial epilepsy (69%) Mean age (range): 29 (2‐68) years | |
| Interventions | Monotherapy with PHB or CBZ Minimum maintenance doses by age groups: 2‐5 years: PHB: 15 mg/d, CBZ: 150 mg/d; 6‐0 years: PHB: 30 mg/d, CBZ: 300 mg/d; 11‐15 years: PHB: 45 mg/d, CBZ: 500 mg/d; > 16 years: PHB: 60 mg/d, CBZ: 600 mg/d. Doses gradually increased Doses achieved not stated | |
| Outcomes | Proportion seizure‐free at 3‐, 6‐, and 12‐month follow‐ups Proportion seizure‐free, with more than 50% seizure reduction and no change in seizure frequency in 6‐ to 12‐month follow‐up period Incidence of adverse effects Trial duration: 12 months Range of follow‐up: 3.5‐23 months | |
| Notes | We received IPD for all outcomes used in this review from the trial author. Results in the published paper were given for 139 participants who completed 6 months' follow‐up, but we received IPD for all 192 participants randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised with random number list, no information provided on method of generating random list |
| Allocation concealment (selection bias) | High risk | Allocation concealed using sealed, opaque envelopes but method not used for all participants (information provided by trial author) |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported or calculated with the IPD provided (see footnote 2) |
| Other bias | High risk | Inconsistencies between number and reasons of withdrawals between the data and the published paper, which could not be resolved by the author |
| Methods | Multinational, randomised, double‐blind trial was conducted at 115 centres across the USA, Canada, Europe and South America Four treatments: CBZ, VPS and TPM (2 arms, 100 mg/d and 200 mg/d) ‐ see Notes | |
| Participants | Participants > 6 years and > 30 kg in weight, with a diagnosis of epilepsy within the 3 months before trial entry and no previous AED treatment except emergency treatment Number randomised (ITT population): CBZ = 126, TPM = 266 (CBZ branch), VPS = 78, TPM = 147 (VPS branch) 327 male participants (53%) 363 participants with partial epilepsy (59%) Mean age (range): 34 (6‐84 years) | |
| Interventions | Monotherapy with CBZ, VPS or TPM Starting doses: CBZ = 200 mg/d, VPS = 250 mg/d, TPM = 25 mg/d Target doses (after 4‐week titration): CBZ = 600 mg/d, VPS = 1000 mg/d, TPM = 100 or 200 mg/d (see Notes) Range of follow‐up: 0‐29 months | |
| Outcomes | Time to exit Time to first seizure Proportion of seizure‐free participants during the last 6 months of double‐blind treatment Safety assessment: most commonly occurring adverse events | |
| Notes | IPD provided for all outcomes of this review by trial sponsor Johnson & Johnson. Trial designed in 2 strata based on whether recommended treatment would be CBZ or VPS. Within the 2 strata, participants were randomised to 10 mg/d TPM, 200 mg/d TPM or CBZ/VPS depending on the strata. Data analysed according to the separate strata in this review with the 2 TPM doses analysed together (see Data extraction and management) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was balanced using permuted blocks of size three and stratified by trial centre, according to a computer‐generated randomisation schedule prepared by the trial sponsor |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Trial was double‐blinded for the first 6 months, followed by an open‐label phase |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants from the ITT population analysed from IPD provided (see footnote 2). Eight participants with no follow‐up data were excluded from ITT population |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Single‐centre, randomised, parallel‐group trial of participants, referrals to the outpatient department of neurology of the Central Hospital of Paijat‐Hame, Finland. 2 treatment arms: CBZ and PHT | |
| Participants | Adults (eligible age range 15‐57) with newly diagnosed epilepsy Number randomised: PHT = 20, CBZ = 23 20 male participants (47%) 10 participants with partial epilepsy (23%) Mean age (SD) years: PHT = 31.5 (11.3), CBZ = 26.8 (13.2) | |
| Interventions | Monotherapy with PHT or CBZ Dose information not reported Trial duration: 6 months, range of follow‐up not stated | |
| Outcomes | Cognitive assessments (visual motor speed, co‐ordination, attention and concentration, verbal and visuospatial learning, visual and recognition memory, reasoning, mood, handedness) Harmful side effects | |
| Notes | 59 participants were randomised but 16 were subsequently excluded. Results were presented only for the 43 participants who completed the entire trial. Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment groups, method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Low risk | Cognitive outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | 16/59 (27%) of participants excluded from analysis. Results presented only for participants who completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in methods section well reported in results section. Adverse effects reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | None identified |
| Methods | Randomised, 'two compartment' parallel trial, conducted in the USA 2 treatment arms: CBZ and PHT | |
| Participants | Adults, previously untreated, with at least 2 seizures or at least 1 seizure and an EEG with paroxysmal features Number randomised: PHT = 45, CBZ = 42 60 male participants (69%) 55 participants with partial epilepsy (63%) Mean age (range) 37.4 (18‐77) years | |
| Interventions | Monotherapy with PHT or CBZ Mean daily dose achieved (for the 54 participants with no major side effects): PHT = 5.35 mg/kg/d, CBZ = 9.32 mg/kg/d Trial duration: 2 years. Range of follow‐up not reported | |
| Outcomes | Laboratory measures Side effects (major and minor) Seizure control/treatment failure | |
| Notes | 7 participants on CBZ and 10 participants on PHT were “dropped for non‐compliance” and excluded from analysis Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to treatment groups, method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (participants and personnel) achieved with additional blank tablet |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | High risk | 17/87 (19.5%) of participants excluded from analysis for "non‐compliance". Results presented only for participants who completed the trial |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the methods sections reported well in the results section. No protocol available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Open‐label, parallel‐design, multicentre RCT conducted at 16 centres in the USA 2 treatment arms: PHT and VPS randomised in a 2:1 ratio | |
| Participants | Participants with at least 2 newly‐diagnosed and previously untreated primary generalised tonic clonic seizures within 14 days of starting the trial Number randomised: PHT = 50, VPS = 86 73 male participants (54%) 0% participants with partial epilepsy (all generalised epilepsy) Mean age (range): 21 (3‐64 years) | |
| Interventions | Monotherapy with PHT or VPS Starting doses PHT: 3 mg/kg/d‐ 5 mg/kg/d, VPS: 10 mg/kd/d‐15 mg/kg/d, doses gradually increased. Doses achieved not stated Range of follow‐up: 0‐11 months | |
| Outcomes | Time to first generalised tonic clonic seizure 6‐month seizure recurrence rates Adverse events | |
| Notes | IPD provided for 3/4 outcomes of this review by the Department of Veteran's Affairs (maximum follow‐up 6 months, therefore trial could not contribute to outcome, 'Time to 12‐month remission') | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised on a 2:1 ratio VPS:PHT using randomisation tables in each centre (information provided by trial author) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial; trial authors state that differences in adverse events of PHT and VPS would "quickly unblind" the trial anyway |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial; trial authors state that differences in adverse events of PHT and VPS would "quickly unblind" the trial anyway |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Double‐blind, multi‐centre, RCT conducted in the USA 2 treatment arms: CBZ and LEV | |
| Participants | Adults > 60 years with new onset partial seizures (previously untreated or under treated) Interim results: 37 participants recruited (numbers recruited to each arm not stated) 28 male participants (76%) 100% of participants had partial epilepsy Age: 20 participants aged 60‐69 years and 17 participants > 70 years | |
| Interventions | Monotherapy with CBZ or LEV Intitial doses: CBZ = 100 mg/d, LEV = 250 mg/d. Target doses: CBZ = 400 mg/d, LEV = 1000 mg/d Interim results, range of follow‐up not stated | |
| Outcomes | Discontinuations from the trial Treatment‐emergent side effects Seizure control | |
| Notes | Trial available as abstract only. Attempts to contact the principal investigator and trial sponsor for further information were unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial ‐ trial drugs were over encapsulated and all participants received similar appearing active medication |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Interim report, 7/37 participants recruited had discontinued treatment. Unclear if an ITT approach would be taken to analysis |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient information to make a judgement |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, double‐blind trial conducted in the USA 2 treatment arms: PHT and TPM | |
| Participants | Participants 12–65 years (inclusive), weighed at least 50 kg and experienced 1–20 unprovoked, complex partial or primary/secondarily generalised tonic–clonic seizures within the past 3 months, either as newly diagnosed epilepsy or as epilepsy relapse from remission Number randomised: PHT = 128, TPM = 133 126 male participants (48%) 53 participants with partial epilepsy (20%) Mean age (range): 34 (12‐78 years) | |
| Interventions | Monotherapy with PHT or TPM Short titration (1 day) to target dose of PHT = 300 mg/d and TPM = 100 mg/d Range of follow‐up: 0‐2.5 months | |
| Outcomes | Time to first complex partial seizure or generalised tonic clonic seizure Participant retention (time to discontinuation of treatment) Incidence and summary of adverse events | |
| Notes | IPD provided by trial sponsor Johnson & Johnson for time to withdrawal and time to first seizure, trial duration insufficient to measure remission outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded assessment of results and serum AED level |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Parallel‐design RCT conducted in Meerut, India 2 treatment arms: PHT and VPS | |
| Participants | Participants with at least 2 partial or generalised tonic‐clonic seizures per month Unclear if participants were newly diagnosed Number randomised: PHT = 45; VPS = 49 70 male participants (74%) 27 participants with partial epilepsy (29%) Age range: PHT: 12‐42 years; VPS: 8‐52 years | |
| Interventions | Monotherapy with PHT or VPS Average daily dose achieved: PHT: 5.6 mg/kg/d, VPS: 18.8 mg/kg/d Participants were evaluated after 4, 12 and 24 weeks of treatment No information on range of follow‐up | |
| Outcomes | Reduction in frequency of seizures:
Adverse effects Seizure control | |
| Notes | Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly allocated irrespective of seizure type," no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Frequency of seizures reported for all randomised participants, no information provided on withdrawal rates/attrition rates etc |
| Selective reporting (reporting bias) | Low risk | Frequency of seizures during treatment well reported, most common adverse events reported No protocol available to compare with a priori analysis plan, outcomes for this review not reported |
| Other bias | Low risk | None identified |
| Methods | Single‐centre, randomised, parallel‐group trial of participants referred to the Neurology Clinic of Nehru Hospital, Chandigarh, India. 2 treatment arms: CBZ and PHT | |
| Participants | Newly diagnosed and drug‐naive adult participants > 14 attending the Neurology Clinic of Nehru Hospital, Chandigarh, India Number randomised: PHT = 20, CBZ = 20 28 male participants (70%) 11 participants with partial epilepsy (27.5%) Mean age (range): PHT group 23.4 (14‐44 years), CBZ 24.4 (14‐45 years) | |
| Interventions | Monotherapy with PHT or CBZ Initial daily dose: PHT = 5 mg/kg/d, CBZ = 10 mg/kg/d Trial duration 10‐12 weeks. Range of follow‐up not reported | |
| Outcomes | Cognitive measures before and after treatments (verbal, performance, memory, visuomotor, perceptomotor organisation, visual organisation, dysfunction) | |
| Notes | 6 participants on CBZ and 8 participants on PHT were excluded from final analysis of cognitive assessments who were lost to follow‐up or who had uncontrolled seizures Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were randomised to one of the two trial groups," no further information given on methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | 14/40 (35%) of participants excluded from analysis who were lost to follow‐up or experienced uncontrolled seizures. Results presented only for participants who completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in methods section well reported in results section. No seizure outcomes or adverse events reported and outcomes chosen for this review not reported. No protocol available, so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | None identified |
| Methods | Randomised, open‐label trial conducted in several hospitals in Mexico 2 treatment arms: CBZ and TPM | |
| Participants | Participants aged 2‐18 years with newly diagnosed partial epilepsy with or without secondary generalisation with at least two unprovoked seizures > 24 h apart and at least 1 seizure in the last 6 months. Participants must have no established treatment and have received no antiepileptic treatment within the past 30 days Number randomised: CBZ = 42, TPM = 46. Number included in analysis CBZ = 32, TPM = 33 100% partial epilepsy 33 male participants (60%) included in analysis Mean age (range): CBZ = 10 (5‐17) years, TPM = 8 (2‐16) years for participants included in analysis | |
| Interventions | Monotherapy with CBZ or TPM Treatments titrated to a maximum of CBZ = 20 mg/kg/d‐25 mg/kg/d, TPM = 9 mg/kg/d Follow‐up assessments at 6 and 9 months, range of follow‐up not stated | |
| Outcomes | Seizure freedom and frequency of seizures during the trial Adverse events during the trial Laboratory results | |
| Notes | The trial was published in Spanish; the characteristics and outcomes were translated. Outcomes chosen for this review were not reported; contact could not be made with trial author to provide IPD Results presented only for those who completed the trial. Those with less than 35% reduction of seizures were excluded from analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Random number tables used to assign participants to treatment groups |
| Blinding of participants and personnel (performance bias) | High risk | No information provided |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Open‐label trial |
| Selective reporting (reporting bias) | Low risk | Attrition rates reported (23 drops outs, 10 for CBZ and 13 for TPM). Only those who completed the trial were included in analysis (non responders to treatment excluded), this is not an ITT approach |
| Other bias | Low risk | No protocol available. Seizure outcomes and adverse events well reported |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 56 centres in Europe and Australia. 3 treatment arms: LTG (200 mg/d), LTG (100 mg/d) and CBZ | |
| Participants | Adults and children > 12 years with newly diagnosed, currently untreated or recurrent epilepsy with ≥ two seizures in the previous 6 months and with at least 1 seizure in the last 3 months. Participants must not have taken AEDs in the previous 6 months Number randomised: LTG (200 mg) = 115, LTG (100 mg) = 115, CBZ = 121 188 male participants (54%) 237 participants with partial epilepsy (68%) Not stated how many participants had received previous AED treatment Mean age (range): 32 (12‐72) years | |
| Interventions | Monotherapy with LTG or CBZ for 30 weeks 4‐week escalation phase leading to LTG = 100 mg/d, LTG = 200 mg/d, CBZ = 600 mg/d Range of follow‐up: 0‐378 days | |
| Outcomes | Proportion completing seizure‐free after the first 6 weeks of treatment Time to first seizure Time to withdrawal Frequency of adverse events with at least 5% incidence in any treatment group | |
| Notes | IPD provided by trial sponsor Glaxo Smith Kline for time to treatment withdrawal, time to first seizure and time to six‐month remission. Participants considered to complete the trial if they experienced a seizure after the first 6 weeks. In primary analysis, two arms of LTG pooled and compared to CBZ and separate doses of LTG compared to CBZ in sensitivity analysis (see Data extraction and management) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐ generated random sequence (information provided by drug manufacturer) |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual, sealed, opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | High risk | Open‐ label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐ label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Open‐label, multicentre trial across 22 centres in the UK 2 treatment arms: CBZ and VPS | |
| Participants | Adults with newly onset primary generalised epilepsy or partial epilepsy with/without generalisation or with a recurrence of seizures following withdrawal of AED treatment were eligible given that no anticonvulsants had been received in the previous 6 months Number randomised: CBZ = 151, VPS = 149 153 (51%) male participants (51%) 147 participants with partial epilepsy (49%) Mean age (range): 33 (16‐79) years | |
| Interventions | Monotherapy with CBZ or VPS Mean daily dose achieved by month 24: CBZ = 516 mg/d, VPS = 924 mg/d Range of follow‐up: 0.5‐90 months | |
| Outcomes | Remission analysis (time to 6‐, 12‐ and 24‐month remission) Retention analysis (time to treatment failure) Adverse event incidence Incidence of treatment failures due to poor seizure control and adverse events | |
| Notes | IPD provided for all outcomes of this review by trial sponsor Sanofi. Participants with other generalised seizure types (e.g. myoclonic/absence) were included in the trial, but efficacy analyses were based solely on generalised tonic clonic seizures. Results in the published paper were given for 181 participants out of 300 analysed by ITT (participants randomised and with data for at least 1 follow‐up visit). IPD is provided for all 300 participants randomised and used for analyses in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to treatment using a computerised minimisation programme with stratification for age, sex, seizure type and centre |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed via central telephone allocation from the Trial Office at Sanofi Winthrop LTD |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 18 Veterans Affairs Medical Centres in the USA 3 treatment arms: LTG, CBZ and GBP | |
| Participants | Adults > 60 years with newly diagnosed seizures, untreated or treated with sub‐therapeutic AED levels, with at least 1 seizure in the previous 3 months. Number randomised: CBZ = 198, GBP = 195, LTG = 200 570 male participants (96%) 446 participants with partial epilepsy (75%) Not stated how many participants had received previous AED treatment Mean age (years): CBZ = 71.9, GBP = 72.9, LTG = 71.9. Range not stated | |
| Interventions | Monotherapy with CBZ, GBP, LTG 6‐week escalation phase leading to CBZ = 600 mg/d, GBP = 1500 mg/d, LTG = 150 mg/d Trial duration: 12 months. Range of follow‐up: not stated | |
| Outcomes | Retention in the trial for 12 months Seizure freedom at 12 months Time to first, second, fifth and tenth seizure (time to seizures) Drug toxicity (incidence of systemic and neurologic toxicities) Serum drug levels and compliance Seizure‐free retention rates | |
| Notes | IPD requested from trial sponsor, the Department of Veterans Affairs, USA. At the time of review, IPD has not been received. Aggregate data extracted from graphs in the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (varying sizes) performed by site via a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation used and pharmacy dispensed a prescription of the allocated drug (part of a blinded drug kit) to participants |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind achieved with double dummy tablets, doses of both increased and decreased simultaneously |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported. Most of the randomised participants included in analysis, 3 excluded due to site closure (not related to treatment) |
| Selective reporting (reporting bias) | Low risk | No protocol available but case report forms of data collected provided by the sponsor. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 29 centres across Croatia, Finland, France, Finland and Norway 2 treatment arms: LTG and CBZ | |
| Participants | Adults > 65 years with newly diagnosed seizures, with a history of at least 2 seizures and at least 1 seizure in the previous 6 months. Participants must not have taken AEDs for more than 2 weeks in the previous 6 months and never taken CBZ or LTG Number randomised: LTG = 93, CBZ = 92 102 male participants (54%) Proportion with partial epilepsy not stated Not stated how many participants had received previous AED treatment Mean age: 74 (65‐91) years | |
| Interventions | Monotherapy with LTG or CBZ 4‐week escalation phase leading to LTG = 100 mg/d, CBZ = 400 mg/d Trial duration 40 weeks. Range of follow‐up: not stated | |
| Outcomes | Retention in the trial (time to treatment withdrawal for any cause) Seizure freedom after week 4 Seizure freedom after week 20 Time to first seizure Adverse event reports Tolerability according to the Liverpool Adverse Event profile (AEP) | |
| Notes | IPD requested from trial sponsor Glaxo Smith Kline but data could not be located. Aggregate summary data extracted from the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind achieved with double dummy tablets, packaged together |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all participants who received trial treatment were included in an ITT analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available but clinical trial summary provided by the sponsor. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in the UK 5 treatment arms: LTG, CBZ, GBP, TPM and OXC | |
| Participants | Adults and children > 4 years with newly diagnosed partial epilepsy, relapsed partial epilepsy or failed treatment with a previous drug not used in this trial. Number randomised: CBZ = 378, LTG = 378, OXC = 210, TPM = 378, GBP = 377 922 male participants (54%) 1491 partial epilepsy (87%) 309 had received previous AED treatment (18%) Mean age(range): 38 (5‐86) years | |
| Interventions | Monotherapy for LTG, CBZ, GBP, TPM or OXC Titration doses and maintenance doses decided by treating clinician Range of follow‐up: 0‐86 months | |
| Outcomes | Time to treatment failure Time to 1‐year (12 month) remission Time to 2‐year remission Time to first seizure Health‐related quality of life via the NEWQOL (Newly Diagnosed Epilepsy Quality of Life Battery) Health economic assessment and cost effectiveness of the drugs (cost per QALY gained and cost per seizure avoided) Frequency of clinically important adverse events | |
| Notes | IPD provided for time to treatment withdrawal, time to first seizure, time to six‐month, time to 12‐month and time to 24‐month remission (trial conducted at our site) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer minimisation programme stratified by centre, sex and treatment history |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation to a central randomisation allocation service |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in the UK 3 treatment arms: LTG, GBP, TPM | |
| Participants | Adults and children > 4 years with newly diagnosed or relapsed generalised or unclassified epilepsy, or failed treatment with a previous drug not used in this trial. Number randomised: LTG = 239, VPS = 238; TPM = 239 420 male participants (59%) 52 partial epilepsy (7%) 108 had received previous AED treatment (15%) Mean age (range): 22.5 (5‐77) years | |
| Interventions | Monotherapy for LTG, GBP or TPM Titration doses and maintenance doses decided by treating clinician Range of follow‐up: 0‐83.5 months | |
| Outcomes | Time to treatment failure Time to 1‐year (12‐month) remission Time to 2‐year remission Time to first seizure Health‐related quality of life via the NEWQOL (Newly Diagnosed Epilepsy Quality of Life Battery) Health economic assessment and cost effectiveness of the drugs (cost per QALY gained and cost per seizure avoided) Frequency of clinically important adverse events | |
| Notes | IPD provided for time to treatment withdrawal, time to first seizure, time to six‐month, time to 12‐month and time to 24‐month remission (trial conducted at our site) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer minimisation programme stratified by centre, sex and treatment history |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation to a central randomisation allocation service |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Parallel‐design RCT conducted at 2 centres (Glasgow, Scotland and Wellington, New Zealand) 2 treatment arms: PHT and VPS | |
| Participants | 21 (64%) of participants previously untreated, 12 (36%) of participants continued to have seizures on previous drug therapies. Original treatments gradually withdrawn before PHT or VPS treatment introduced. Number randomised: PHT = 15, VPS = 18 12 male participants (36%) 19 participants with partial epilepsy (58%) Mean age (range): 23 (7‐55 years) | |
| Interventions | Monotherapy with PHT or VPS Starting doses: PHT: < 12 years 150 mg/d, older participants: 300 mg/d, VPS: < 12 years 300‐400 mg/d, older participants: 800‐1200 mg/d. Doses achieved not stated. Mean follow‐up (range): 30 (9‐48 months) | |
| Outcomes | Seizures during treatment Adverse events | |
| Notes | Outcomes chosen for this review were not reported IPD not available but could be constructed from the publication for the outcome 'Time to treatment withdrawal.' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "randomly divided", using telephone randomisation (information provided by trial author) |
| Allocation concealment (selection bias) | Low risk | Centralised telephone randomisation used (information provided by trial author) |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Results reported for all randomised participants, time on treatment reported for all randomised participants. No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | No protocol available, outcomes chosen for this review not reported, Seizure and adverse event outcomes well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised double‐blind study conducted in the USA 2 treatment arms: CBZ and VPS | |
| Participants | Participants between the ages of 10 and 70 who had experienced at least two complex partial seizures who were previously untreated or insufficiently treated Number randomised: CBZ = 17, VPS = 16 15 male participants (45%) 100% of participants with partial epilepsy Mean age (range): CBZ = 32.5 (13‐65), VPS = 31.3 (17‐57) | |
| Interventions | Monotherapy with CBZ or VPS Doses started or achieved not stated 4‐week titration period followed by a 24‐week maintenance period. Range of follow‐up not stated | |
| Outcomes | Proportion of participants free of complex partial seizures during the maintenance period Proportion of participants reporting specific adverse events | |
| Notes | Outcomes for this review were not reported; IPD were not available due to time elapsed since the trial was conducted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised in a 1:1 ratio, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates reported. Only those who entered the maintenance period were included in analysis; this is not an ITT analysis |
| Selective reporting (reporting bias) | Low risk | Efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, multicentre trial conducted in the UK 2 treatment arms: LTG and PHT | |
| Participants | Participants aged 14‐75 years with two or more partial, secondarily generalised, or primary generalised tonic‐clonic seizures Number randomised: PHT = 95, LTG = 86 101 male participants (56%) 90 participants with partial epilepsy (50%) Mean age (range): 34 (13‐75 years) | |
| Interventions | Monotherapy with LTG or PHT Titrated for 2 weeks to a target dose of LTG = 150 mg/d, PHT = 300 mg/d Range of follow‐up: 0‐15 months | |
| Outcomes | Percentage of participants remaining on treatment Percentage of participants remaining seizure free in the last 24 and last 16 weeks of treatment Number of seizures (percentage change from baseline) in the last 24 weeks and 16 weeks of treatment Time to first seizure after the first 6 weeks of treatment (dose‐titration period) Time to discontinuation Incidence of adverse events and adverse events leading to discontinuation Quality of Life according to the Side Effects and Life Satisfaction (SEALs) inventory | |
| Notes | IPD provided by trial sponsor Glaxo Smith Kline for time to treatment withdrawal, time to first seizure and time to six‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified according to seizure type, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | All participants, personnel and outcome assessors involved in the trial were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All participants, personnel and outcome assessors involved in the trial were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, open‐label, parallel‐group trial conducted in 24 centres across Germany. 4 treatment arms: LTG (two arms), CBZ and VPS Participants with partial and generalised epilepsy randomised separately to LTG or CBZ and LTG or VPS respectively | |
| Participants | Adults and children > 12 years with newly diagnosed epilepsy; at least 1 seizure and EEG imaging suggesting epilepsy Number randomised not stated, number included in analysis: LTG = 88, CBZ = 88 (partial); LTG = 33, VPS = 30 (generalised) 106 male participants (64%) in partial epilepsy group, 27 male participants (43%) in the generalised epilepsy group 166 out of 239 total included in analysis have partial epilepsy (69%) Not stated how many participants had received previous AED treatment Mean age (years): LTG (partial) = 46.6, CBZ = 43.1, LTG (generalised) = 22.3, VPS = 23.3 Range not stated | |
| Interventions | Monotherapy with LTG, CBZ or VPS 4‐week escalation phase leading to LTG = 100 mg/d‐200 mg/d, CBZ = 600 mg/d‐1200 mg/d in adults and 600 mg/d‐1000 mg/d in children aged 11‐15, VPS = 600 mg/d‐1200 mg/d for children aged 6‐14, 600 mg/d‐1500 mg/d for adolescents over 14 years and 1200 mg/d‐2100 mg/d for adults Trial duration: 26 weeks, range of follow‐up: not stated | |
| Outcomes | Number of seizure‐free patients during trial weeks 17‐24 "Leaving the study" (retention rates) Adverse event rates | |
| Notes | IPD requested from trial sponsor Glaxo Smith Kline but data could not be provided due to restrictions over the de‐identification of datasets from trials conducted in Germany. Aggregate data extracted from graphs in the publication. Data from participants with partial epilepsy is the randomised comparison of LTG and CBZ and data from participants with generalised epilepsy is the randomised comparison of LTG and VPS (see Data extraction and management) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Number of participants randomised to each group not reported (254 randomised and 239 analysed in the four arms of the trial). Reasons for exclusion stated but not which drug these participants were randomised to |
| Selective reporting (reporting bias) | Low risk | No protocol available but clinical trial summary provided by the sponsor. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, single‐centre, open‐label trial conducted in Scotland, UK 2 treatment arms LTG and VPS | |
| Participants | Participants of at least 13 years with a minimum of 2 newly onset unprovoked seizures of any type and no previous exposure to LTG or VPS Number randomised: LTG = 117, VPS =109 114 male participants (50%) 154 participants with partial epilepsy (68%) Mean age (range): 36 (13 ‐ 80 years) | |
| Interventions | Monotherapy with LTG or VPS Titration of 5‐10 weeks to target doses of LTG = 200 mg/d and VPS = 1000 mg/d Range of follow‐up: 0‐51 months | |
| Outcomes | Percentage of randomised participants achieving a minimum period of 12 months' seizure freedom Percentage of randomised participants withdrawing due to adverse events Percentage of randomised participants with lack of efficacy at maximum tolerated dose Changes in levels of androgenic hormone levels (testosterone, androstenedione and sex hormone‐binding globulin levels) Changes in weight and BMI from baseline | |
| Notes | IPD provided for all outcomes of this review by trial author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants from the ITT population analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | High risk | There were inconsistencies between rates of seizure recurrence and reasons for withdrawal between the data provided and the published paper, which the authors could not resolve |
| Methods | Randomised, single‐centre, open‐label trial conducted in Bengaluru, India. 2 treatment arms: CBZ and LEV | |
| Participants | Participants aged 18‐60 years diagnosed newly with focal or partial seizures with or without secondary generalisation referred to the Department of Neurology at Vydehi Institute of Medical Sciences and Research Center Number randomised CBZ = 30, LEV = 30 30 male participants (50%) 100% participants with partial epilepsy Mean age (range): not provided for all randomised participants | |
| Interventions | Monotherapy with CBZ or LEV Starting dose of CBZ = 200 mg/d, LEV = 500 mg/d titrated to a maximum dose of CBZ 1200 mg/d, LEV 300 mg/d Trial duration: 1 year, range of follow‐up: not stated | |
| Outcomes | Quality of Life by the QOLIE‐10 questionnaire before and after 26 weeks of therapy Treatment efficacy (seizure freedom at 4 weeks, 12 weeks, 26 weeks and 6 months) Treatment safety (proportion of participants experiencing at least 1 adverse event) | |
| Notes | Outcomes chosen for this review were not reported; contact could not be made with trial author to provide IPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition rates reported, two participants lost to follow‐up in each group not included in analysis. This is not an ITT approach but unlikely that this small amount of missing data would influence the overall results |
| Selective reporting (reporting bias) | High risk | Only one outcome is predefined in the methods section (Quality of Life), other results reported were not predefined |
| Other bias | Low risk | None identified |
| Methods | Parallel‐design RCT conducted in Madras (Chennai), India Three treatment arms: PHB, PHT, VPS | |
| Participants | Children with more than 1 previously untreated generalised tonic clonic (afebrile) seizure Number randomised: PHB group = 51, PHT = 52, VPS = 48 81 boys (54%) 0% partial epilepsy (all had generalised epilepsy) Age range: 4‐12 years | |
| Interventions | Monotherapy with PHT or VPS Starting doses: PHB: 3 mg/kg/d‐ 5 mg/kg/d PHT: 5 mg/kg/d‐ 8 mg/kg/d, VPS: 15 mg/kg/d‐ 50 mg/kg/d Dose achieved not stated Range of follow‐up (months): 22‐36 | |
| Outcomes | Proportion with recurrence of seizures Adverse events | |
| Notes | Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised via a computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double–blinded using additional placebo tablets, unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double–blinded using additional placebo tablets, unclear who was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed |
| Selective reporting (reporting bias) | Low risk | No protocol available, outcomes chosen for this review not reported, Seizure and adverse event outcomes well reported |
| Other bias | Low risk | None identified |
| Methods | Multi‐centre, open label, randomised, two parallel group stratified trial carried out in a community setting between February 2005 and October 2007 in 269 centres across 23 European countries and Australia. Four treatment arms: CBZ (controlled release), LEV (two arms) and VPS (extended release) ‐ see notes | |
| Participants | Patients aged ≥16 years were included if they had two or more unprovoked seizures in the previous 2 years with at least one during the previous 6 months. Participants must not have received one of the trial drugs previously or treated for epilepsy with any other AED in the previous 6 months Number randomised (ITT population): CBZ = 503, LEV = 492 (CBZ branch), LEV = 349, VPS = 353 (VPS branch). 949 male participants (56%) 1048 participants with partial epilepsy (62%) Mean age (range): 40 (16 ‐ 89 years). | |
| Interventions | Monotherapy with CBZ, LEV or VPS Titration over two weeks to target doses CBZ‐CR=600 mg/day, LEV=1000 mg/day, VPS‐ER=1000 mg/day, Range of follow up: 0 to 28.5 months | |
| Outcomes | Time to withdrawal from trial medication (treatment withdrawal) after randomisation Time to first seizure after randomisation Treatment withdrawal rates at 6 and 12 months Seizure‐freedom rates at 6 and 12 months Change of baseline in quality of life measures (QOLIE‐31‐P and EQ‐5D) Treatment emergent adverse events (intensity and seriousness) | |
| Notes | IPD provided for all outcomes of this review by trial sponsor UCB. Trial designed in 2 strata based on whether recommended treatment would be CBZ or VPS. Data analysed according to the separate strata in this review (see Data extraction and management) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified, no further information provided |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed by use of an interactive voice‐response system via telephone to manage the randomisation process |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants from the ITT population analysed from IPD provided (see footnote 2). 8 randomised participants excluded from ITT population due to no informed consent or lack of compliance with good clinical practice. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Single‐centre, parallel‐design RCT conducted in Newcastle, UK 2 treatment arms: PHT and VPS | |
| Participants | Participants with ≥ 2 partial or generalised tonic‐clonic seizures in the past 3 years. Participants were previously untreated but started on AED treatment within 3 months of their most recent seizure Number randomised: PHT = 70, VPS = 70 73 male participants (52%) 63 participants with partial epilepsy (45%) Mean age (range): 35 (14‐70 years) | |
| Interventions | Monotherapy with PHT or VPS Starting doses: PHT 300 mg/d, VPS 600 mg/d. Dose achieved not stated Range of follow‐up: 3.5‐52 months | |
| Outcomes | Time to 2‐year remission Time to first seizure Adverse events | |
| Notes | IPD provided for all outcomes included in this review by trial author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised with stratification for age group, gender and seizure type. Method of randomisation not stated or provided by author |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Open‐label, multicentre trial across 63 centres in UK and Ireland 2 treatment arms: CBZ and VPS | |
| Participants | Children with newly onset primary generalised epilepsy or partial epilepsy with/without generalisation or with a recurrence of seizures following withdrawal of AED treatment were eligible given that no anticonvulsants had been received in the previous 6 months Number randomised: CBZ = 130, VPS = 130 122 boys (47%) 108 participants with partial epilepsy (42%) Mean age (range): 10 (5‐16) years | |
| Interventions | Monotherapy with CBZ or VPS Mean daily dose achieved by month 24 CBZ = 450 mg/d, VPS = 700 mg/d Range of follow‐up: 2‐59 months | |
| Outcomes | Remission analysis (time to 6‐, 12‐ and 24‐month remission) Retention analysis (time to treatment failure) Adverse event incidence Incidence of treatment failures due to poor seizure control and adverse events | |
| Notes | IPD provided for all outcomes of this review by trial sponsor Sanofi. Results in the published paper are given for 244 children out of 260 analysed by "intention to treat" (children randomised and with data for at least one follow‐up visit). IPD is provided for all 260 children randomised and will be used for analyses in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to treatment using a computerised minimisation programme with stratification for age, sex, seizure type and centre |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed via central telephone allocation from the Trial Office at Sanofi Winthrop LTD |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 47 centres across Germany, Austria and Switzerland. 3 treatment arms: LTG, CBZ and LEV | |
| Participants | Adults > 60 years with newly diagnosed partial seizures, with a history of at least 2 seizures and at least 1 seizure in the previous 6 months. Participants must not have taken AEDs for more than 4 weeks Number randomised: LTG = 118, CBZ = 121, LEV = 122 215 male participants (60%) 100% of participants with partial epilepsy Not stated how many participants had received previous AED treatment Mean age(range): 71.5 (60‐95) years | |
| Interventions | Monotherapy with LEV, LTG or CBZ for 58 weeks 6‐week escalation phase leading to CBZ = 400 mg/d. LEV+ 1000 mg/d, LTG = 100 mg/d Range of follow‐up: 0‐54 months | |
| Outcomes | Retention rate at week 58 Time to discontinuation from randomisation Seizure‐freedom rates at week 30 and week 58 Time to first seizure from randomisation Time to first drug‐related adverse event Adverse events (by severity) | |
| Notes | IPD provided by trial author for time to treatment withdrawal, time to first seizure, time to six‐month and time to 12‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list for each centre (random permuted blocks) was prepared by the Interdisciplinary Centre for Clinical Trials (IZKS), Mainz, Germany |
| Allocation concealment (selection bias) | Low risk | The pharmacy of the University Hospital Mainz encapsulated the trial drugs and labelled the blinded medication including the randomisation number. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and trial investigator blinded by the use of matching capsules |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
1. Abbreviations: ADL: activities of daily living; AED: antiepileptic drug; BMI: body mass index; CBCL: child behavior checklist; CBZ: carbamazepine; EEG: electroencephalography; GBP: gabapentin; IPD: Iindividual participant data; ITT: intention to treat; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; PSB: polysomnography; RCT: randomised controlled trial; REM: rapid eye movement; TPM: topiramate; VPS: sodium valproate; WISC: Wechsler Intelligence Scale for Children; ZNS: zonisamide
2. Attrition bias and reporting bias are reduced in trials for which IPD were provided, as attrition rates and unpublished outcome data were requested
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Participants randomised to LTG and physician's choice of CBZ or VPS. No fully randomised comparison between the drugs | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Cross‐over design is not appropriate for measuring long‐term outcomes | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Trial terminated early, no results available | |
| Trial terminated early, no results available | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Cross‐over design is not appropriate for measuring long‐term outcomes | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Trial terminated early, no results available | |
| Participants randomised to LTG and physician's choice of CBZ PHT or VPS. No fully randomised comparison between the drugs | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Cross‐over design is not appropriate for measuring long‐term outcomes | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Conversion to monotherapy design, monotherapy comparison not possible | |
| Included participants primarily had dementia, only a subset had epilepsy |
CBZ: carbamazepine; LTG: lamotrigine; PHT: phenytoin; VPS: sodium valproate
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised trial conducted in China 2 treatment arms: CBZ and OXC |
| Participants | Children aged 2‐14 years, who were newly diagnosed with focal epilepsy between October 2009 and December 2011 Number randomised: CBZ = 60, OXC = 58 |
| Interventions | Monotherapy with CBZ or OXC Doses started and achieved not stated |
| Outcomes | Response rates Seizure‐free rates Adverse event rates |
| Notes | 2 publications of the trial available only in Chinese (English abstract). Awaiting translation of the full text |
| Methods | Randomised, double‐blind trial conducted at Neurology clinic of Ahvaz Golestan Hospital, Iran 2 treatment arms: OXC or PHT |
| Participants | Participants > 65 years with partial and secondary generalised epilepsy |
| Interventions | Monotherapy with PHT or OXC for 6 months Maximum dose: PHT = 600 mg/d, OXC = 600 mg/d |
| Outcomes | Seizure symptoms Adverse events |
| Notes | Trial registered as IRCT201202068943N1 on the Iranian Registry of Clinical Trials. We have attempted to contact the trial authors for more information |
| Methods | Randomised, double‐blind, parallel‐design trial 2 treatment arms: CBZ and ZNS |
| Participants | People newly diagnosed with epilepsy Number randomised: 171 (not stated by treatment group) Number entering dose escalation phase: CBZ = 82, ZNS = 73 |
| Interventions | Monotherapy with CBZ or ZNS 4 weeks titration to maximum dose of CBZ: 600 mg/d, ZNS: 300 mg/d |
| Outcomes | Terminal remission rate at week 24 Time interval to first seizure recurrence Adverse events |
| Notes | Full text of the trial available only in Korean (English Abstract). Awaiting translation of the full text |
| Methods | Phase 4, randomised, parallel‐design, open‐label safety trial 2 treatment arms: TPM and ZNS |
| Participants | Participants > 13 years with at least 2 seizures and 1 in the 3 months prior to screening and no AEDs in the previous 4 months Estimated number enrolled = 140 |
| Interventions | Monotherapy with TPM or ZNS Initial doses: TPM = 25 mg/d, ZNS = 100 mg/d. Maximum doses: TPM = 400 mg/d, ZNS = 600 mg/d |
| Outcomes | Cognitive function (change from baseline at 24 weeks) |
| Notes | Trial registered as NCT00154076 on ClincalTrials.gov and listed as completed but no results published. Trial sponsored by Eisai Korea, inquiries regarding this trial made to the sponsor but no data could be provided. If more information on this trial can be found, this trial will be included in future updates of the review. |
| Methods | Randomised, open‐label, parallel‐group trial conducted in Republic of Korea 2 treatment arms: OXC and TPM |
| Participants | Children with newly diagnosed epilepsy Number randomised: OXC = 20, TPM = 25 |
| Interventions | Monotherapy OXC or TPM Doses started and achieved not stated Trial duration: 16 weeks |
| Outcomes | Seizure freedom Seizure frequency Adverse events |
| Notes | Full text of the trial available only in Korean (English abstract). Awaiting translation of the full text |
| Methods | 2‐arm trial of CBZ and PHT. Unclear from information provided in the English abstract if the trial is randomised |
| Participants | 64 participants with untreated partial (n = 9), partial complex (n = 27), partial secondary generalised (n = 22), or primary generalised seizures (n = 6) |
| Interventions | Monotherapy with CBZ or PHT. Unclear how many participants were allocated to each drug |
| Outcomes | Somatosensoric evoked potentials (mean wave amplitude, mean proximal conduction time, mean central conduction time) |
| Notes | Full‐text available only in Polish, abstract available in English. Full‐text is awaiting translation before eligibility can be judged |
| Methods | Randomised trial conducted in China 4 treatment arms: LEV, LTG, OXC and TPM |
| Participants | Participants with newly diagnosed, complex partial epilepsy/complex partial secondary generalized seizures Number randomised: LEV = 68, LTG = 70, OXC = 57, TPM = 58 |
| Interventions | Monotherapy with LEV, LTG, OXC or TPM Doses started and achieved not stated |
| Outcomes | "Effective rate" (assumed to be efficacy/seizure freedom) One‐year retention rate Cause of drug withdrawal |
| Notes | Full text of the trial available only in Chinese (English abstract). Awaiting translation of the full text |
Abbreviations: AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHT: phenytoin; TPM: topiramate; ZNS: zonisamide
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | EpiNet‐First Trial 2: Comparison of efficacy of levetiracetam and sodium valproate in people with previously untreated epilepsy who have generalised seizures |
| Methods | Phase 4 randomised, open‐label, pragmatic trial conducted across sites in New Zealand and Europe 2 treatment arms: LEV and VPS |
| Participants | Individuals > 5 years with ≥ 2 spontaneous generalised seizures that require AED treatment (provided all seizures have not been absence seizures) Target sample size = 506 |
| Interventions | Monotherapy with LEV or VPS Target doses LEV: 250 mg‐4000 mg, VPS: 250 mg‐400 mg |
| Outcomes | Time to 12‐month remission from seizures Proportion of participants who achieve a seizure‐free 12‐month remission by 18 months AND who have not changed to a different AED Time to treatment failure due to either inadequate seizure control, or due to unacceptable adverse events Time to treatment failure due to inadequate seizure control. Time to treatment failure due to unacceptable adverse events. Time to first seizure Time to 24‐month remission. Serious adverse events attributed to the trial medication or other AED. Quality of life as assessed by the QOLIE31 and QOLIE48 questionnaires |
| Starting date | May 2015 |
| Contact information | Dr Peter Bergin ([email protected]) |
| Notes | Trial is registered as ACTRN12615000556549 on the Australian New Zealand Clinical Trials Registry and is listed as currently recruiting participants (correct to August 2016) Estimated finish date is May 2018 |
| Trial name or title | EpiNet‐First Trial 3: Comparison of efficacy of levetiracetam and lamotrigine in people with previously untreated epilepsy who have generalised seizures, and for whom sodium valproate is not deemed an acceptable anti‐epileptic drug |
| Methods | Phase 4 randomised, open‐label, pragmatic trial conducted across sites in New Zealand and Europe 2 treatment arms: LEV and LTG |
| Participants | Individuals > 5 years with ≥ 2 spontaneous generalised seizures that require AED treatment (provided all seizures have not been absence seizures) Target sample size = 664 |
| Interventions | Monotherapy with LEV or LTG Target doses LEV: 250 mg‐4000 mg, LTG: 250 mg‐400 mg |
| Outcomes | Time to 12‐month remission from seizures Proportion of participants who achieve a seizure‐free 12‐month remission by 18 months AND who have not changed to a different AED Time to treatment failure due to either inadequate seizure control, or due to unacceptable adverse events Time to treatment failure due to inadequate seizure control. Time to treatment failure due to unacceptable adverse events. Time to first seizure Time to 24‐month remission Serious adverse events attributed to the trial medication or other antiepileptic medication Quality of life as assessed by the QOLIE31 and QOLIE48 questionnaires |
| Starting date | May 2015 |
| Contact information | Dr Peter Bergin ([email protected]) |
| Notes | Trial is registered as ACTRN12615000639527 on the Australian New Zealand Clinical Trials Registry and is listed as currently recruiting participants (correct to August 2016) Estimated finish date is May 2018 |
| Trial name or title | EpiNet‐First Trial 4: Comparison of efficacy of levetiracetam, lamotrigine and sodium valproate in people with previously untreated epilepsy who have unclassified seizures |
| Methods | Phase 4 randomised, open‐label, pragmatic trial conducted across sites in New Zealand and Europe Three treatment arms: LEV, LTG and VPS |
| Participants | Individuals > 5 years with ≥ 2 spontaneous generalised seizures that require AED treatment (provided all seizures have not been absence seizures) Target sample size = 1176 |
| Interventions | Monotherapy with LEV, LTG or VPS Target doses LEV: 250 mg‐4000 mg, LTG 250 mg‐400 mg, VPS: 250 mg‐400 mg |
| Outcomes | Time to 12‐month remission from seizures Proportion of participants who achieve a seizure‐free 12‐month remission by 18 months AND who have not changed to a different AED Time to treatment failure due to either inadequate seizure control, or due to unacceptable adverse events Time to treatment failure due to inadequate seizure control. Time to treatment failure due to unacceptable adverse events. Time to first seizure Time to 24‐month remission Serious adverse events attributed to the trial medication or other AED Quality of life as assessed by the QOLIE31 and QOLIE48 questionnaires |
| Starting date | May 2015 |
| Contact information | Dr Peter Bergin ([email protected]) |
| Notes | Trial is registered as ACTRN12615000640505 on the Australian New Zealand Clinical Trials Registry and is listed as currently recruiting participants (correct to August 2016) Estimated finish date is May 2018 |
| Trial name or title | EpiNet‐First Trial 5: Comparison of efficacy of levetiracetam and lamotrigine in people with previously untreated epilepsy who have unclassified seizures, and for whom sodium valproate is not deemed an acceptable AED |
| Methods | Phase 4 randomised, open‐label, pragmatic trial conducted across sites in New Zealand and Europe 2 treatment arms: LEV and LTG |
| Participants | Individuals > 5 years with ≥ 2 spontaneous generalised seizures that require AED treatment (provided all seizures have not been absence seizures) Target sample size = 664 |
| Interventions | Monotherapy with LEV or LTG Target doses LEV: 250 mg‐4000 mg, LTG: 250 mg‐400 mg |
| Outcomes | Time to 12‐month remission from seizures Proportion of participants who achieve a seizure‐free 12‐month remission by 18 months AND who have not changed to a different AED Time to treatment failure due to either inadequate seizure control, or due to unacceptable adverse events Time to treatment failure due to inadequate seizure control. Time to treatment failure due to unacceptable adverse events. Time to first seizure Time to 24‐month remission Serious adverse events attributed to the trial medication or other antiepileptic medication Quality of life as assessed by the QOLIE31 and QOLIE48 questionnaires |
| Starting date | May 2015 |
| Contact information | Dr Peter Bergin ([email protected]) |
| Notes | Trial is registered as ACTRN12615000641594 on the Australian New Zealand Clinical Trials Registry and is listed as currently recruiting participants (correct to August 2016) . Estimated finish date is May 2018 |
| Trial name or title | EpiNet‐First Trial 1: Comparison of efficacy of levetiracetam, lamotrigine and carbamazepine in people with previously untreated epilepsy who have focal seizures |
| Methods | Phase 4 randomised, open‐label, pragmatic trial conducted across sites in New Zealand and Europe 3 treatment arms: CBZ, LEV and LTG |
| Participants | Individuals > 5 years with ≥ 2 spontaneous generalised seizures that require AED treatment (provided all seizures have not been absence seizures) Target sample size = 1467 |
| Interventions | Monotherapy with CBZ, LEV or LTG Target doses CBZ: 250 mg‐4000 mg, LEV: 250 mg‐4000 mg, LTG: 250 mg‐400 mg |
| Outcomes | Time to 12‐month remission from seizures Proportion of participants who achieve a seizure‐free 12‐month remission by 18 months AND who have not changed to a different AED Time to treatment failure due to either inadequate seizure control, or due to unacceptable adverse events Time to treatment failure due to inadequate seizure control. Time to treatment failure due to unacceptable adverse events. Time to first seizure Time to 24‐month remission Serious adverse events attributed to the trial medication or other antiepileptic medication Quality of life as assessed by the QOLIE31 and QOLIE48 questionnaires |
| Starting date | May 2015 |
| Contact information | Dr Peter Bergin ([email protected]) |
| Notes | Trial is registered as ACTRN12615000643572 on the Australian New Zealand Clinical Trials Registry and is listed as currently recruiting participants (correct to August 2016) Estimated finish date is May 2018 |
| Trial name or title | Cognitive AED outcomes in pediatric localization related epilepsy (COPE) |
| Methods | Phase 3 randomised, single‐blinded (outcome assessor) trial conducted at 12 sites in the USA 3 treatment arms: LEV, LTG and OXC |
| Participants | Children aged 5‐16 at the time of enrolment with localisation‐related partial epilepsy with or without secondary generalised Participants must be AED naive or have had less than a weeks' exposure to AEDs Estimated enrolment = 300 |
| Interventions | Monotherapy with LEV, LTG or OXC Assessments made at 3 and 6 months |
| Outcomes | Conners' Continuous Performance Test (CPT) Confidence Interval Child Behavior Checklist Weschler Intelligence Scale for Children‐IV Processing Speed Story Memory Symbol Digit Modalities Test Grooved Pegboard Columbia Suicidality Severity Rating Scale Youth Self Report Affective Reactivity Scale Pediatric Neuro‐QOL Parenting Stress Index Pediatric Inventory for Parents |
| Starting date | August 2013 |
| Contact information | Emory University |
| Notes | Trial registered as NCT01891890 on ClincalTrials.gov and listed as ongoing but not recruiting participants (correct to August 2016). Estimated finishing date is April 2017. |
| Trial name or title | A study to investigate the safety of the drugs topiramate and levetiracetam in treating children recently diagnosed with epilepsy |
| Methods | Phase 3, randomised, open‐label, parallel‐group trial conducted in multiple centres in the USA, South America, Asia and Europe 2 treatment arms: LEV and TPM |
| Participants | Participants with a clinical diagnosis of new‐onset or recent‐onset epilepsy characterised by partial‐onset seizures (with or without secondary generalisation) or primary generalised tonic‐clonic seizures with no previous treatment for epilepsy (except emergency treatment) Estimated enrolment = 282 |
| Interventions | Monotherapy with LEV or TPM Maximum recommended doses: LEV = 3000 mg/d, TPM = 400 mg/d |
| Outcomes | Percentage of participants with kidney stones Change from baseline in weight Z‐score at month 12 Change from baseline in height at month 12 Change from baseline in bone mineral density (BMD) at Month 12 (other measures of weight, height and bone density specified on trial registration page) |
| Starting date | October 2014 |
| Contact information | Janssen Research & Development |
| Notes | Trial registered as NCT012201251 on ClincalTrials.gov and listed as currently recruiting participants (correct to August 2016). Estimated finishing date is March 2018. |
Abbreviations: AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; VPS: sodium valproate; TPM: topiramate

Network plot of pairwise comparisons in all included studies, studies providing individual participant data (IPD) and studies without IPD
Note that the size of the node indicates the number of studies the drug is included in and the thickness of the edges corresponds to the number of participants contributing to the comparison (i.e. larger node = more studies, thicker edge = more participants).
CBZ: carbamazepine; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

Network plot of pairwise comparisons for all included participants (total 17,961 participants), participants with partial seizures and participants with generalised tonic‐clonic seizures with or without other seizure types (shortened to 'generalised seizures' for brevity).
11978 participants were classified as experiencing partial seizures (66.7% of total), 4407 participants were classified as experiencing generalised seizures (24.5% of total) and 1576 had an unclassified or missing seizure type (8.8% of total).
Note that the size of the node indicates the number of studies the drug is included in and the thickness of the edges corresponds to the number of participants contributing to the comparison (i.e. larger node = more studies, thicker edge = more participants).
CBZ: carbamazepine; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
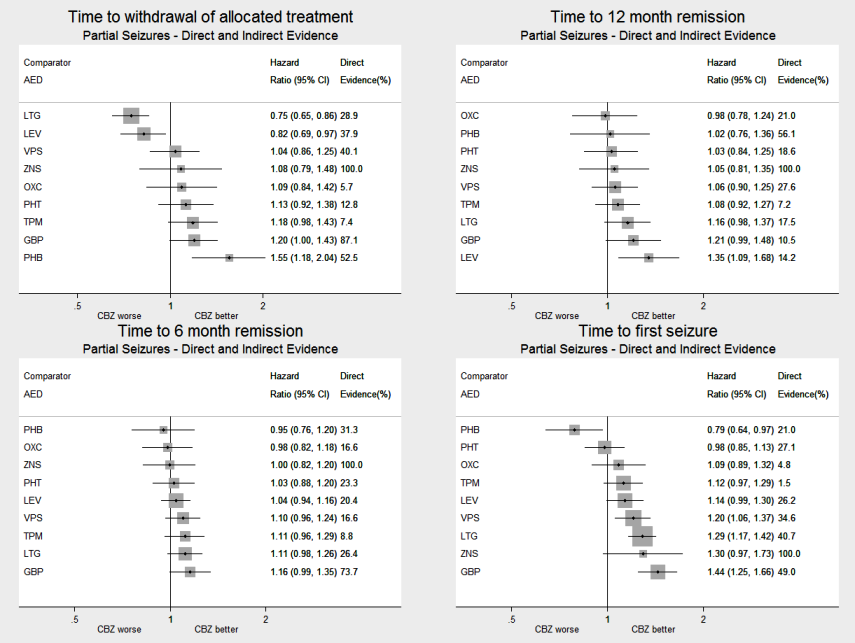
AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with partial seizures, all drugs compared to carbamazepine (CBZ)
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with partial seizures, all drugs compared to lamotrigine (LTG)
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with generalised seizures, all drugs compared to sodium valproate (VPS)
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with partial seizures, all pairwise comparisons for time to withdrawal of allocated treatment and time to 12‐month remission.
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.
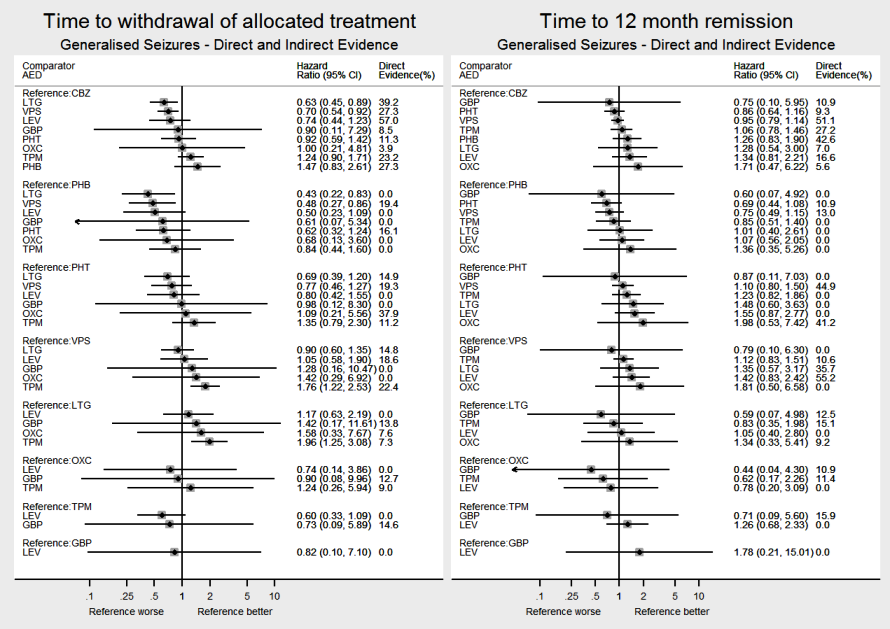
AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with generalised seizures, all pairwise comparisons for time to withdrawal of allocated treatment and time to 12‐month remission.
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with partial seizures, all pairwise comparisons for time to six‐month remission and time to first seizure.
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.
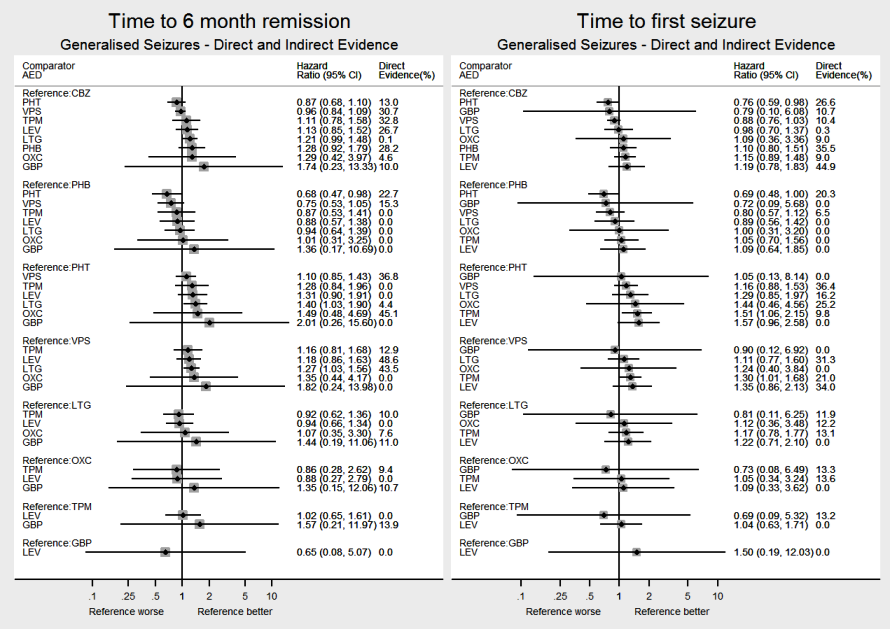
AED: antiepileptic drug; CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Network meta‐analysis results (direct and indirect evidence combined) for individuals with generalised seizures, all pairwise comparisons for time to six‐month remission and time to first seizure.
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Consistency: direct, indirect and network estimates for individuals with partial seizures compared to carbamazepine (CBZ) for time to withdrawal of allocated treatment and time to 12‐month remission.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.
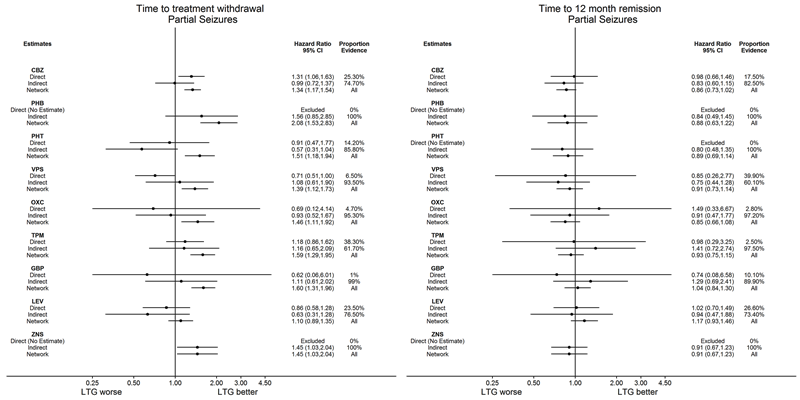
CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Consistency: direct, indirect and network estimates for individuals with partial seizures compared to lamotrigine (LTG) for time to withdrawal of allocated treatment and time to 12‐month remission.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.
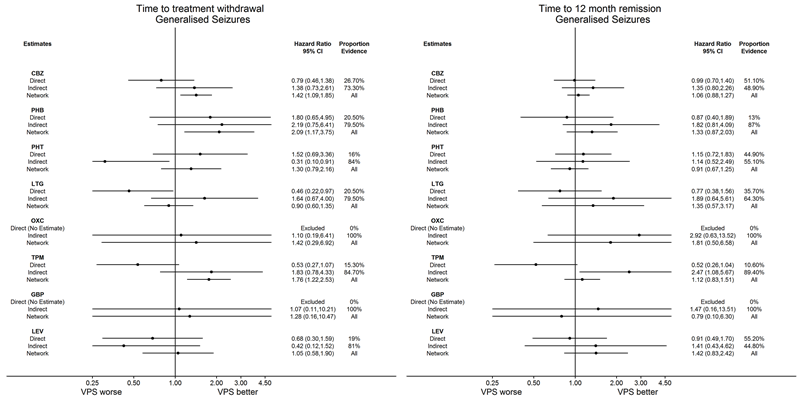
CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Consistency: Direct, Indirect and Network estimates for individuals with generalised seizures compared to sodium valproate (VPS) for time to withdrawal of allocated treatment and time to 12‐month remission.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.
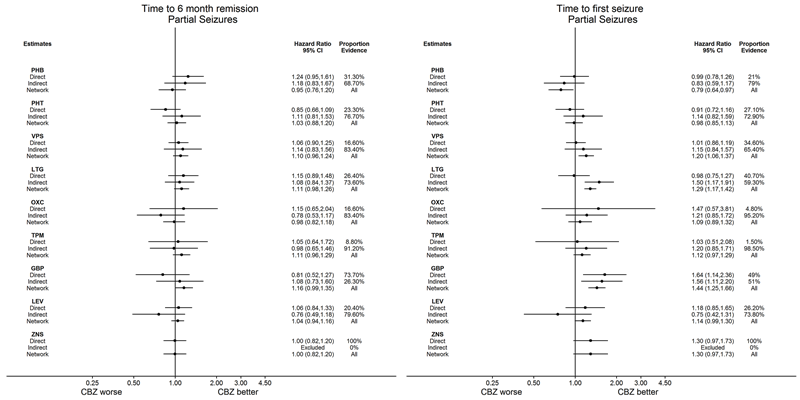
CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Consistency: direct, indirect and network estimates for individuals with partial seizures compared to carbamazepine (CBZ) for time to six‐month remission and time to first seizure.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Consistency: direct, indirect and network estimates for individuals with partial seizures compared to lamotrigine (LTG) for time to six‐month remission and time to first seizure.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.

CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
Consistency: direct, indirect and network estimates for individuals with generalised seizures compared to sodium valproate (VPS) for time to six‐month remission and time to first seizure.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network‐meta‐analysis‐figures.
| Antiepileptic drug monotherapy for epilepsy: time to withdrawal of allocated treatment for individuals with partial seizures | ||||||
| Patient or population: adults and children with partial seizures Settings: outpatients Intervention: phenobarbitone, phenytoin, sodium valproate, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam and zonisamide Comparison: carbamazepine | ||||||
| Intervention (experimental treatment)a,b | Comparison (reference treatment) | No of participants | Relative effect Direct evidence Heterogeneity: I2 | Relative effect Direct plus indirect evidence | Proportion of | Quality of the evidence |
| Phenobarbitone | Carbamazepine | 520 (4 studies) | 1.57 (1.16 to 2.13) I2 = 0% | 1.55 (1.18 to 2.04) | 52.5% | ⊕⊕⊕⊕ |
| Phenytoin | Carbamazepine | 428 (3 studies) | 1.03 (0.74 to 1.42) I2 = 63.6% | 1.13 (0.92 to 1.38) | 12.8% | ⊕⊕⊕⊕ |
| Sodium Valproate | Carbamazepine | 814 (5 studies) | 0.94 (0.73 to 1.19) I2 = 0% | 1.04 (0.86 to 1.25) | 40.1% | ⊕⊕⊕⊕ |
| Lamotrigine | Carbamazepine | 2268 (9 studies) | 0.76 (0.61 to 0.95) I2 = 39.3% | 0.75 (0.65 to 0.86) | 28.9% | ⊕⊕⊕⊕ |
| Oxcarbazepine | Carbamazepine | 562 (2 studies) | 4.62 (0.95 to 22.4) I2 = 0% | 1.09 (0.84 to 1.42) | 5.7% | ⊕⊕⊕⊕ |
| Topiramate | Carbamazepine | 937 (2 studies) | 1.04 (0.52 to 2.07) I2 = 0% | 1.18 (0.98 to 1.43) | 7.4% | ⊕⊕⊕⊕ |
| Gabapentin | Carbamazepine | 954 (2 studies) | 1.14 (0.84 to 1.55) I2 = 0% | 1.20 (1.00 to 1.43) | 87.1% | ⊕⊕⊕⊕ |
| Levetiracetam | Carbamazepine | 1567 (3 studies) | 0.70 (0.52 to 0.94) I2 = 0% | 0.82 (0.69 to 0.97) | 37.9% | ⊕⊕⊕⊕ |
| Zonisamide | Carbamazepine | 583 (1 study) | 1.08 (0.81 to 1.44) I2 = NA) | 1.08 (0.79 to 1.48) | 100% | ⊕⊕⊕⊕ |
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOrder of drugs in the table: drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Antiepileptic drug monotherapy for epilepsy: time to withdrawal of allocated treatment for individuals with partial seizures | ||||||
| Patient or population: adults and children with partial seizures Settings: outpatients Intervention: carbamazepine, phenobarbitone, phenytoin, sodium valproate, oxcarbazepine, topiramate, gabapentin, levetiracetam and zonisamide Comparison: lamotrigine | ||||||
| Intervention (experimental treatment)a,b | Comparison (reference | No of participants | Relative effect Direct evidence Heterogeneity: I2 | Relative effect Direct plus | Proportion of | Quality of the evidence |
| Carbamazepine | Lamotrigine | 2268 (9 studies) | 1.31 (1.05 to 1.64) I2 = 39.3% | 1.34 (1.17 to 1.53) | 28.9% | ⊕⊕⊕⊕ |
| Phenobarbitone | Lamotrigine | No direct evidence | No direct evidence I2: NA | 2.08 (1.52 to 2.86) | 0% | ⊕⊕⊕⊕ |
| Phenytoin | Lamotrigine | 90 (1 study) | 0.91 (0.47 to 1.76) I2: NA | 1.52 (1.18 to 1.92) | 11.6% | ⊕⊕⊕⊕ |
| Sodium Valproate | Lamotrigine | 221 (3 studies) | 0.71 (0.51 to 1.00) I2 = 45.1% | 1.39 (1.11 to 1.72) | 5.1% | ⊕⊕⊕⊝ |
| Oxcarbazepine | Lamotrigine | 506 (1 study) | 0.69 (0.12 to 4.14) I2: NA | 1.46 (1.11 to 1.92) | 4.4% | ⊕⊕⊕⊕ |
| Topiramate | Lamotrigine | 648 (1 study) | 1.18 (0.86 to 1.62) I2: NA | 1.59 (1.29 to 1.95) | 20.9% | ⊕⊕⊕⊕ |
| Gabapentin | Lamotrigine | 659 (1 study) | 0.62 (0.06 to 6.01) I2: NA | 1.60 (1.31 to 1.96) | 1% | ⊕⊕⊕⊕ |
| Levetiracetam | Lamotrigine | 240 (1 study) | 0.86 (0.58 to 1.28) I2: NA | 1.10 (0.89 to 1.35) | 23.7% | ⊕⊕⊕⊕ |
| Zonisamide | Lamotrigine | No direct evidence | No direct evidence I2: NA | 1.45 (1.03 to 2.04) | 0% | ⊕⊕⊕⊕ |
| Abbreviations: CI: confidence interval; HR: hazard Ratio; NA: not applicable | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOrder of drugs in the table: drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Antiepileptic drug monotherapy for epilepsy: time to withdrawal of allocated treatment for individuals with generalised seizures | ||||||
| Patient or population: adults and children with generalised seizures* Settings: outpatients Intervention: carbamazepine, phenobarbitone, phenytoin, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam and zonisamide. Comparison: sodium valproate | ||||||
| Intervention (experimental treatment)a,b | Comparison (reference | No of | Relative effect Direct evidence Heterogeneity: I2 | Relative effect Direct plus | Proportion of | Quality of the evidence |
| Carbamazepine | Sodium Valproate | 405 (4 studies) | 0.79 (0.45 to 1.37) I2 = 6.6% | 1.42 (1.09 to 1.85) | 27.3% | ⊕⊕⊕⊕ |
| Phenobarbitone | Sodium Valproate | 94 (2 studies) | 1.79 (0.65 to 5.00) I2 = 0% | 2.09 (1.17 to 3.75) | 19.4% | ⊕⊕⊕⊝ |
| Phenytoin | Sodium Valproate | 326 (3 studies) | 1.52 (0.68 to 3.33) I2 = 22.6% | 1.30 (0.79 to 2.15) | 19.3% | ⊕⊕⊕⊕ |
| Lamotrigine | Sodium Valproate | 387 (3 studies) | 0.46 (0.22 to 0.97) I2 = 0% | 0.90 (0.60 to 1.35) | 14.8% | ⊕⊕⊕⊕ |
| Oxcarbazepine | Sodium Valproate | No direct evidence | No direct evidence I2: NA | 1.42 (0.29 to 6.92) | 0% | ⊕⊕⊕⊝ |
| Topiramate | Sodium Valproate | 443 (2 studies) | 1.04 (0.52 to 2.07) I2 = 48.5% | 1.76 (1.22 to 2.53) | 22.4% | ⊕⊕⊕⊝ |
| Gabapentin | Sodium Valproate | No direct evidence | No direct evidence I2: NA | 1.28 (0.16 to 10.5) | 0% | ⊕⊕⊕⊝ |
| Levetiracetam | Sodium Valproate | 512 (1 study) | 0.68 (0.30 to 1.59) I2: NA) | 1.05 (0.58 to 1.90) | 18.6% | ⊕⊕⊕⊕ |
| Abbreviations: CI: confidence interval; HR: hazard Ratio; NA: not applicable | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Antiepileptic drug monotherapy for epilepsy: time to 12‐month remission for individuals with partial seizures | ||||||
| Patient or population: adults and children with partial seizures Settings: outpatients Intervention: phenobarbitone, phenytoin, sodium valproate, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam and zonisamide Comparison: carbamazepine | ||||||
| Intervention (experimental treatment)a,b | Comparison (reference treatment) | No of participants | Relative effect Direct evidence Heterogeneity: I2 | Relative effect Direct plus | Proportion of | Quality of the evidence |
| Phenobarbitone | Carbamazepine | 525 (4 studies) | 1.41 (1.04 to 1.91) I2 = 0% | 1.02 (0.76 to 1.35) | 56.1% | ⊕⊕⊕⊕ |
| Phenytoin | Carbamazepine | 430 (3 studies) | 1.00 (0.76 to 1.32) I2 = 54.8% | 1.03 (0.85 to 1.25) | 18.6% | ⊕⊕⊕⊕ |
| Sodium Valproate | Carbamazepine | 816 (5 studies) | 1.03 (0.85 to 1.25) I2 = 46.4% | 1.05 (0.89 to 1.25) | 27.6% | ⊕⊕⊕⊕ |
| Lamotrigine | Carbamazepine | 891 (2 studies) | 1.02 (0.69 to 1.50) I2 = 0% | 1.16 (0.98 to 1.37) | 17.5% | ⊕⊕⊕⊕ |
| Oxcarbazepine | Carbamazepine | 555 (2 studies) | 1.13 (0.62 to 2.05) I2 = 0% | 0.98 (0.78 to 1.25) | 21% | ⊕⊕⊕⊕ |
| Topiramate | Carbamazepine | 925 (2 studies) | 0.94 (0.48 to 1.83) I2 = 0% | 1.08 (0.92 to 1.27) | 7.2% | ⊕⊕⊕⊕ |
| Gabapentin | Carbamazepine | 651 (1 study) | 0.61 (0.06 to 5.82) I2: NA | 1.20 (0.99 to 1.47) | 10.5% | ⊕⊕⊕⊕ |
| Levetiracetam | Carbamazepine | 1567 (3 studies) | 1.08 (0.81 to 1.42) I2 = 60.8% | 1.35 (1.09 to 1.69) | 14.2% | ⊕⊕⊕⊕ |
| Zonisamide | Carbamazepine | 582 (1 study) | 1.05 (0.85 to 1.30) I2: NA | 1.05 (0.81 to 1.35) | 100% | ⊕⊕⊕⊕ |
| Abbreviations: CI: confidence interval; HR: hazard Ratio; NA: not applicable | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOrder of drugs in the table: drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Antiepileptic drug monotherapy for epilepsy: time to 12‐month remission for individuals with partial seizures | ||||||
| Patient or population: adults and children with partial seizures Settings: outpatients Intervention: carbamazepine, phenobarbitone, phenytoin, sodium valproate, oxcarbazepine, topiramate, gabapentin, levetiracetam and zonisamide Comparison: lamotrigine | ||||||
| Intervention (experimental treatment)a,b | Comparison (reference treatment) | No of participants | Relative effect Direct evidence Heterogeneity: I2 | Relative effect Direct plus | Proportion of | Quality of the evidence |
| Carbamazepine | Lamotrigine | 891 (2 studies) | 0.98 (0.67 to 1.45) I2 = 0% | 0.86 (0.72 to 1.02) | 17.5% | ⊕⊕⊕⊕ |
| Phenobarbitone | Lamotrigine | No direct evidence | No direct evidence I2: NA | 0.88 (0.62 to 1.22) | 0% | ⊕⊕⊕⊕ |
| Phenytoin | Lamotrigine | No direct evidence | No direct evidence I2: NA | 0.89 (0.68 to 1.13) | 0% | ⊕⊕⊕⊕ |
| Sodium Valproate | Lamotrigine | 221 (3 studies) | 0.72 (0.56 to 0.93) I2 = 0% | 0.91 (0.73 to 1.33) | 39.9% | ⊕⊕⊕⊕ |
| Oxcarbazepine | Lamotrigine | 499 (1 study) | 1.49 (0.33 to 6.67) I2: NA | 0.85 (0.66 to 1.09) | 2.8% | ⊕⊕⊕⊕ |
| Topiramate | Lamotrigine | 636 (1 study) | 0.98 (0.29 to 3.25) I2: NA | 0.93 (0.75 to 1.15) | 2.5% | ⊕⊕⊕⊕ |
| Gabapentin | Lamotrigine | 647 (1 study) | 0.74 (0.08 to 6.58) I2: NA | 1.04 (0.84 to 1.30) | 10.1% | ⊕⊕⊕⊕ |
| Levetiracetam | Lamotrigine | 240 (1 study) | 1.02 (0.70 to 1.49) I2: NA | 1.16 (0.93 to 1.47) | 26.6% | ⊕⊕⊕⊕ |
| Zonisamide | Lamotrigine | No direct evidence | No direct evidence I2: NA | 0.91 (0.67 to 1.22) | 0% | ⊕⊕⊕⊕ |
| Abbreviations: CI: confidence interval; HR: hazard Ratio; NA: not applicable | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOrder of drugs in the table: drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Antiepileptic drug monotherapy for epilepsy: time to withdrawal of allocated treatment for individuals with generalised seizures | ||||||
| Patient or population: adults and children with generalised seizures* Settings: outpatients Intervention: carbamazepine, phenobarbitone, phenytoin, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam and zonisamide Comparison: sodium valproate | ||||||
| Intervention (experimental treatment)a,b | Comparison (reference treatment) | No of participants | Relative effect Direct evidence | Relative effect Direct plus | Proportion of | Quality of the evidence |
| Carbamazepine | Sodium Valproate | 412 (4 studies) | 0.99 (0.69 to 1.39) I2 = 0% | 1.06 (0.88 to 1.27) | 51.1% | ⊕⊕⊕⊕ |
| Phenobarbitone | Sodium Valproate | 98 (2 studies) | 0.86 (0.40 to 1.89) I2 = 42.3% | 1.33 (0.87 to 2.04) | 13% | ⊕⊕⊕⊕ |
| Phenytoin | Sodium Valproate | 269 (4 studies) | 1.15 (0.71 to 1.82) I2 = 0% | 0.91 (0.67 to 1.25) | 44.9% | ⊕⊕⊕⊕ |
| Lamotrigine | Sodium Valproate | 387 (3 studies) | 0.77 (0.38 to 1.56) I2 = 0% | 1.35 (0.57 to 3.13) | 35.7% | ⊕⊕⊕⊕ |
| Oxcarbazepine | Sodium Valproate | No direct evidence | No direct evidence I2: NA | 1.82 (0.50 to 6.67) | 0% | ⊕⊕⊕⊝ |
| Topiramate | Sodium Valproate | 441 (2 studies) | 0.52 (0.26 to 1.04) I2 = 58.5% | 1.12 (0.83 to 1.52) | 10.6% | ⊕⊕⊕⊕ |
| Gabapentin | Sodium Valproate | No direct evidence | No direct evidence I2: NA | 0.79 (0.10 to 6.25) | 0% | ⊕⊕⊕⊝ |
| Levetiracetam | Sodium Valproate | 512 (1 study) | 0.91 (0.49 to 1.70) I2: NA | 1.41 (0.83 to 2.44) | 55.2% | ⊕⊕⊕⊕ |
| Abbreviations: CI: confidence interval; HR: hazard Ratio; NA: not applicable | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity. aOrder of drugs in the table: drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Trial\Drug | CBZ | PHB | PHT | VPS | LTG | OXC | LEV | TPM | GBP | ZNS | Total | Total randomiseda |
| Trials providing individual participant data | ||||||||||||
| 54 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 108 | 108 | |
| 301 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 282 | 583 | 583 | |
| 0 | 0 | 144 | 0 | 0 | 143 | 0 | 0 | 0 | 0 | 287 | 287 | |
| 0 | 0 | 0 | 69 | 66 | 0 | 0 | 0 | 0 | 0 | 135 | 136 | |
| 66 | 0 | 0 | 0 | 70 | 0 | 0 | 0 | 0 | 0 | 136 | 136 | |
| 63 | 0 | 0 | 0 | 61 | 0 | 0 | 0 | 0 | 0 | 124 | 124 | |
| 48 | 0 | 0 | 0 | 102 | 0 | 0 | 0 | 0 | 0 | 150 | 150 | |
| 291 | 0 | 0 | 0 | 0 | 0 | 288 | 0 | 0 | 0 | 579 | 579 | |
| 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 218 | 0 | 292 | 292 | |
| 0 | 0 | 81 | 85 | 0 | 0 | 0 | 0 | 0 | 0 | 166 | 166 | |
| 54 | 10 | 54 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 167 | 173 | |
| 26 | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 | 52 | 52 | |
| 41 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 | 84 | 84 | |
| 0 | 0 | 94 | 0 | 0 | 99 | 0 | 0 | 0 | 0 | 193 | 193 | |
| 61 | 58 | 63 | 61 | 0 | 0 | 0 | 0 | 0 | 0 | 243 | 243 | |
| 0 | 0 | 0 | 44 | 37 | 0 | 0 | 0 | 0 | 0 | 81 | 81 | |
| 53 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 110 | 110 | |
| 155 | 155 | 165 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 475 | 475 | |
| 236 | 0 | 0 | 244 | 0 | 0 | 0 | 0 | 0 | 0 | 480 | 480 | |
| 202 | 0 | 0 | 0 | 420 | 0 | 0 | 0 | 0 | 0 | 622 | 622 | |
| 19 | 18 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 55 | 55 | |
| 0 | 47 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 94 | 94 | |
| 95 | 97 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 192 | 192 | |
| Privitera 2003 (CBZ branch)b | 129 | 0 | 0 | 0 | 0 | 0 | 0 | 266 | 0 | 0 | 395 | 395 |
| Privitera 2003 (VPS branch)b | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 147 | 0 | 0 | 225 | 225 |
| 0 | 0 | 50 | 86 | 0 | 0 | 0 | 0 | 0 | 0 | 136 | 136 | |
| 0 | 0 | 128 | 0 | 0 | 0 | 0 | 133 | 0 | 0 | 261 | 261 | |
| 121 | 0 | 0 | 0 | 230 | 0 | 0 | 0 | 0 | 0 | 351 | 351 | |
| 151 | 0 | 0 | 149 | 0 | 0 | 0 | 0 | 0 | 0 | 300 | 300 | |
| 378 | 0 | 0 | 0 | 378 | 210 | 0 | 378 | 377 | 0 | 1721 | 1721 | |
| 0 | 0 | 0 | 238 | 239 | 0 | 0 | 239 | 0 | 0 | 716 | 716 | |
| 0 | 0 | 95 | 0 | 86 | 0 | 0 | 0 | 0 | 0 | 181 | 181 | |
| 0 | 0 | 0 | 109 | 117 | 0 | 0 | 0 | 0 | 0 | 226 | 227 | |
| Trinka 2013 (CBZ branch)b | 503 | 0 | 0 | 0 | 0 | 0 | 493 | 0 | 0 | 0 | 996 | 999 |
| Trinka 2013 (VPS branch)b | 0 | 0 | 0 | 353 | 0 | 0 | 350 | 0 | 0 | 0 | 703 | 703 |
| 0 | 0 | 70 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 140 | 140 | |
| 130 | 0 | 0 | 130 | 0 | 0 | 0 | 0 | 0 | 0 | 260 | 260 | |
| 121 | 0 | 0 | 0 | 118 | 0 | 122 | 0 | 0 | 0 | 361 | 361 | |
| Total | 3372 | 439 | 1009 | 1765 | 2024 | 478 | 1253 | 1163 | 595 | 282 | 12,380 | 12,391 |
| Trials not providing individual participant data | ||||||||||||
| Trial\Drug | CBZ | PHB | PHT | VPS | LTG | OXC | LEV | TPM | GBP | ZNS | Total | Total randomiseda |
| 0 | 0 | 18 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 37 | 37 | |
| 36 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 71 | 71 | |
| 0 | 0 | 0 | 0 | 151 | 0 | 0 | 0 | 158 | 0 | 309 | 309 | |
| 59 | 0 | 58 | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 181 | 181 | |
| 17 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 35 | 35 | |
| 14 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 27 | 27 | |
| 26 | 25 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 76 | 76 | |
| 15 | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 31 | 31 | |
| 0 | 0 | 0 | 121 | 0 | 128 | 0 | 0 | 0 | 0 | 249 | 249 | |
| 66 | 0 | 0 | 0 | 0 | 0 | 62 | 0 | 0 | 0 | 128 | 128 | |
| 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 12 | |
| 30 | 30 | 30 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 120 | 120 | |
| 100 | 0 | 0 | 0 | 0 | 94 | 0 | 0 | 0 | 0 | 194 | 194 | |
| 28 | 0 | 0 | 29 | 0 | 55 | 0 | 0 | 0 | 0 | 112 | 112 | |
| 152 | 150 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 302 | 302 | |
| 23 | 0 | 20 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | 64 | |
| 0 | 0 | 0 | 0 | 21 | 27 | 0 | 0 | 0 | 0 | 48 | 48 | |
| 32 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 64 | 64 | |
| 64 | 0 | 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 121 | 121 | |
| 70 | 0 | 0 | 0 | 73 | 0 | 0 | 0 | 0 | 0 | 143 | 143 | |
| 6 | 0 | 0 | 3 | 0 | 0 | 6 | 0 | 0 | 0 | 15 | 15 | |
| 129 | 0 | 0 | 0 | 264 | 0 | 0 | 0 | 0 | 0 | 393 | 393 | |
| 0 | 0 | 0 | 38 | 35 | 0 | 0 | 0 | 0 | 0 | 73 | 73 | |
| 15 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 33 | |
| 66 | 0 | 51 | 46 | 0 | 0 | 0 | 0 | 0 | 0 | 163 | 163 | |
| 0 | 0 | 0 | 0 | 50 | 0 | 50 | 0 | 0 | 0 | 100 | 100 | |
| 0 | 0 | 0 | 0 | 0 | 178 | 175 | 0 | 0 | 0 | 353 | 353 | |
| 215 | 0 | 0 | 0 | 0 | 0 | 218 | 0 | 0 | 0 | 433 | 433 | |
| 23 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 | 43 | |
| 42 | 0 | 45 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 87 | 87 | |
| ? | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 37 | 37 | |
| 0 | 0 | 45 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 94 | 94 | |
| 20 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 40 | |
| 42 | 0 | 0 | 0 | 0 | 0 | 0 | 46 | 0 | 0 | 88 | 88 | |
| 198 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 195 | 0 | 593 | 593 | |
| 92 | 0 | 0 | 0 | 93 | 0 | 0 | 0 | 0 | 0 | 185 | 185 | |
| 0 | 0 | 15 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 33 | |
| 17 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 33 | |
| 30 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 60 | 60 | |
| 88 | 0 | 0 | 30 | 121 | 0 | 0 | 0 | 0 | 0 | 239 | 239 | |
| 0 | 51 | 52 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 151 | 151 | |
| Totalc | 1721 | 315 | 374 | 538 | 1040 | 501 | 645 | 46 | 353 | 0 | 5570 | 5570 |
| Grand totalc | 5093 | 754 | 1383 | 2303 | 3064 | 979 | 1898 | 1209 | 948 | 282 | 17,950 | 17,961 |
| CBZ: carbamazepine; GBP: gabapentin; IPD: individual participant data; ITT: intention to treat; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aDrug allocated missing for 11 participants in the IPD provided. | ||||||||||||
| Trial | Gender | Epilepsy type | Epilepsy type reclassifiedc | ||||||
| Male | Female | Missing | Genb | Partial | Missing | Genb | Partial | Unclassifiedd | |
| 61 (56%) | 47 (44%) | 0 (0%) | 49 (45%) | 59 (55%) | 0 (0%) | 49 (45%) | 59 (55%) | 0 (0%) | |
| 347 (60%) | 236 (40%) | 0 (0%) | 0 (0%) | 583 (100%) | 0 (0%) | 0 (0%) | 583 (100%) | 0 (0%) | |
| 174 (61%) | 113 (39%) | 0 (0%) | 105 (37%) | 182 (63%) | 0 (0%) | 75 (26%) | 182 (63%) | 30 (10%) | |
| 60 (44%) | 75 (55%) | 1 (1%) | 46 (34%) | 82 (60%) | 8 (6%) | 33 (24%) | 82 (60%) | 21 (15%) | |
| 56 (41%) | 80 (59%) | 0 (0%) | 54 (40%) | 82 (60%) | 0 (0%) | 34 (25%) | 82 (60%) | 20 (15%) | |
| 56 (45%) | 68 (55%) | 0 (0%) | 62 (50%) | 62 (50%) | 0 (0%) | 39 (31%) | 62 (50%) | 23 (19%) | |
| 83 (55%) | 67 (45%) | 0 (0%) | 45 (30%) | 105 (70%) | 0 (0%) | 0 (0%) | 105 (70%) | 45 (30%) | |
| 319 (55%) | 260 (45%) | 0 (0%) | 113 (20%) | 466 (80%) | 0 (0%) | 50 (9%) | 466 (80%) | 63 (11%) | |
| 157 (54%) | 135 (46%) | 0 (0%) | 0 (0%) | 292 (100%) | 0 (0%) | 0 (0%) | 292 (100%) | 0 (0%) | |
| 71 (43%) | 92 (55%) | 3 (2%) | 86 (52%) | 80 (48%) | 0 (0%) | 2 (1%) | 80 (48%) | 84 (51%) | |
| 86 (50%) | 81 (47%) | 6 (3%) | 84 (49%) | 89 (51%) | 0 (0%) | 84 (49%) | 89 (51%) | 0 (0%) | |
| 21 (40%) | 31 (60%) | 0 (0%) | 0 (0%) | 52 (100%) | 0 (0%) | 0 (0%) | 52 (100%) | 0 (0%) | |
| 48 (57%) | 36 (43%) | 0 (0%) | 0 (0%) | 84 (100%) | 0 (0%) | 0 (0%) | 84 (100%) | 0 (0%) | |
| 100 (52%) | 93 (48%) | 0 (0%) | 50 (26%) | 143 (74%) | 0 (0%) | 45 (23%) | 143 (74%) | 5 (3%) | |
| 117 (48%) | 126 (52%) | 0 (0%) | 141 (58%) | 102 (42%) | 0 (0%) | 82 (34%) | 102 (42%) | 59 (24%) | |
| 40 (49%) | 41 (51%) | 0 (0%) | 48 (59%) | 29 (36%) | 4 (5%) | 25 (31%) | 29 (36%) | 27 (33%) | |
| 57 (52%) | 53 (48%) | 0 (0%) | 15 (14%) | 95 (86%) | 0 (0%) | 6 (5%) | 95 (86%) | 9 (8%) | |
| 413 (87%) | 58 (12%) | 4 (1%) | 1 (0%) | 474 (100%) | 0 (0%) | 1 (0%) | 474 (100%) | 0 (0%) | |
| 445 (93%) | 35 (7%) | 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | |
| 329 (53%) | 293 (47%) | 0 (0%) | 3 (1%) | 619 (99%) | 0 (0%) | 1 (0%) | 619 (100%) | 2 (0%) | |
| 34 (62%) | 21 (38%) | 0 (0%) | 45 (82%) | 10 (18%) | 0 (0%) | 26 (47%) | 10 (18%) | 19 (35%) | |
| 47 (50%) | 45 (48%) | 2 (2%) | 34 (36%) | 60 (64%) | 0 (0%) | 34 (36%) | 60 (64%) | 0 (0%) | |
| 67 (35%) | 125 (65%) | 0 (0%) | 59 (31%) | 133 (69%) | 0 (0%) | 35 (18%) | 133 (69%) | 24 (13%) | |
| (CBZ branch)a | 215 (54%) | 180 (46%) | 0 (0%) | 88 (22%) | 285 (72%) | 22 (6%) | 51 (13%) | 285 (72%) | 59 (15%) |
| (VPS branch)a | 112 (50%) | 113 (50%) | 0 (0%) | 131 (58%) | 78 (35%) | 16 (7%) | 86 (38%) | 78 (35%) | 61 (27%) |
| 73 (54%) | 63 (46%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 110 (81%) | 0 (0%) | 26 (19%) | |
| 126 (48%) | 135 (52%) | 0 (0%) | 150 (57%) | 53 (20%) | 58 (22%) | 80 (31%) | 53 (20%) | 128 (49%) | |
| 188 (54%) | 163 (46%) | 0 (0%) | 114 (32%) | 237 (68%) | 0 (0%) | 71 (20%) | 237 (68%) | 43 (12%) | |
| 153 (51%) | 147 (49%) | 0 (0%) | 154 (51%) | 146 (49%) | 0 (0%) | 87 (29%) | 146 (49%) | 67 (22%) | |
| 922 (54%) | 755 (44%) | 44 (3%) | 25 (1%) | 1491 (87%) | 205 (12%) | 16 (1%) | 1491 (87%) | 214 (12%) | |
| 420 (59%) | 282 (39%) | 14 (2%) | 463 (65%) | 52 (7%) | 201 (28%) | 397 (55%) | 52 (7%) | 267 (37%) | |
| 101 (56%) | 80 (44%) | 0 (0%) | 91 (50%) | 90 (50%) | 0 (0%) | 55 (30%) | 90 (50%) | 36 (20%) | |
| 114 (50%) | 112 (49%) | 1 (0%) | 32 (14%) | 154 (68%) | 41 (18%) | 29 (13%) | 154 (68%) | 44 (19%) | |
| (CBZ branch)a | 551 (55%) | 448 (45%) | 0 (0%) | 141 (14%) | 858 (86%) | 0 (0%) | 48 (5%) | 858 (86%) | 93 (9%) |
| (VPS branch)a | 398 (57%) | 305 (43%) | 0 (0%) | 513 (73%) | 190 (27%) | 0 (0%) | 285 (41%) | 190 (27%) | 228 (32%) |
| 73 (52%) | 67 (48%) | 0 (0%) | 77 (55%) | 63 (45%) | 0 (0%) | 42 (30%) | 63 (45%) | 35 (25%) | |
| 122 (47%) | 138 (53%) | 0 (0%) | 152 (58%) | 108 (42%) | 0 (0%) | 152 (58%) | 108 (42%) | 0 (0%) | |
| 215 (60%) | 146 (40%) | 0 (0%) | 0 (0%) | 361 (100%) | 0 (0%) | 0 (0%) | 361 (100%) | 0 (0%) | |
| Total | 6971(56%) | 5345 (43%) | 75 (1%) | 3307 (27%) | 8529 (69%) | 555 (4%) | 2130 (17%) | 8529 (69%) | 1732 (14%) |
| aTrials designed in two strata based on whether recommended treatment would be CBZ or VPS. Within the two strata, participants were randomised to TPM in Privitera 2003/LEV in Trinka 2013 or CBZ/VPS depending on the strata. Data analysed according to the separate strata (CBZ branch or VPS branch) in this review. | |||||||||
| Trial | Age (years) | Epilepsy duration (years) | Number of seizures in the last 6 months | |||||||
| Mean | SD | Range | Missing | Median | Range | Missing | Median | Range | Missing | |
| 5.7 | 3.5 | 1 to 15 | 0 | 1.2 | 0 to 11.5 | 0 | 24 | 1 to 7200 | 5 | |
| 36.4 | 15.9 | 18 to 75 | 0 | 0.2 | 0 to 17.7 | 30 | 2 | 1 to 30 | 1 | |
| 26.8 | 10.7 | 15 to 91 | 1 | 0.4 | 0 to 25 | 0 | 3 | 0 to 252 | 0 | |
| 32 | 14.5 | 12 to 76 | 0 | 1 | 0 to 53 | 27 | 2 | 0 to 100 | 2 | |
| 34 | 15.8 | 14 to 71 | 0 | 1 | 0 to 18 | 0 | 4 | 1 to 960 | 0 | |
| 30 | 14.1 | 14 to 81 | 0 | 0.5 | 0 to 19.4 | 0 | 3 | 1 to 1020 | 0 | |
| 76.9 | 6 | 65 to 94 | 0 | NA | NA | 150 | 3 | 0 to 163 | 0 | |
| 39 | 16.2 | 15 to 82 | 0 | NA | NA | 579 | 3 | 1 to 1410 | 4 | |
| 35 | 16.6 | 12 to 86 | 0 | 0.5 | 0 to 7.7 | 5 | 4 | 1 to 146 | 6 | |
| 78.2 | 7.1 | 61 to 95 | 3 | NA | NA | 166 | 3 | 0 to 99 | 3 | |
| 9.9 | 3.6 | 3 to 16 | 6 | 0.5 | 0 to 13.7 | 6 | 3 | 1 to 900 | 6 | |
| 10.8 | 2.3 | 4 to 15 | 0 | NA | NA | 52 | 3 | 1 to 60 | 0 | |
| 8.8 | 2.1 | 5 to 13 | 0 | 0.4 | 0 to 4.5 | 0 | 3 | 2 to 11 | 0 | |
| 18.6 | 9.7 | 5 to 53 | 1 | 0.4 | 0 to 20 | 0 | 2 | 0 to 157 | 0 | |
| 32.3 | 14.8 | 13 to 77 | 3 | 1 | 0 to 40 | 4 | 2 | 1 to 579 | 3 | |
| 33.9 | 10.9 | 16 to 56 | 0 | NA | NA | 81 | 1 | 0 to 540 | 0 | |
| 35.8 | 12.2 | 16 to 60 | 0 | NA | NA | 110 | 2 | 0 to 200 | 0 | |
| 41 | 15.5 | 18 to 82 | 4 | 2 | 0.5 to 59 | 5 | 1 | 1 to 100 | 7 | |
| 47.1 | 16.1 | 18 to 83 | 0 | 3 | 1 to 68 | 19 | 12 | 1 to 2248 | 38 | |
| 27.2 | 21.4 | 2 to 84 | 1 | NA | NA | 622 | 3 | 1 to 9000 | 0 | |
| 27.5 | 8.5 | 14 to 55 | 0 | 7 | 3 to 11.5 | 18 | 12 | 6 to 42 | 0 | |
| 11.4 | 5 | 2 to 18 | 0 | 2.5 | 0.5 to 17 | 2 | NA | NA | 94 | |
| 29 | 17.6 | 2 to 68 | 0 | 5 | 0.5 to 44 | 0 | 2 | 0 to 100 | 0 | |
| (CBZ branch)a | 34.4 | 18.4 | 6 to 80 | 0 | NA | NA | 395 | 4 | 0 to 2400 | 0 |
| (VPS branch)a | 32.8 | 19.4 | 6 to 84 | 0 | NA | NA | 225 | 4 | 0 to 20000 | 0 |
| 20.9 | 14.2 | 3 to 64 | 0 | 0 | 0 to 3 | 0 | NA | NA | 136 | |
| 34.1 | 14.8 | 12 to 78 | 0 | NA | NA | 261 | 4 | 0 to 570 | 0 | |
| 32.1 | 14.2 | 12 to 72 | 2 | 0.7 | 0 to 27 | 3 | 3 | 1 to 145 | 1 | |
| 33 | 14.9 | 16 to 79 | 2 | NA | NA | 300 | 4 | 2 to 101 | 5 | |
| 38.4 | 18.3 | 5 to 86 | 44 | NA | NA | 1721 | 4 | 0 to 1185 | 49 | |
| 22.5 | 14.1 | 5 to 77 | 14 | NA | NA | 716 | 3 | 0 to 2813 | 17 | |
| 34.1 | 16.7 | 13 to 75 | 1 | 1.3 | 0 to 28.5 | 1 | 3 | 1 to 600 | 0 | |
| 36 | 16.9 | 13 to 80 | 2 | NA | NA | 227 | 18 | 6 to 1080 | 37 | |
| (CBZ branch)1 | 42.8 | 17.2 | 16 to 89 | 0 | NA | NA | 999 | NA | NA | 999 |
| (VPS branch)1 | 36.5 | 17.8 | 16 to 85 | 1 | NA | NA | 703 | NA | NA | 703 |
| 35.2 | 16.1 | 14 to 70 | 0 | 0.75 | 0.1 to 30 | 0 | 2 | 0 to 60 | 0 | |
| 10.1 | 2.9 | 5 to 16 | 13 | 0.3 | 0 to 5.9 | 32 | 3 | 1 to 104 | 12 | |
| 71.5 | 7.2 | 60 to 95 | 0 | NA | NA | 361 | 2 | 1 to 96 | 7 | |
| Total (missing) | 98 | 7820 | 2135 | |||||||
| Abbreviations: SD: Standard deviation aTrials designed in two strata based on whether recommended treatment would be CBZ or VPS. Within the two strata, participants were randomised to TPM in Privitera 2003/LEV in Trinka 2013 or CBZ/VPS depending on the strata. Data analysed according to the separate strata (CBZ branch or VPS branch) in this review. | ||||||||||
| Trial | Electroencephalographic (EEG) | Computerised Tomography (CT) /Magnetic Resonance Imaging (MRI) | Neurological exams | ||||||
| Normal | Abnormal | Missing | Normal | Abnormal | Missing | Normal | Abnormal | Missing | |
| 49 (45%) | 54 (50%) | 5 (5%) | 21 (19%) | 5 (5%) | 82 (76%) | 0 (0%) | 0 (0%) | 108 (100%) | |
| 0 (0%) | 0 (0%) | 583 (100%) | 0 (0%) | 0 (0%) | 583 (100%) | 478 (82%) | 103 (18%) | 2 (0%) | |
| 126 (44%) | 152 (53%) | 9 (3%) | 173 (60%) | 69 (24%) | 45 (16%) | 0 (0%) | 0 (0%) | 287 (100%) | |
| 0 (0%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 136 (100%) | 89 (65%) | 46 (34%) | 1 (1%) | |
| 62 (46%) | 72 (53%) | 2 (1%) | 82 (60%) | 12 (9%) | 42 (31%) | 123 (90%) | 13 (10%) | 0 (0%) | |
| 76 (61%) | 42 (34%) | 6 (5%) | 72 (58%) | 20 (16%) | 32 (26%) | 108 (87%) | 16 (13%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 150 (100%) | 62 (41%) | 87 (58%) | 1 (1%) | 90 (60%) | 60 (40%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 579 (100%) | 0 (0%) | 0 (0%) | 579 (100%) | 493 (85%) | 86 (15%) | 0 (0%) | |
| 107 (37%) | 179 (61%) | 6 (2%) | 0 (0%) | 0 (0%) | 292 (100%) | 0 (0%) | 0 (0%) | 292 (100%) | |
| 28 (17%) | 74 (45%) | 64 (39%) | 0 (0%) | 0 (0%) | 166 (100%) | 0 (0%) | 0 (0%) | 166 (100%) | |
| 0 (0%) | 0 (0%) | 173 (100%) | 0 (0%) | 0 (0%) | 173 (100%) | 152 (88%) | 15 (9%) | 6 (3%) | |
| 18 (35%) | 34 (65%) | 0 (0%) | 50 (96%) | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 52 (100%) | |
| 6 (7%) | 78 (93%) | 0 (0%) | 75 (89%) | 9 (11%) | 0 (0%) | 83 (99%) | 1 (1%) | 0 (0%) | |
| 92 (48%) | 99 (51%) | 2 (1%) | 126 (65%) | 12 (6%) | 55 (28%) | 0 (0%) | 0 (0%) | 193 (100%) | |
| 0 (0%) | 0 (0%) | 243 (100%) | 0 (0%) | 0 (0%) | 243 (100%) | 222 (91%) | 19 (8%) | 2 (1%) | |
| 0 (0%) | 0 (0%) | 81 (100%) | 0 (0%) | 0 (0%) | 81 (100%) | 0 (0%) | 0 (0%) | 81 (100%) | |
| 58 (53%) | 52 (47%) | 0 (0%) | 74 (67%) | 36 (33%) | 0 (0%) | 110 (100%) | 0 (0%) | 0 (0%) | |
| 126 (27%) | 343 (72%) | 6 (1%) | 308 (65%) | 119 (25%) | 48 (10%) | 0 (0%) | 0 (0%) | 475 (100%) | |
| 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | 0 (0%) | 480 (100%) | |
| 0 (0%) | 0 (0%) | 622 (100%) | 0 (0%) | 0 (0%) | 622 (100%) | 0 (0%) | 0 (0%) | 622 (100%) | |
| 0 (0%) | 0 (0%) | 55 (100%) | 37 (67%) | 0 (0%) | 18 (33%) | 55 (100%) | 0 (0%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 94 (100%) | 0 (0%) | 0 (0%) | 94 (100%) | 24 (26%) | 70 (74%) | 0 (0%) | |
| 180 (94%) | 12 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 192 (100%) | 0 (0%) | 0 (0%) | 192 (100%) | |
| (CBZ branch)a | 0 (0%) | 0 (0%) | 395 (100%) | 0 (0%) | 0 (0%) | 395 (100%) | 0 (0%) | 0 (0%) | 395 (100%) |
| (VPS branch)a | 0 (0%) | 0 (0%) | 225 (100%) | 0 (0%) | 0 (0%) | 225 (100%) | 0 (0%) | 0 (0%) | 225 (100%) |
| 0 (0%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 136 (100%) | |
| 0 (0%) | 0 (0%) | 261 (100%) | 0 (0%) | 0 (0%) | 261 (100%) | 0 (0%) | 0 (0%) | 261 (100%) | |
| 13 (4%) | 13 (4%) | 325 (93%) | 16 (5%) | 5 (1%) | 330 (94%) | 305 (87%) | 46 (13%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 300 (100%) | 0 (0%) | 0 (0%) | 300 (100%) | 0 (0%) | 0 (0%) | 300 (100%) | |
| 0 (0%) | 0 (0%) | 1721 (100%) | 0 (0%) | 0 (0%) | 1721 (100%) | 1267 (74%) | 410 (24%) | 44 (3%) | |
| 0 (0%) | 0 (0%) | 716 (100%) | 0 (0%) | 0 (0%) | 716 (100%) | 595 (83%) | 107 (15%) | 14 (2%) | |
| 103 (57%) | 71 (39%) | 7 (4%) | 111 (61%) | 33 (18%) | 37 (20%) | 165 (91%) | 16 (9%) | 0 (0%) | |
| 51 (22%) | 121 (53%) | 55 (24%) | 0 (0%) | 0 (0%) | 227 (100%) | 0 (0%) | 0 (0%) | 227 (100%) | |
| (CBZ branch)1 | 0 (0%) | 0 (0%) | 999 (100%) | 0 (0%) | 0 (0%) | 999 (100%) | 0 (0%) | 0 (0%) | 999 (100%) |
| (VPS branch)1 | 0 (0%) | 0 (0%) | 703 (100%) | 0 (0%) | 0 (0%) | 703 (100%) | 0 (0%) | 0 (0%) | 703 (100%) |
| 70 (50%) | 70 (50%) | 0 (0%) | 17 (12%) | 10 (7%) | 113 (81%) | 0 (0%) | 0 (0%) | 140 (100%) | |
| 0 (0%) | 0 (0%) | 260 (100%) | 0 (0%) | 0 (0%) | 260 (100%) | 0 (0%) | 0 (0%) | 260 (100%) | |
| 117 (32%) | 242 (67%) | 2 (1%) | 78 (22%) | 282 (78%) | 1 (0%) | 0 (0%) | 0 (0%) | 361 (100%) | |
| Total | 1282 (10%) | 1708 (14%) | 9401 (75%) | 1302 (11%) | 701 (6%) | 10,388 (83%) | 4359 (36%) | 1008 (8%) | 7024 (56%) |
| aTrials designed in two strata based on whether recommended treatment would be CBZ or VPS. Within the two strata, participants were randomised to TPM in Privitera 2003/LEV in Trinka 2013 or CBZ/VPS depending on the strata. Data analysed according to the separate strata (CBZ branch or VPS branch) in this review. | |||||||||
| Trial | Summary of resultsb |
| 1. MANOVA revealed no significant interaction effect of group and time 2. MANOVA revealed no significant interaction effect of group and time 3. MANOVA revealed no significant interaction effect of group and time 4. MANOVA revealed no significant interaction effect of group and time | |
| 1. CBZ: 64%, PHB: 63% 2. No statistically significant difference between groups 3. No statistically significant difference between groups 4. Mean seizure frequency: CBZ: 0.66, PHB: 0.8 5. Mean duration (seconds): CBZ: 12.63; PHB: 15 | |
| 1. Median time to exit: GBP: 69 days, LTG: 48 days HR: 1.043 (95% confidence interval 0.602 to 1.809) 2. Proportion of evaluable population completing the study – GBP: 71.6%, LTG: 67.1% No difference between groups for time to withdrawal for any reason 3. No difference between groups for time to first seizure 4. GBP: 76.1%, LTG: 76.8% (ITT population) 5. Withdrawals during titration: GBP: 7, LTG: 10 Withdrawals after titration: GBP: 10, LTG: 13 | |
| 1a. PHT: 67%; CBZ: 37%; VPS: 53% 2. PHT: 10%; CBZ: 8%; VPS:11% | |
| 1. CBZ: 76%, LEV: 76% 2. Proportion with AEs: CBZ: 65%, LEV: 50% 3. CBZ: 2 discontinuations due to failure to control seizures and interactions with other medications LEV: 3 discontinuations – 1 death from stroke and 2 due to AEs | |
| 1. No significant difference between groups 2. No significant difference between groups | |
| 1. No significant difference between groups 3. 2 children from PHB group, 1 child from CBZ group and no children from VPS group withdrew from the study because of allergic reactions 4. No significant difference between groups | |
| 1. Overall effect on sleep parameters was comparable between groups. LEV group PSG significant increase post treatment compared to baseline in sleep efficiency (P = 0.039) and in decrease of wake time after sleep onset (P = 0.047), no significant change in other sleep parameters. CBZ group post treatment compared to baseline significant increases in the percentage of slow wave sleep (P = 0.038), no significant change in other sleep parameters 2. No significant difference between baseline and post‐treatment between the 2 groups | |
| 1. OXC 56.6% ; VPS 53.8% 2. No significant difference between groups 3. OXC 40.6% ; VPS 33.9% 4. Efficacy no significant difference between groups Tolerability no significant difference between groups Therapeutic effect no significant difference between groups 5. Proportion of participants experiencing at least 1 AE regardless of relationship to trial drug OXC 89.8%; VPS 87.6% 6. Seizure frequency per week OXC (n = 106) mean 0.17 median 0, VPS (n = 106) mean 0.40, median 0 | |
| 1. No significant difference between groups 2. Completed study LEV 52/62, CBZ 54/66, withdrawals: 8 poor compliance (LEV 4, CBZ 4); 7 severe adverse effect (LEV 3, CBZ 4); 7 unknown cause (LEV 3, CBZ 4) 3. Attention deficit on digital span end of follow up greater in CBZ group than LEV (P = 0.03) Stroop test worse in CBZ than LEV (P = 0.02) No significant difference between groups for other scales. Impairment of activities of daily living greater CBZ than LEV (P = 0.05) 4. 4 participants (LEV 2, CBZ 2) had abnormal EEG at baseline, normal at end of treatment. Drug dose reduction (LEV 4, CBZ 2). Remaining participants unmodified versus baseline 5. No significant difference between groups | |
| 1. Significant decrease in visual‐verbal memory for CBZ and acoustic memory for PHB. No significant differences for other tests | |
| 1. PHB: 60%, PHT: 59%; CBZ: 62%; VPS: 64% | |
| 1. Baseline OXC mean 2.9 (SD 7.0), median 1, range 0‐60 CBZ mean 5.8 (SD 14.7) median 1, range 0‐99 Maintenance phases OXC mean 0.4 (SD 3.0) median 0, range 0‐27 CBZ mean 0.3 (SD 1.4) median 0, range 0‐12 2. Severe side effects CBZ 25, OXC 13, statistically significant difference favouring OXC (P = 0.04) Participants without any side effects CBZ 25, OXC 29 no significant difference between groups (P = 0.22) 3. Global efficacy no significant difference between groups (P = 0.77); global tolerability (P = 0.11) Participants very good/good CBZ 69 (73%), OXC 76 (84%) Participants poor/very poor CBZ 26 (27%), OXC 15 (16%) 4. Nature of side effects same between groups, included tiredness, headache, dizziness, ataxia. Participants withdrawn due to severe side effects CBZ 16, OXC 9 5. Clinically relevant changes observed in 2 participants only, both CBZ group, both stopped treatment | |
| 1. Comparison of cognitive results no significant difference between treatment groups (P = 0.195) No significant difference between treatment groups for secondary variables (psychomotor speed, alertness, memory and learning, attention, intelligence scores) 2. OXC 58%; CBZ 46%; VPS 54% 3. Most common (> 10% reported) side effects OXC fatigue and headache; CBZ fatigue and rash VPS headache, increased appetite, alopecia 4. Good/very good: OXC investigators 84%, participants 82%, parents/carers 86%; Combined CBZ/VPS investigators 77%, participants 73%, parents/carers 80% | |
| 1. Minor adverse effects reported in PHB: 58 participants (39%) reported 86 AEs, CBZ: 46 participants (30%) reported 68 AEs 2. All withdrawals: PHB: 18%, CBZ: 17% Withdrawals due to side‐effects: PHB: 5%, CBZ: 3% 3. Seizure‐free: PHB: 54%, CBZ: 52% > 50% reduction of seizures: PHB: 23%, CBZ: 29% 50% reduction‐50% increase in seizures: PHB: 15%, CBZ: 13% > 50% increase in seizures: PHB: 8%, CBZ: 6% | |
| 1. Significant difference favouring VPS test of speed of information processing No significant differences between treatment groups for any other cognitive tests 2. PHT: 30%; CBZ: 39%; VPS:33% | |
| 1. Seizure freedom: LTG: 38%, OXC: 44% < 50% seizure reduction: LTG: 48%, OXC: 55% 2. Both groups showed improvement in verbal learning and in 1/4 measures of attention. In addition, participants under OXC improved in word fluency. Improved mood was reported with OXC only. | |
| 1. Number of participants experiencing early seizures as first event: LTG 2/32, CBZ 3/32 Number of participants remaining seizure‐free in the follow‐up period: LTG 23/32 (72%), CBZ 14/32 (44%) P = 0.05 2. Incidence of side effects: LTG 2/32 (6.25%), CBZ 12/32 (37.5%) P = 0.05 3. Withdrawals from study due to side effects LTG 1/32 (3%), CBZ 10/32 (31%), P = 0.02 | |
| 1. No difference between groups in terms of social competence; school competence; internalising behaviour problems; externalising behaviour problems; total behaviour problems and anxiety. Significant decrease in depression in LEV group compared to CBZ group (P = 0.027) 2. LEV 95.7% , CBZ 97.1% , P = 0.686 3. LEV 66.7%, CBZ 57.8% , P = 0.317 4. LEV 33.3%, CBZ 46.9%. Number of AEs not significantly different between groups | |
| 1.CBZ: 53% LTG: 56% 2. No significant difference between groups in overall cognitive score. In terms of individual assessments, only Stroop test B showed a statistically significant advantage for LTG. | |
| 1. No significant difference between groups 2. No significant difference between groups | |
| 1. LTG: 65% CBZ: 70% 2. Total seizure‐free rate LTG: 62% CBZ: 63% Time to first seizure: mean (SD): weeks LTG: 10 (5.09), CBZ: 10.82 (6.44) | |
| 1. LTG: 54%, VPS: 55 %, no difference by seizure type 2. LTG: 69%, VPS:68 % | |
| 1. No significant differences between treatment groups 2. Compliance: trend towards better compliance in CBZ group (not significant) Randomised participants only: trend towards higher rate withdrawal from treatment in PHB group (not significant). More mild systemic side‐effects in CBZ group (significant). 3 children switched from CBZ to PHB and 1 from PHB to CB following adverse reactions 3. 6 months: excellent/good: PHB = 15, CBZ = 13 12 months: excellent/good: PHB = 13, CBZ = 9 | |
| 1. Partial seizures ‐ PHT: 32%; CBZ: 40%; VPS : 41% Generalised seizures ‐ PHT :35%; CBZ: 15%; VPS: 7% 1. Partial seizures ‐ PHT: 24%; CBZ: 24%; VPS : 25% Generalised seizures ‐ PHT :13%; CBZ: 0%; VPS: 0% | |
| 1. Seizure recurrence at 2 weeks ‐ LTG: 43% LEV: 35%, p=0.42 Seizure recurrence at 4 weeks ‐ LTG: 39% LEV: 33%, p=0.53 Seizure recurrence at 8 weeks ‐ LTG: 35% LEV: 28%, p=0.50 Seizure recurrence at 12 weeks ‐ LTG: 33% LEV: 24%, p=0.35 Seizure recurrence at 20 weeks ‐ LTG: 31% LEV: 13%, p=0.03 2. No significant difference between groups 3. Proportion with AEs ‐ LTG: 53%, LEV: 67% | |
| 1. LEV: 12.7%, OXC: 23.4% 2. Median months: LEV: 7.6, OXC: NA (fewer than 50% of participants in the OXC group had seizure recurrence) 3. LEV: 53.8%, OXC: 58.5% 4. LEV: 34.7%, OXC: 40.9% | |
| 1. LEV: 47.3%, CBZ: 68.4% 2. LEV: 48.4%, CBZ: 70.2% 3. Number of events: LEV: 88, CBZ: 45 4. Number of events: LEV: 87, CBZ: 39 5. Number of events: LEV: 97, CBZ: 57 | |
| 1. Compared to CBZ, participants on PHT became slower (motor speed of the hand) and their visual memory decreased. There was an equal decrease in negative mood (helplessness, irritability, depression) on PHT and CBZ 2. 3 participants taking PHT complained of tiredness, and 1 participant taking CBZ complained of facial skin problems, another tiredness and memory problems | |
| 1. Incidence of major side effects (proportion of analysed participants): PHT 23%; CBZ: 23% Minor side effects: cognitive impairment and sedation twice as likely on CBZ compared to PHT. Other minor side effects similar between groups. 2. Treatment failures among analysed participants: Seizure control (among analysed participants with no major side effects): PHT: 86%; CBZ: 82% 3. Significantly lower mean LDH level at 24 weeks in CBZ participants than PHT participants. Other laboratory results similar across treatment groups | |
| 1. 8 discontinuations; due to generalised rash (n = 1), excessive tiredness (n = 1), withdrew consent (n = 2), renal transplant (n = 1), lost to follow‐up (n = 2), died (n = 1) 2. 6 participants reported treatment‐emergent side effects. 3. No participants withdrew due to lack of seizure control | |
| 1(a). PHT: 51%, VPS: 49% 1(b). PHT : 24%, VPS: 35% 1(c). PHT: 18%, VPS: 10% 1(d). PHT: 2%, VPS: 6% 2. All reported AEs were minor and similar rates between groups | |
| 1. No significant differences between any tests of cognitive function taken before treatment and after 10‐12 weeks for both treatment groups | |
| 1. Six months of seizure freedom: CBZ: 81%, TPM: 91% 50% reduction of seizures: CBZ: 84% TPM: 97% The average number of seizures was significantly less in the TPM group compared to the CBZ group at 6 and 9 months 2. AEs were mild and similar between groups 3. No significant differences between groups | |
| 1. Significant difference between 3 treatment groups (P = 0.00022) CBZ more early terminators than GBP (P = 0.008) or LTG (P < 0.0001) 2. LTG 51.4%, GBP 47.4%, CBZ 64.3% no significant difference between groups P = 0.09 3. No difference between groups for time to first, second, fifth and tenth seizure (P values = 0.18, 0.13, 0.74, 0.95 respectively) 4. More systemic toxicities on GBP than CBZ or LTG No significant differences in neuro‐toxicities between treatment groups over 12 months 5. Mean serum levels: 6 weeks GBP 8.67 ± 4.83; µg/mL, CBZ 6.79 ± 2.92 µg/mL and LTG 2.87 ± 1.60 µg/mL 52 weeks GBP 8.54 ± 5.57 µg/mL, CBZ 6.48 ± 3.72 µg/mL and LTG 3.46 ± 1.68 µg/mL Overall medical compliance 89% without significant group differences 6. 3 months LTG 49.7%, GBP 43.3%, CBZ 36.0% significant difference between groups P = 0.02 6 months LTG 37.2%, GBP 33.0%, CBZ 28.9% no significant difference between groups P = 0.22 12 months LTG 28.6%, GBP 23.2%, CBZ 22.8% no significant difference between groups P = 0.33 | |
| 1. LTG 68 (73%), CBZ 61 (67%), no significant difference between groups 2. LTG 59 (63%), CBZ 69 (76%), not significant difference P = 0.068 ITT analysis 3. LTG 71 (76%), CBZ 81 (89%), significant difference, P = 0.0234 ITT analysis 4.Hazard ratio (lamotrigine/carbamazepine) 1.50, 95% CI 0.94–2.40, p value 0.092 5. During treatment period LTG 82 (88%) reported 378 AEs, CBZ 79 (86%) reported 310 AEs. No significant differences between groups for any AEs except for immune system Withdrew due to AE LTG 13 (14%), CBZ 23 (25%), P = 0.078 6. No difference between groups even when changes over time corrected for age, gender and baseline score | |
| 1. PHT: 33%; VPS: 39% 2. All reported AEs were minor and similar rates between groups | |
| 1. VPS 7/11 (64%), CBZ 9/14 (64%) 2. At least one AE reported VPS 15/16 (94%), CBZ 16/17 (94%) | |
| 1. FE CBZ group 83/88 (94.3%), LTG group 78/88 (88.6%) no significant difference between groups GE VPS group 25/30 (83.3%) LTG group 20/33 (60.6%) no significant difference between groups 2. FE CBZ group 81%, LTG group 91%, not a significant difference between groups GE VPS group 97%, LTG group 88%, not stated as significant or non‐significant difference 3. At least 1 AE FE CBZ 81 participants (91%), LTG 68 participants (77.3%) GE VPS 25 participants (83.3%), LTG 24 participants (72.7%) Serious AEs FE CBZ 8 participants (9%), LTG 6 participants (7%) GE VPS 1 participant (3%), LTG 5 participants (15%) AEs considered related to study drug FE CBZ 65 participants (74%), LTG 38 participants (43%) GE VPS 16 participants (53%), LTG 15 participants (45.5%) | |
| 1. Mean quality‐of‐life score at baseline CBZ group 31.14 ± 1.83, LEV group 29.76 ± 1.71 (P value = 0.5861) Mean quality of life score after 26 weeks of treatment CBZ group 58.41 ± 1.89, LEV 64.58 ± 2.02 (P value = 0.0302) 2.28 participants in CBZ group, 28 in LEV group Seizure freedom 4 weeks CBZ group 85.72%, LEV group 85.72% (P value = 1); 12 weeks CBZ group 89.29%, LEV group 93.34% (P value = 0.4595); 26 weeks CBZ group 96.43%, LEV group 100% (P value = 0.1212); 6 months CBZ group 71.42% (20 participants), LEV group 78.57% (22 participants) (P value = 0.2529) 3. Participants experiencing at least 1 AE, CBZ group 36.66%, LEV group 40% (P value = 0.77) | |
| 1. PHB: 31%, PHT: 27%, VPS: 21% 2. PHB: 33%, PHT: 63%, VPS: 31% | |
| AE: adverse event; CBZ: carbamazepine; EEG: electroencephalogram; FE: focal epilepsies; GBP: gabapentin; GE: generalised epilepsies; ITT: intention to treat; LDH: lactic acid dehydrogenase; LEV: levetiracetam; LTG: lamotrigine; MANOVA: repeated measures analysis of variance; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; SD: standard deviation; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aFor further details of adverse events see Table 16 and Table 17. | |
| Trial | Time to withdrawal from allocated treatmentc | Time to first seizure | Time to 12‐month remissiond | Time to six‐month remissiond | ||||||||||||
| Cens | Event | Total | Missing | Cens | Event | Total | Missing | Cens | Event | Total | Missing | Cens | Event | Total | Missing | |
| 0 | 0 | 0 | 108 | 39 | 69 | 108 | 0 | 0 | 0 | 0 | 108 | 0 | 0 | 0 | 108 | |
| 392 | 191 | 583 | 0 | 388 | 186 | 574 | 9 | 251 | 323 | 574 | 9 | 194 | 380 | 574 | 9 | |
| 232 | 55 | 287 | 0 | 137 | 145 | 282 | 5 | 190 | 92 | 282 | 5 | 136 | 146 | 282 | 5 | |
| 99 | 36 | 135 | 1 | 64 | 71 | 135 | 1 | 0 | 0 | 0 | 136 | 90 | 45 | 135 | 1 | |
| 78 | 58 | 136 | 0 | 69 | 67 | 136 | 0 | 0 | 0 | 0 | 136 | 122 | 14 | 136 | 0 | |
| 79 | 45 | 124 | 0 | 52 | 72 | 124 | 0 | 0 | 0 | 0 | 124 | 96 | 28 | 124 | 0 | |
| 95 | 55 | 150 | 0 | 70 | 80 | 150 | 0 | 0 | 0 | 0 | 150 | 106 | 44 | 150 | 0 | |
| 323 | 256 | 579 | 0 | 350 | 229 | 579 | 0 | 260 | 319 | 579 | 0 | 177 | 402 | 579 | 0 | |
| 69 | 223 | 292 | 0 | 102 | 190 | 292 | 0 | 0 | 0 | 0 | 292 | 193 | 99 | 292 | 0 | |
| 0 | 0 | 0 | 166 | 68 | 81 | 149 | 17 | 117 | 30 | 147 | 19 | 58 | 89 | 147 | 19 | |
| 100 | 67 | 167 | 6 | 18 | 149 | 167 | 6 | 22 | 145 | 167 | 6 | 19 | 148 | 167 | 6 | |
| 44 | 8 | 52 | 0 | 40 | 12 | 52 | 0 | 11 | 41 | 52 | 0 | 8 | 44 | 52 | 0 | |
| 75 | 9 | 84 | 0 | 52 | 32 | 84 | 0 | 0 | 0 | 0 | 84 | 35 | 49 | 84 | 0 | |
| 151 | 42 | 193 | 0 | 106 | 84 | 190 | 3 | 112 | 78 | 190 | 3 | 84 | 106 | 190 | 3 | |
| 166 | 77 | 243 | 0 | 66 | 177 | 243 | 0 | 78 | 165 | 243 | 0 | 49 | 194 | 243 | 0 | |
| 60 | 21 | 81 | 0 | 38 | 29 | 67 | 14 | 68 | 13 | 81 | 0 | 30 | 50 | 80 | 1 | |
| 73 | 37 | 110 | 0 | 79 | 31 | 110 | 0 | 0 | 0 | 0 | 110 | 39 | 71 | 110 | 0 | |
| 267 | 208 | 475 | 0 | 226 | 238 | 464 | 11 | 325 | 149 | 474 | 1 | 281 | 193 | 474 | 1 | |
| 308 | 172 | 480 | 0 | 165 | 303 | 468 | 12 | 334 | 133 | 467 | 13 | 242 | 225 | 467 | 13 | |
| 511 | 111 | 622 | 0 | 310 | 312 | 622 | 0 | 0 | 0 | 0 | 622 | 431 | 191 | 622 | 0 | |
| 0 | 0 | 0 | 55 | 29 | 26 | 55 | 0 | 0 | 0 | 0 | 55 | 0 | 0 | 0 | 55 | |
| 0 | 0 | 0 | 94 | 41 | 49 | 90 | 4 | 82 | 8 | 90 | 4 | 63 | 27 | 90 | 4 | |
| 158 | 32 | 190 | 2 | 121 | 71 | 192 | 0 | 132 | 60 | 192 | 0 | 69 | 123 | 192 | 0 | |
| (CBZ branch)b | 221 | 174 | 395 | 0 | 208 | 187 | 395 | 0 | 316 | 79 | 395 | 0 | 194 | 201 | 395 | 0 |
| (VPS branch)b | 111 | 114 | 225 | 0 | 119 | 106 | 225 | 0 | 180 | 45 | 225 | 0 | 106 | 119 | 225 | 0 |
| 113 | 23 | 136 | 0 | 81 | 44 | 125 | 11 | 0 | 0 | 0 | 136 | 78 | 47 | 125 | 11 | |
| 192 | 69 | 261 | 0 | 197 | 64 | 261 | 0 | 0 | 0 | 0 | 261 | 0 | 0 | 0 | 261 | |
| 288 | 63 | 351 | 0 | 216 | 135 | 351 | 0 | 0 | 0 | 0 | 351 | 328 | 23 | 351 | 0 | |
| 210 | 76 | 286 | 14 | 91 | 199 | 290 | 10 | 92 | 198 | 290 | 10 | 77 | 213 | 290 | 10 | |
| 857 | 815 | 1672 | 49 | 383 | 1261 | 1644 | 77 | 577 | 1067 | 1644 | 77 | 355 | 1326 | 1681 | 40 | |
| 400 | 299 | 699 | 17 | 182 | 511 | 693 | 23 | 167 | 526 | 693 | 23 | 96 | 610 | 706 | 10 | |
| 108 | 73 | 181 | 0 | 100 | 81 | 181 | 0 | 0 | 0 | 0 | 181 | 157 | 24 | 181 | 0 | |
| 160 | 67 | 227 | 0 | 81 | 140 | 221 | 6 | 172 | 55 | 227 | 0 | 137 | 90 | 227 | 0 | |
| (CBZ branch)b | 760 | 239 | 999 | 0 | 572 | 427 | 999 | 0 | 780 | 219 | 999 | 0 | 336 | 663 | 999 | 0 |
| (VPS branch)b | 583 | 120 | 703 | 0 | 456 | 247 | 703 | 0 | 484 | 219 | 703 | 0 | 191 | 512 | 703 | 0 |
| 91 | 49 | 140 | 0 | 75 | 65 | 140 | 0 | 47 | 93 | 140 | 0 | 36 | 104 | 140 | 0 | |
| 187 | 59 | 246 | 14 | 59 | 187 | 246 | 14 | 84 | 162 | 246 | 14 | 19 | 227 | 246 | 14 | |
| 195 | 166 | 361 | 0 | 249 | 96 | 345 | 16 | 211 | 150 | 361 | 0 | 178 | 183 | 361 | 0 | |
| Total | 7756 | 4109 | 11,865 | 526 | 5699 | 6453 | 12,152 | 239 | 5092 | 4369 | 9461 | 2930 | 4810 | 7010 | 11,820 | 571 |
| Abbreviation: cens = censored aFor two studies we could only calculate 'Time to first seizure'; the study duration of Ogunrin 2005 was 12 weeks, and all randomised participants completed the study without withdrawing; and Banu 2007 did not record the dates of all seizures after randomisation and dates of withdrawal for allocated treatment for all participants. | ||||||||||||||||
| Reason for withdrawal | Classification for analysis | Randomised drug4b | ||||||||||
| CBZ | PHB | PHT | VPS | LTG | OXC | TPM | GBP | LEV | ZNS | Total | ||
| Adverse events | Event | 505 (45%) | 24 (20%) | 93 (35%) | 132 (28%) | 235 (41%) | 56 (41%) | 259 (48%) | 73 (20%) | 134 (39%) | 31 (32%) | 1542 (38%) |
| Inadequate response | Event | 232 (20%) | 20 (16%) | 46 (17%) | 140 (29%) | 144 (26%) | 36 (26%) | 148 (27%) | 223 (62%) | 89 (26%) | 23 (24%) | 1101 (27%) |
| Both adverse events and inadequate response | Event | 148 (13%) | 51 (41%) | 54 (20%) | 107 (22%) | 32 (6%) | 11 (8%) | 46 (8%) | 32 (9%) | 0 (0%) | 0 (0%) | 481 (12%) |
| Protocol violation/non compliance | Event | 102 (9%) | 15 (12%) | 41 (15%) | 11 (2%) | 68 (12%) | 27 (20%) | 0 (0%) | 21 (6%) | 21 (6%) | 3 (3%) | 309 (8%) |
| Withdrew consent | Event | 121 (11%) | 13 (11%) | 25 (9%) | 64 (13%) | 65 (11%) | 2 (1%) | 55 (10%) | 4 (1%) | 68 (20%) | 35 (36%) | 452 (11%) |
| Othera | Event | 29 (3%) | 0 (0%) | 7 (3%) | 24 (5%) | 26 (5%) | 5 (4%) | 37 (7%) | 9 (2%) | 32 (9%) | 4 (4%) | 173 (4%) |
| Total eventsb | 1137 (35%) | 123 (38%) | 266 (31%) | 478 (28%) | 570 (29%) | 137 (29%) | 545 (47%) | 362 (61%) | 344 (27%) | 96 (34%) | 4058 (34%) | |
| Illness or death | Censored | 34 (2%) | 10 (5%) | 17 (3%) | 7 (1%) | 20 (1%) | 1 (0%) | 10 (2%) | 9 (4%) | 0 (0%) | 0 (0%) | 108 (1%) |
| Remission of seizures | Censored | 49 (2%) | 4 (2%) | 38 (6%) | 75 (6%) | 40 (3%) | 12 (4%) | 44 (7%) | 21 (9%) | 0 (0%) | 0 (0%) | 283 (4%) |
| Lost to follow‐up | Censored | 81 (4%) | 31 (16%) | 51 (9%) | 63 (5%) | 33 (3%) | 24 (7%) | 18 (3%) | 0 (0%) | 41 (5%) | 0 (0%) | 342 (4%) |
| Otherc | Censored | 104 (5%) | 6 (3%) | 22 (4%) | 82 (7%) | 31 (2%) | 5 (2%) | 26 (4%) | 26 (12%) | 0 (0%) | 25 (13%) | 327 (4%) |
| Completed study | Censored | 1829 (87%) | 139 (73%) | 468 (79%) | 949 (81%) | 1272 (91%) | 291 (87%) | 501 (84%) | 166 (75%) | 868 (95%) | 161 (87%) | 6644 (86%) |
| Total censoredb | 2097 (65%) | 190 (62%) | 596 (69%) | 1176 (72%) | 1396 (71%) | 333 (71%) | 599 (53%) | 222 (39%) | 909 (73%) | 186 (66%) | 7704 (66%) | |
| Missingd | 24 | 7 | 1 | 26 | 12 | 8 | 14 | 11 | 0 | 0 | 103 | |
| Totale | 3258 | 320 | 863 | 1680 | 1978 | 478 | 1158 | 595 | 1253 | 282 | 11,865 | |
| CBZ: carbamazepine; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aOther treatment‐related reasons included: physician's decision, drug‐related death, pregnancy or perceived remission, or non specific (drug‐related) reason. | ||||||||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number of | Number of | HR (95% CI)b,c | I² statisticd | Direct evidence (%)e | HR (95% CI)b,c | |
| CBZ vs PHB | 4 | 520 | 1.57 (1.16 to 2.13) | 0% | 52.5% | 1.55 (1.18 to 2.04) |
| CBZ vs PHT | 3 | 428 | 1.03 (0.74 to 1.42) | 63.6% | 12.8% | 1.13 (0.92 to 1.38) |
| CBZ vs VPS | 5 | 814 | 0.94 (0.73 to 1.19) | 0% | 40.1% | 1.04 (0.86 to 1.25) |
| CBZ vs LTG | 9 | 2268 | 0.76 (0.61 to 0.95) | 39.3% | 28.9% | 0.75 (0.65 to 0.86) |
| CBZ vs OXC | 2 | 562 | 4.62 (0.95 to 22.4) | 0% | 5.7% | 1.09 (0.84 to 1.42) |
| CBZ vs TPM | 2 | 937 | 1.04 (0.52 to 2.07) | 0% | 7.4% | 1.18 (0.98 to 1.43) |
| CBZ vs GBP | 2 | 954 | 1.14 (0.84 to 1.55) | 0% | 87.1% | 1.20 (1.00 to 1.43) |
| CBZ vs LEV | 3 | 1567 | 0.70 (0.52 to 0.94) | 0% | 37.9% | 0.82 (0.69 to 0.97) |
| CBZ vs ZNS | 1 | 583 | 1.08 (0.81 to 1.44) | NA | 100% | 1.08 (0.79 to 1.48) |
| PHB vs PHT | 3 | 404 | 0.67 (0.50 to 0.91) | 65% | 15.2% | 0.73 (0.55 to 0.96) |
| PHB vs VPS | 2 | 75 | 0.68 (0.34 to 1.36) | 23% | 8.8% | 0.67 (0.48 to 0.92) |
| PHB vs LTG | No direct evidence | 0% | 0.48 (0.35 to 0.66) | |||
| PHB vs OXC | No direct evidence | 0% | 0.70 (0.48 to 1.03) | |||
| PHB vs TPM | No direct evidence | 0% | 0.76 (0.55 to 1.06) | |||
| PHB vs GBP | No direct evidence | 0% | 0.77 (0.55 to 1.07) | |||
| PHB vs LEV | No direct evidence | 0% | 0.53 (0.38 to 0.73) | |||
| PHB vs ZNS | No direct evidence | 0% | 0.70 (0.46 to 1.06) | |||
| PHT vs VPS | 4 | 168 | 1.00 (0.60 to 1.64) | 58.5% | 9% | 0.92 (0.70 to 1.21) |
| PHT vs LTG | 1 | 90 | 1.10 (0.57 to 2.14) | NA | 11.6% | 0.66 (0.52 to 0.85) |
| PHT vs OXC | 2 | 325 | 0.65 (0.32 to 1.32) | 0% | 40.4% | 0.97 (0.69 to 1.35) |
| PHT vs TPM | 1 | 53 | 0.77 (0.38 to 1.57) | NA | 10.9% | 1.05 (0.80 to 1.39) |
| PHT vs GBP | No direct evidence | 0% | 1.06 (0.81 to 1.40) | |||
| PHT vs LEV | No direct evidence | 0% | 0.73 (0.56 to 0.95) | |||
| PHT vs ZNS | No direct evidence | 0% | 0.96 (0.66 to 1.39) | |||
| VPS vs LTG* | 3 | 221 | 1.40 (1.00 to 1.96) | 45.1% | 5.1% | 0.72 (0.58 to 0.90) |
| VPS vs OXC | No direct evidence | 0% | 1.05 (0.76 to 1.44) | |||
| VPS vs TPM | 2 | 111 | 1.66 (1.24 to 2.23) | 48.1% | 33.7% | 1.14 (0.88 to 1.48) |
| VPS vs GBP | No direct evidence | 0% | 1.15 (0.89 to 1.49) | |||
| VPS vs LEV | 1 | 190 | 1.14 (0.73 to 1.75) | NA | 17.2% | 0.79 (0.61 to 1.03) |
| VPS vs ZNS | No direct evidence | 0% | 1.04 (0.73 to 1.50) | |||
| LTG vs OXC | 1 | 506 | 0.69 (0.12 to 4.14) | NA | 4.4% | 1.46 (1.11 to 1.92) |
| LTG vs TPM | 1 | 648 | 1.18 (0.86 to 1.62) | NA | 20.9% | 1.59 (1.29 to 1.95) |
| LTG vs GBP | 1 | 659 | 0.62 (0.06 to 6.01) | NA | 1% | 1.60 (1.31 to 1.96) |
| LTG vs LEV | 1 | 240 | 0.86 (0.58 to 1.28) | NA | 23.7% | 1.10 (0.89 to 1.35) |
| LTG vs ZNS | No direct evidence | 0% | 1.45 (1.03 to 2.04) | |||
| OXC vs TPM | 1 | 496 | 0.87 (0.16 to 4.73) | NA | 4.9% | 1.09 (0.82 to 1.44) |
| OXC vs GBP | 1 | 507 | 0.90 (0.08 to 9.96) | NA | 2.3% | 1.10 (0.83 to 1.45) |
| OXC vs LEV | No direct evidence | 0% | 0.75 (0.55 to 1.03) | |||
| OXC vs ZNS | No direct evidence | 0% | 0.99 (0.66 to 1.49) | |||
| TPM vs GBP | 1 | 649 | 1.04 (0.12 to 9.33) | NA | 1.1% | 1.01 (0.82 to 1.25) |
| TPM vs LEV | No direct evidence | 0% | 0.69 (0.54 to 0.89) | |||
| TPM vs ZNS | No direct evidence | 0% | 0.91 (0.64 to 1.31) | |||
| GBP vs LEV | No direct evidence | 0% | 0.69 (0.54 to 0.88) | |||
| GBP vs ZNS | No direct evidence | 0% | 0.90 (0.63 to 1.30) | |||
| LEV vs ZNS | No direct evidence | 0% | 1.32 (0.93 to 1.88) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). For comparisons marked with a *, confidence intervals of direct evidence and network meta‐analysis do not overlap indicating that inconsistency may be present in the results. | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number of | Number of | HR (95% CI)b,c | I² statisticd | Direct | HR (95% CI)b,c | |
| CBZ vs PHB | 3 | 156 | 1.21 (0.51 to 2.86) | 11.8% | 27.3% | 1.47 (0.83 to 2.61) |
| CBZ vs PHT | 2 | 118 | 2.68 (0.95 to 7.57) | 0% | 11.3% | 0.92 (0.59 to 1.42) |
| CBZ vs VPS | 4 | 405 | 1.26 (0.73 to 2.20) | 6.6% | 27.3% | 0.70 (0.54 to 0.92) |
| CBZ vs LTG | 7 | 302 | 1.23 (0.72 to 2.10) | 0% | 39.2% | 0.63 (0.45 to 0.89) |
| CBZ vs OXC | 1 | 9 | 0.39 (0.03 to 4.35) | NA | 3.9% | 1.00 (0.21 to 4.81) |
| CBZ vs TPM | 2 | 101 | 1.10 (0.51 to 2.36) | 0% | 23.2% | 1.24 (0.90 to 1.71) |
| CBZ vs GBP | 1 | 6 | 0.49 (0.03 to 7.90) | NA | 8.5% | 0.90 (0.11 to 7.29) |
| CBZ vs LEV | 2 | 251 | 1.22 (0.74 to 2.02) | 0% | 57% | 0.74 (0.44 to 1.23) |
| PHB vs PHT | 2 | 95 | 1.56 (0.49 to 4.99) | 0% | 16.1% | 0.62 (0.32 to 1.24) |
| PHB vs VPS | 2 | 94 | 0.56 (0.20 to 1.54) | 0% | 19.4% | 0.48 (0.27 to 0.86) |
| PHB vs LTG | No direct evidence | 0% | 0.43 (0.22 to 0.83) | |||
| PHB vs OXC | No direct evidence | 0% | 0.68 (0.13 to 3.60) | |||
| PHB vs TPM | No direct evidence | 0% | 0.84 (0.44 to 1.60) | |||
| PHB vs GBP | No direct evidence | 0% | 0.61 (0.07 to 5.34) | |||
| PHB vs LEV | No direct evidence | 0% | 0.50 (0.23 to 1.09) | |||
| PHT vs VPS | 3 | 326 | 0.66 (0.30 to 1.45) | 22.6% | 19.3% | 0.77 (0.46 to 1.27) |
| PHT vs LTG | 1 | 91 | 1.11 (0.42 to 2.94) | NA | 14.9% | 0.69 (0.39 to 1.20) |
| PHT vs OXC | 2 | 155 | 1.05 (0.44 to 2.52) | 0% | 37.9% | 1.09 (0.21 to 5.56) |
| PHT vs TPM | 1 | 150 | 1.68 (0.49 to 5.69) | NA | 11.2% | 1.35 (0.79 to 2.30) |
| PHT vs GBP | No direct evidence | 0% | 0.98 (0.12 to 8.30) | |||
| PHT vs LEV | No direct evidence | 0% | 0.80 (0.42 to 1.55) | |||
| VPS vs LTG | 3 | 387 | 0.46 (0.22 to 0.97) | 0% | 14.8% | 0.90 (0.60 to 1.35) |
| VPS vs OXC | No direct evidence | 0% | 1.42 (0.29 to 6.92) | |||
| VPS vs TPM* | 2 | 443 | 0.53 (0.27 to 1.07) | 48.5% | 22.4% | 1.76 (1.22 to 2.53) |
| VPS vs GBP | No direct evidence | 0% | 1.28 (0.16 to 10.5) | |||
| VPS vs LEV | 1 | 512 | 0.68 (0.30 to 1.59) | NA | 18.6% | 1.05 (0.58 to 1.90) |
| LTG vs OXC | 1 | 10 | 2.09 (0.34 to 12.8) | NA | 7.6% | 1.58 (0.33 to 7.67) |
| LTG vs TPM | 1 | 14 | 1.10 (0.42 to 2.89) | NA | 7.3% | 1.96 (1.25 to 3.08) |
| LTG vs GBP | 1 | 7 | 2.63 (0.27 to 25.7) | NA | 13.8% | 1.42 (0.17 to 11.6) |
| LTG vs LEV | No direct evidence | 0% | 1.17 (0.63 to 2.19) | |||
| OXC vs TPM | 1 | 14 | 1.31 (0.24 to 7.32) | NA | 9% | 1.24 (0.26 to 5.94) |
| OXC vs GBP | 1 | 7 | 1.26 (0.11 to 14.1) | NA | 12.7% | 0.90 (0.08 to 9.96) |
| OXC vs LEV | No direct evidence | 0% | 0.74 (0.14 to 3.86) | |||
| TPM vs GBP | 1 | 11 | 0.96 (0.11 to 8.67) | NA | 14.6% | 0.73 (0.09 to 5.89) |
| TPM vs LEV | No direct evidence | 0% | 0.60 (0.33 to 1.09) | |||
| GBP vs LEV | No direct evidence | 0% | 0.82 (0.10 to 7.10) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). For comparisons marked with a *, confidence intervals of direct evidence and network meta‐analysis do not overlap indicating that inconsistency may be present in the results | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number of | Number of | HR (95% CI)b,c | I² statisticd | Direct | HR (95% CI)b,c | |
| CBZ vs PHB | 4 | 525 | 1.41 (1.04 to 1.91) | 0% | 56.1% | 1.02 (0.76 to 1.35) |
| CBZ vs PHT | 3 | 430 | 1.00 (0.76 to 1.32) | 54.8% | 18.6% | 1.03 (0.85 to 1.25) |
| CBZ vs VPS | 5 | 816 | 1.03 (0.85 to 1.25) | 46.4% | 27.6% | 1.05 (0.89 to 1.25) |
| CBZ vs LTG | 2 | 891 | 1.02 (0.69 to 1.50) | 0% | 17.5% | 1.16 (0.98 to 1.37) |
| CBZ vs OXC | 2 | 555 | 1.13 (0.62 to 2.05) | 0% | 21% | 0.98 (0.78 to 1.25) |
| CBZ vs TPM | 2 | 925 | 0.94 (0.48 to 1.83) | 0% | 7.2% | 1.08 (0.92 to 1.27) |
| CBZ vs GBP | 1 | 651 | 0.61 (0.06 to 5.82) | NA | 10.5% | 1.20 (0.99 to 1.47) |
| CBZ vs LEV | 3 | 1567 | 1.08 (0.81 to 1.42) | 60.8% | 14.2% | 1.35 (1.09 to 1.69) |
| CBZ vs ZNS | 1 | 582 | 1.05 (0.85 to 1.30) | NA | 100% | 1.05 (0.81 to 1.35) |
| PHB vs PHT | 4 | 465 | 0.80 (0.59 to 1.10) | 0% | 0.2% | 1.01 (0.75 to 1.37) |
| PHB vs VPS | 2 | 80 | 0.85 (0.51 to 1.40) | 4.4% | 15.6% | 1.04 (0.75 to 1.43) |
| PHB vs LTG | No direct evidence | 0% | 1.14 (0.82 to 1.59) | |||
| PHB vs OXC | No direct evidence | 0% | 0.96 (0.67 to 1.41) | |||
| PHB vs TPM | No direct evidence | 0% | 1.06 (0.76 to 1.47) | |||
| PHB vs GBP | No direct evidence | 0% | 1.19 (0.83 to 1.69) | |||
| PHB vs LEV | No direct evidence | 0% | 1.33 (0.93 to 1.92) | |||
| PHB vs ZNS | No direct evidence | 0% | 1.03 (0.70 to 1.52) | |||
| PHT vs VPS | 4 | 245 | 1.04 (0.78 to 1.40) | 0% | 41.6% | 1.03 (0.80 to 1.32) |
| PHT vs LTG | No direct evidence | 0% | 1.12 (0.88 to 1.45) | |||
| PHT vs OXC | 2 | 318 | 1.21 (0.73 to 2.03) | 0% | 29.9% | 0.95 (0.70 to 1.30) |
| PHT vs TPM | No direct evidence | 0% | 1.05 (0.81 to 1.35) | |||
| PHT vs GBP | No direct evidence | 0% | 1.18 (0.88 to 1.56) | |||
| PHT vs LEV | No direct evidence | 0% | 1.32 (0.98 to 1.75) | |||
| PHT vs ZNS | No direct evidence | 0% | 1.02 (0.74 to 1.41) | |||
| VPS vs LTG | 3 | 221 | 1.37 (1.07 to 1.77) | 0% | 39.9% | 1.10 (0.88 to 1.37) |
| VPS vs OXC | No direct evidence | 0% | 0.93 (0.70 to 1.23) | |||
| VPS vs TPM | 2 | 111 | 1.11 (0.87 to 1.40) | 0% | 67.8% | 1.02 (0.80 to 1.30) |
| VPS vs GBP | No direct evidence | 0% | 1.14 (0.88 to 1.47) | |||
| VPS vs LEV | 1 | 190 | 1.14 (0.84 to 1.55) | NA | 34.7% | 1.28 (0.97 to 1.67) |
| VPS vs ZNS | No direct evidence | 0% | 0.99 (0.74 to 1.35) | |||
| LTG vs OXC | 1 | 499 | 1.49 (0.33 to 6.67) | NA | 2.8% | 0.85 (0.66 to 1.09) |
| LTG vs TPM | 1 | 636 | 0.98 (0.29 to 3.25) | NA | 2.5% | 0.93 (0.75 to 1.15) |
| LTG vs GBP | 1 | 647 | 0.74 (0.08 to 6.58) | NA | 10.1% | 1.04 (0.84 to 1.30) |
| LTG vs LEV | 1 | 240 | 1.02 (0.70 to 1.49) | NA | 26.6% | 1.16 (0.93 to 1.47) |
| LTG vs ZNS | No direct evidence | 0% | 0.91 (0.67 to 1.22) | |||
| OXC vs TPM | 1 | 487 | 0.66 (0.17 to 2.47) | NA | 3.7% | 1.10 (0.83 to 1.45) |
| OXC vs GBP | 1 | 498 | 0.49 (0.05 to 4.74) | NA | 9.8% | 1.23 (0.95 to 1.59) |
| OXC vs LEV | No direct evidence | 0% | 1.37 (1.05 to 1.79) | |||
| OXC vs ZNS | No direct evidence | 0% | 1.06 (0.76 to 1.52) | |||
| TPM vs GBP | 1 | 635 | 0.75 (0.09 to 6.00) | NA | 11.2% | 1.12 (0.87 to 1.45) |
| TPM vs LEV | No direct evidence | 0% | 1.25 (0.96 to 1.64) | |||
| TPM vs ZNS | No direct evidence | 0% | 0.97 (0.72 to 1.32) | |||
| GBP vs LEV | No direct evidence | 0% | 1.12 (0.88 to 1.43) | |||
| GBP vs ZNS | No direct evidence | 0% | 0.87 (0.63 to 1.20) | |||
| LEV vs ZNS | No direct evidence | 0% | 0.78 (0.56 to 1.09) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number of | Number of | HR (95% CI)b,c | I² statisticd | Direct | HR (95% CI)b,c | |
| CBZ vs PHB | 3 | 158 | 0.53 (0.28 to 1.00) | 0% | 42.6% | 1.25 (0.83 to 1.89) |
| CBZ vs PHT | 2 | 121 | 1.11 (0.61 to 2.02) | 64.5% | 9.3% | 0.86 (0.65 to 1.16) |
| CBZ vs VPS | 4 | 412 | 1.01 (0.72 to 1.43) | 0% | 51.1% | 0.94 (0.79 to 1.14) |
| CBZ vs LTG | 1 | 9 | 1.33 (0.29 to 6.03) | NA | 7% | 1.28 (0.54 to 3.03) |
| CBZ vs OXC | 1 | 9 | 0.77 (0.15 to 3.89) | NA | 5.6% | 1.72 (0.47 to 6.25) |
| CBZ vs TPM | 2 | 101 | 1.15 (0.52 to 2.53) | 0% | 27.2% | 1.06 (0.78 to 1.45) |
| CBZ vs GBP | 1 | 6 | 2.19 (0.23 to 21.2) | NA | 10.9% | 0.75 (0.10 to 5.88) |
| CBZ vs LEV | 2 | 251 | 1.02 (0.65 to 1.59) | 77.4% | 16.6% | 1.33 (0.81 to 2.22) |
| PHB vs PHT | 3 | 130 | 1.30 (0.61 to 2.78) | 53% | 10.9% | 0.68 (0.44 to 1.08) |
| PHB vs VPS | 2 | 98 | 1.15 (0.53 to 2.49) | 42.3% | 13% | 0.75 (0.49 to 1.15) |
| PHB vs LTG | No direct evidence | 0% | 1.01 (0.40 to 2.63) | |||
| PHB vs OXC | No direct evidence | 0% | 1.37 (0.35 to 5.26) | |||
| PHB vs TPM | No direct evidence | 0% | 0.85 (0.51 to 1.41) | |||
| PHB vs GBP | No direct evidence | 0% | 0.60 (0.07 to 5.00) | |||
| PHB vs LEV | No direct evidence | 0% | 1.06 (0.56 to 2.04) | |||
| PHT vs VPS | 4 | 269 | 0.87 (0.55 to 1.40) | 0% | 44.9% | 1.10 (0.80 to 1.49) |
| PHT vs LTG | No direct evidence | 0% | 1.47 (0.60 to 3.57) | |||
| PHT vs OXC | 2 | 154 | 0.77 (0.41 to 1.47) | 0% | 41.2% | 2.00 (0.53 to 7.69) |
| PHT vs TPM | No direct evidence | 0% | 1.23 (0.81 to 1.85) | |||
| PHT vs GBP | No direct evidence | 0% | 0.87 (0.11 to 7.14) | |||
| PHT vs LEV | No direct evidence | 0% | 1.56 (0.87 to 2.78) | |||
| VPS vs LTG | 3 | 387 | 0.77 (0.38 to 1.56) | 0% | 35.7% | 1.35 (0.57 to 3.13) |
| VPS vs OXC | No direct evidence | 0% | 1.82 (0.50 to 6.67) | |||
| VPS vs TPM | 2 | 441 | 0.52 (0.26 to 1.04) | 58.5% | 10.6% | 1.12 (0.83 to 1.52) |
| VPS vs GBP | No direct evidence | 0% | 0.79 (0.10 to 6.25) | |||
| VPS vs LEV | 1 | 512 | 0.91 (0.49 to 1.70) | NA | 55.2% | 1.41 (0.83 to 2.44) |
| LTG vs OXC | 1 | 10 | 0.58 (0.13 to 2.64) | NA | 9.2% | 1.35 (0.33 to 5.56) |
| LTG vs TPM | 1 | 14 | 1.13 (0.33 to 3.82) | NA | 15.1% | 0.83 (0.35 to 2.00) |
| LTG vs GBP | 1 | 7 | 1.64 (0.18 to 14.8) | NA | 12.5% | 0.59 (0.07 to 5.00) |
| LTG vs LEV | No direct evidence | 0% | 1.05 (0.40 to 2.78) | |||
| OXC vs TPM | 1 | 14 | 1.95 (0.51 to 7.50) | NA | 11.4% | 0.62 (0.17 to 2.27) |
| OXC vs GBP | 1 | 7 | 2.83 (0.29 to 27.6) | NA | 10.9% | 0.44 (0.04 to 4.35) |
| OXC vs LEV | No direct evidence | 0% | 0.78 (0.20 to 3.13) | |||
| TPM vs GBP | 1 | 11 | 1.45 (0.18 to 11.7) | NA | 15.9% | 0.71 (0.09 to 5.56) |
| TPM vs LEV | No direct evidence | 0% | 1.27 (0.68 to 2.33) | |||
| GBP vs LEV | No direct evidence | 0% | 1.79 (0.21 to 14.3) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number of | Number of | HR (95% CI)b,c | I² statisticd | Direct | HR (95% CI)b,c | |
| CBZ vs PHB | 4 | 525 | 1.24 (0.95 to 1.61) | 0% | 31.3% | 0.95 (0.76 to 1.19) |
| CBZ vs PHT | 3 | 430 | 0.85 (0.66 to 1.09) | 4.2% | 23.3% | 1.03 (0.88 to 1.20) |
| CBZ vs VPS | 5 | 816 | 1.06 (0.90 to 1.25) | 56.5% | 16.6% | 1.10 (0.96 to 1.25) |
| CBZ vs LTG | 7 | 1535 | 1.15 (0.89 to 1.48) | 0% | 26.4% | 1.11 (0.98 to 1.27) |
| CBZ vs OXC | 2 | 555 | 1.15 (0.65 to 2.04) | 0% | 16.6% | 0.98 (0.82 to 1.18) |
| CBZ vs TPM | 2 | 925 | 1.05 (0.64 to 1.72) | 0% | 8.8% | 1.11 (0.96 to 1.28) |
| CBZ vs GBP | 2 | 943 | 0.81 (0.52 to 1.27) | 0% | 73.7% | 1.16 (0.99 to 1.35) |
| CBZ vs LEV | 3 | 1567 | 1.06 (0.84 to 1.33) | 37.9% | 20.4% | 1.04 (0.93 to 1.16) |
| CBZ vs ZNS | 1 | 582 | 1.00 (0.82 to 1.20) | NA | 100% | 1.00 (0.83 to 1.20) |
| PHB vs PHT | 4 | 465 | 0.79 (0.60 to 1.05) | 0% | 31.1% | 1.08 (0.85 to 1.37) |
| PHB vs VPS | 2 | 80 | 0.67 (0.42 to 1.08) | 0% | 9.1% | 1.15 (0.89 to 1.49) |
| PHB vs LTG | No direct evidence | 0% | 1.16 (0.90 to 1.52) | |||
| PHB vs OXC | No direct evidence | 0% | 1.03 (0.77 to 1.39) | |||
| PHB vs TPM | No direct evidence | 0% | 1.16 (0.89 to 1.54) | |||
| PHB vs GBP | No direct evidence | 0% | 1.22 (0.93 to 1.59) | |||
| PHB vs LEV | No direct evidence | 0% | 1.10 (0.85 to 1.41) | |||
| PHB vs ZNS | No direct evidence | 0% | 1.04 (0.78 to 1.41) | |||
| PHT vs VPS | 5 | 245 | 0.90 (0.70 to 1.15) | 0% | 26.5% | 1.06 (0.88 to 1.30) |
| PHT vs LTG | 1 | 90 | 0.88 (0.25 to 3.03) | NA | 1.20% | 1.09 (0.88 to 1.32) |
| PHT vs OXC | 2 | 318 | 1.21 (0.79 to 1.87) | 0% | 33.2% | 0.95 (0.75 to 1.22) |
| PHT vs TPM | No direct evidence | 0% | 1.09 (0.88 to 1.33) | |||
| PHT vs GBP | No direct evidence | 0% | 1.12 (0.91 to 1.39) | |||
| PHT vs LEV | No direct evidence | 0% | 1.02 (0.84 to 1.22) | |||
| PHT vs ZNS | No direct evidence | 0% | 0.97 (0.76 to 1.23) | |||
| VPS vs LTG | 3 | 221 | 1.22 (0.97 to 1.52) | 0% | 32.1% | 1.02 (0.85 to 1.22) |
| VPS vs OXC | No direct evidence | 0% | 0.90 (0.72 to 1.12) | |||
| VPS vs TPM | 2 | 111 | 1.08 (0.87 to 1.34) | 0% | 61.7% | 1.02 (0.83 to 1.23) |
| VPS vs GBP | No direct evidence | 0% | 1.05 (0.87 to 1.28) | |||
| VPS vs LEV | 1 | 190 | 1.09 (0.88 to 1.33) | NA | 40.5% | 0.95 (0.79 to 1.14) |
| VPS vs ZNS | No direct evidence | 0% | 0.91 (0.72 to 1.14) | |||
| LTG vs OXC | 1 | 499 | 1.08 (0.27 to 4.32) | NA | 2.4% | 0.88 (0.73 to 1.08) |
| LTG vs TPM | 1 | 636 | 0.89 (0.70 to 1.13) | NA | 1.7% | 1.00 (0.85 to 1.18) |
| LTG vs GBP | 1 | 647 | 1.46 (0.16 to 13.0) | NA | 1.6% | 1.04 (0.88 to 1.22) |
| LTG vs LEV | 1 | 240 | 0.83 (0.59 to 1.17) | NA | 17.8% | 0.93 (0.80 to 1.10) |
| LTG vs ZNS | No direct evidence | 0% | 0.89 (0.71 to 1.12) | |||
| OXC vs TPM | 1 | 487 | 0.86 (0.26 to 2.86) | NA | 3.3% | 1.14 (0.93 to 1.37) |
| OXC vs GBP | 1 | 498 | 1.35 (0.15 to 12.1) | NA | 2.1% | 1.18 (0.96 to 1.43) |
| OXC vs LEV | No direct evidence | 0% | 1.06 (0.86 to 1.32) | |||
| OXC vs ZNS | No direct evidence | 0% | 1.01 (0.78 to 1.32) | |||
| TPM vs GBP | 1 | 635 | 1.56 (0.2 to 12.5) | NA | 1.6% | 1.04 (0.88 to 1.23) |
| TPM vs LEV | No direct evidence | 0% | 0.93 (0.79 to 1.12) | |||
| TPM vs ZNS | No direct evidence | 0% | 0.89 (0.70 to 1.14) | |||
| GBP vs LEV | No direct evidence | 0% | 0.90 (0.75 to 1.09) | |||
| GBP vs ZNS | No direct evidence | 0% | 0.86 (0.68 to 1.10) | |||
| LEV vs ZNS | No direct evidence | 0% | 0.95 (0.77 to 1.19) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number | Number | HR (95% CI)b,c | I² statisticd | Direct | HR (95% CI)b,c | |
| CBZ vs PHB | 3 | 158 | 0.56 (0.33 to 0.96) | 13.2% | 28.2% | 1.28 (0.92 to 1.79) |
| CBZ vs PHT | 2 | 121 | 1.44 (0.82 to 2.55) | 31.4% | 13% | 0.87 (0.68 to 1.10) |
| CBZ vs VPS | 4 | 412 | 1.11 (0.81 to 1.53) | 29.9% | 30.7% | 0.95 (0.84 to 1.09) |
| CBZ vs LTG | 5 | 254 | 0.58 (0.25 to 1.32) | 0% | 0.1% | 1.20 (0.99 to 1.49) |
| CBZ vs OXC | 1 | 9 | 0.79 (0.17 to 3.56) | NA | 4.6% | 1.30 (0.42 to 4.00) |
| CBZ vs TPM | 2 | 101 | 1.00 (0.55 to 1.79) | 0% | 32.8% | 1.11 (0.78 to 1.59) |
| CBZ vs GBP | 1 | 6 | 0.71 (0.07 to 6.90) | NA | 10% | 1.75 (0.23 to 12.5) |
| CBZ vs LEV | 2 | 251 | 1.00 (0.72 to 1.37) | 57.9% | 26.7% | 1.14 (0.85 to 1.52) |
| PHB vs PHT | 3 | 130 | 1.31 (0.67 to 2.53) | 0% | 22.7% | 0.68 (0.47 to 0.98) |
| PHB vs VPS | 2 | 98 | 1.50 (0.72 to 3.11) | 7.5% | 15.3% | 0.75 (0.53 to 1.05) |
| PHB vs LTG | No direct evidence | 0% | 0.94 (0.64 to 1.39) | |||
| PHB vs OXC | No direct evidence | 0% | 1.01 (0.31 to 3.23) | |||
| PHB vs TPM | No direct evidence | 0% | 0.87 (0.53 to 1.41) | |||
| PHB vs GBP | No direct evidence | 0% | 1.37 (0.17 to 11.1) | |||
| PHB vs LEV | No direct evidence | 0% | 0.88 (0.57 to 1.37) | |||
| PHT vs VPS | 4 | 394 | 1.03 (0.68 to 1.54) | 0% | 36.8% | 1.10 (0.85 to 1.43) |
| PHT vs LTG | 1 | 91 | 1.96 (0.37 to 10.2) | NA | 4.4% | 1.39 (1.03 to 1.89) |
| PHT vs OXC | 2 | 154 | 0.71 (0.42 to 1.21) | 0% | 45.1% | 1.49 (0.48 to 4.76) |
| PHT vs TPM | No direct evidence | 0% | 1.28 (0.84 to 1.96) | |||
| PHT vs GBP | No direct evidence | 0% | 2.00 (0.26 to 16.7) | |||
| PHT vs LEV | No direct evidence | 0% | 1.32 (0.89 to 1.92) | |||
| VPS vs LTG | 3 | 387 | 0.84 (0.48 to 1.47) | 0% | 43.5% | 1.27 (1.03 to 1.56) |
| VPS vs OXC | No direct evidence | 0% | 1.35 (0.44 to 4.17) | |||
| VPS vs TPM | 2 | 441 | 0.67 (0.38 to 1.19) | 58.7% | 12.9% | 1.16 (0.81 to 1.67) |
| VPS vs GBP | No direct evidence | 0% | 1.82 (0.24 to 14.3) | |||
| VPS vs LEV | 1 | 512 | 0.88 (0.60 to 1.30) | NA | 48.6% | 1.19 (0.86 to 1.64) |
| LTG vs OXC | 1 | 10 | 0.80 (0.20 to 3.26) | NA | 7.6% | 1.08 (0.35 to 3.33) |
| LTG vs TPM | 1 | 14 | 0.59 (0.30 to 1.16) | NA | 10% | 0.92 (0.62 to 1.37) |
| LTG vs GBP | 1 | 7 | 0.73 (0.08 to 6.57) | NA | 11% | 1.45 (0.19 to 11.1) |
| LTG vs LEV | No direct evidence | 0% | 0.93 (0.65 to 1.33) | |||
| OXC vs TPM | 1 | 14 | 1.34 (0.40 to 4.54) | NA | 9.4% | 0.86 (0.28 to 2.63) |
| OXC vs GBP | 1 | 7 | 0.91 (0.10 to 8.20) | NA | 10.7% | 1.35 (0.15 to 12.5) |
| OXC vs LEV | No direct evidence | 0% | 0.88 (0.27 to 2.78) | |||
| TPM vs GBP | 1 | 11 | 0.68 (0.08 to 5.45) | NA | 13.9% | 1.56 (0.21 to 12.5) |
| TPM vs LEV | No direct evidence | 0% | 1.02 (0.65 to 1.61) | |||
| GBP vs LEV | No direct evidence | 0% | 0.65 (0.08 to 5.00) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number | Number | HR (95% CI)b,c | I² statisticd | Direct | HR (95% CI)b,c | |
| CBZ vs PHB | 6 | 581 | 0.99 (0.78 to 1.26) | 54.3% | 21% | 0.79 (0.64 to 0.97) |
| CBZ vs PHT | 4 | 432 | 0.91 (0.72 to 1.16) | 16.1% | 27.1% | 0.98 (0.85 to 1.13) |
| CBZ vs VPS | 5 | 813 | 1.01 (0.86 to 1.19) | 32% | 34.6% | 1.20 (1.06 to 1.37) |
| CBZ vs LTG | 9 | 2252 | 0.98 (0.75 to 1.27) | 0% | 40.7% | 1.29 (1.17 to 1.42) |
| CBZ vs OXC | 2 | 555 | 1.47 (0.57 to 3.81) | 57.3% | 4.8% | 1.09 (0.89 to 1.32) |
| CBZ vs TPM | 2 | 925 | 1.03 (0.51 to 2.08) | 69.3% | 1.5% | 1.12 (0.97 to 1.29) |
| CBZ vs GBP | 2 | 943 | 1.64 (1.14 to 2.36) | 17.7% | 49% | 1.44 (1.25 to 1.66) |
| CBZ vs LEV | 3 | 1552 | 1.18 (0.85 to 1.65) | 0% | 26.2% | 1.14 (0.99 to 1.30) |
| CBZ vs ZNS | 1 | 581 | 1.30 (0.97 to 1.73) | NA | 100% | 1.30 (0.97 to 1.73) |
| PHB vs PHT | 5 | 463 | 1.07 (0.83 to 1.37) | 27.7% | 33.6% | 1.24 (0.99 to 1.56) |
| PHB vs VPS* | 2 | 80 | 0.71 (0.43 to 1.17) | 9.1% | 12.8% | 1.53 (1.20 to 1.94) |
| PHB vs LTG | No direct evidence | 0% | 1.63 (1.30 to 2.06) | |||
| PHB vs OXC | No direct evidence | 0% | 1.38 (1.04 to 1.83) | |||
| PHB vs TPM | No direct evidence | 0% | 1.42 (1.11 to 1.83) | |||
| PHB vs GBP | No direct evidence | 0% | 1.83 (1.42 to 2.35) | |||
| PHB vs LEV | No direct evidence | 0% | 1.44 (1.12 to 1.85) | |||
| PHB vs ZNS | No direct evidence | 0% | 1.64 (1.15 to 2.35) | |||
| PHT vs VPS | 5 | 245 | 0.96 (0.72 to 1.29) | 0% | 25.4% | 1.23 (1.02 to 1.48) |
| PHT vs LTG | 1 | 90 | 0.77 (0.38 to 1.54) | NA | 6% | 1.31 (1.10 to 1.57) |
| PHT vs OXC | 2 | 318 | 1.46 (0.88 to 2.44) | 23.9% | 36.1% | 1.11 (0.87 to 1.41) |
| PHT vs TPM | 1 | 53 | 2.32 (0.95 to 5.70) | NA | 4% | 1.14 (0.93 to 1.40) |
| PHT vs GBP | No direct evidence | 0% | 1.47 (1.20 to 1.80) | |||
| PHT vs LEV | No direct evidence | 0% | 1.16 (0.95 to 1.41) | |||
| PHT vs ZNS | No direct evidence | 0% | 1.32 (0.96 to 1.82) | |||
| VPS vs LTG | 3 | 215 | 1.57 (1.23 to 2.00) | 39.4% | 10% | 1.07 (0.92 to 1.24) |
| VPS vs OXC | No direct evidence | 0% | 0.90 (0.72 to 1.14) | |||
| VPS vs TPM | 2 | 111 | 1.18 (0.93 to 1.50) | 0% | 70.2% | 0.93 (0.77 to 1.13) |
| VPS vs GBP | No direct evidence | 0% | 1.20 (0.99 to 1.44) | |||
| VPS vs LEV | 1 | 190 | 1.27 (0.94 to 1.72) | NA | 31% | 0.94 (0.77 to 1.15) |
| VPS vs ZNS | No direct evidence | 0% | 1.08 (0.78 to 1.48) | |||
| LTG vs OXC | 1 | 499 | 0.87 (0.23 to 3.25) | NA | 5.5% | 0.84 (0.69 to 1.03) |
| LTG vs TPM | 1 | 636 | 0.73 (0.57 to 0.93) | NA | 2.3% | 0.87 (0.75 to 1.01) |
| LTG vs GBP | 1 | 647 | 0.63 (0.07 to 5.42) | NA | 4.4% | 1.12 (0.96 to 1.30) |
| LTG vs LEV | 1 | 229 | 0.84 (0.53 to 1.35) | NA | 15.9% | 0.88 (0.75 to 1.04) |
| LTG vs ZNS | No direct evidence | 0% | 1.01 (0.74 to 1.36) | |||
| OXC vs TPM | 1 | 487 | 0.55 (0.15 to 2.06) | NA | 5.4% | 1.03 (0.84 to 1.27) |
| OXC vs GBP | 1 | 498 | 0.73 (0.08 to 6.49) | NA | 4.6% | 1.32 (1.08 to 1.63) |
| OXC vs LEV | No direct evidence | 0% | 1.05 (0.83 to 1.32) | |||
| OXC vs ZNS | No direct evidence | 0% | 1.19 (0.84 to 1.69) | |||
| TPM vs GBP | 1 | 635 | 1.31 (0.15 to 11.2) | NA | 3.5% | 1.28 (1.09 to 1.51) |
| TPM vs LEV | No direct evidence | 0% | 1.01 (0.83 to 1.23) | |||
| TPM vs ZNS | No direct evidence | 0% | 1.15 (0.84 to 1.59) | |||
| GBP vs LEV | No direct evidence | 0% | 0.79 (0.65 to 0.96) | |||
| GBP vs ZNS | No direct evidence | 0% | 0.90 (0.65 to 1.24) | |||
| LEV vs ZNS | No direct evidence | 0% | 1.14 (0.83 to 1.57) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; zNS: Zonisamide aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). For comparisons marked with a *, confidence intervals of direct evidence and network meta‐analysis do not overlap indicating that inconsistency may be present in the results. | ||||||
| Comparisiona | Direct evidence (pairwise meta‐analysis) | Direct plus indirect evidence | ||||
| Number | Number | HR (95% CI)2,3 | I² statistic4 | Direct | HR (95% CI)2,3 | |
| CBZ vs PHB | 5 | 237 | 0.55 (0.33 to 0.92) | 50.4% | 35.5% | 1.10 (0.80 to 1.51) |
| CBZ vs PHT | 3 | 150 | 0.88 (0.51 to 1.54) | 0% | 26.6% | 0.76 (0.59 to 0.98) |
| CBZ vs VPS | 4 | 411 | 1.37 (0.98 to 1.92) | 84.1% | 10.4% | 0.88 (0.76 to 1.03) |
| CBZ vs LTG | 7 | 302 | 1.49 (0.94 to 2.35) | 0% | 0.3% | 0.98 (0.70 to 1.37) |
| CBZ vs OXC | 1 | 9 | 1.55 (0.38 to 6.31) | NA | 9% | 1.09 (0.36 to 3.36) |
| CBZ vs TPM | 2 | 101 | 1.19 (0.56 to 2.50) | 62% | 9% | 1.15 (0.89 to 1.48) |
| CBZ vs GBP | 1 | 6 | 2.83 (0.31 to 25.5) | NA | 10.7% | 0.79 (0.10 to 6.08) |
| CBZ vs LEV | 2 | 251 | 1.04 (0.65 to 1.64) | 0% | 44.9% | 1.19 (0.78 to 1.83) |
| PHB vs PHT | 4 | 161 | 1.41 (0.76 to 2.62) | 46.9% | 20.3% | 0.69 (0.48 to 1.00) |
| PHB vs VPS | 2 | 98 | 1.87 (0.87 to 4.00) | 69.8% | 6.5% | 0.80 (0.57 to 1.12) |
| PHB vs LTG | No direct evidence | 0% | 0.89 (0.56 to 1.42) | |||
| PHB vs OXC | No direct evidence | 0% | 1.00 (0.31 to 3.20) | |||
| PHB vs TPM | No direct evidence | 0% | 1.05 (0.70 to 1.56) | |||
| PHB vs GBP | No direct evidence | 0% | 0.72 (0.09 to 5.68) | |||
| PHB vs LEV | No direct evidence | 0% | 1.09 (0.64 to 1.85) | |||
| PHT vs VPS | 4 | 394 | 1.11 (0.71 to 1.74) | 0% | 36.4% | 1.16 (0.88 to 1.53) |
| PHT vs LTG | 1 | 91 | 1.00 (0.40 to 2.46) | NA | 16.2% | 1.29 (0.85 to 1.97) |
| PHT vs OXC | 2 | 154 | 0.60 (0.33 to 1.10) | 49.7% | 25.2% | 1.44 (0.46 to 4.56) |
| PHT vs TPM | 1 | 150 | 0.63 (0.18 to 2.26) | NA | 9.8% | 1.51 (1.06 to 2.15) |
| PHT vs GBP | No direct evidence | 0% | 1.05 (0.13 to 8.14) | |||
| PHT vs LEV | No direct evidence | 0% | 1.57 (0.96 to 2.58) | |||
| VPS vs LTG | 3 | 377 | 0.64 (0.37 to 1.11) | 23.2% | 31.3% | 1.11 (0.77 to 1.60) |
| VPS vs OXC | No direct evidence | 0% | 1.24 (0.40 to 3.84) | |||
| VPS vs TPM* | 2 | 441 | 0.42 (0.23 to 0.80) | 46.4% | 21% | 1.30 (1.01 to 1.68) |
| VPS vs GBP | No direct evidence | 0% | 0.90 (0.12 to 6.92) | |||
| VPS vs LEV | 1 | 512 | 0.82 (0.48 to 1.40) | NA | 34% | 1.35 (0.86 to 2.13) |
| LTG vs OXC | 1 | 10 | 0.94 (0.25 to 3.57) | NA | 12.2% | 1.12 (0.36 to 3.48) |
| LTG vs TPM | 1 | 14 | 0.61 (0.28 to 1.30) | NA | 13.1% | 1.17 (0.78 to 1.77) |
| LTG vs GBP | 1 | 7 | 1.72 (0.20 to 14.9) | NA | 11.9% | 0.81 (0.11 to 6.25) |
| LTG vs LEV | No direct evidence | 0% | 1.22 (0.71 to 2.10) | |||
| OXC vs TPM | 1 | 14 | 1.90 (0.50 to 7.19) | NA | 13.6% | 1.05 (0.34 to 3.24) |
| OXC vs GBP | 1 | 7 | 1.83 (0.20 to 16.5) | NA | 13.3% | 0.73 (0.08 to 6.49) |
| OXC vs LEV | No direct evidence | 0% | 1.09 (0.33 to 3.62) | |||
| TPM vs GBP | 1 | 11 | 0.96 (0.11 to 8.29) | NA | 13.2% | 0.69 (0.09 to 5.32) |
| TPM vs LEV | No direct evidence | 0% | 1.04 (0.63 to 1.71) | |||
| GBP vs LEV | No direct evidence | 0% | 1.50 (0.19 to 12.0) | |||
| CBZ: carbamazepine; CI: confidence interval; GBP: gabapentin; HR: hazard ratio; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity aOrder of drugs in the table: most commonly used drug first (carbamazepine), then drugs are ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). For comparisons marked with a *, confidence intervals of direct evidence and network meta‐analysis do not overlap indicating that inconsistency may be present in the results | ||||||
| Drug | Number of participants | Number of participants | Number of events |
| CBZ | 5134 | 3023 | 9769 |
| PHB | 754 | 271 | 181 |
| PHT | 1384 | 614 | 1513 |
| VPS | 2303 | 1294 | 3599 |
| LTG | 3107 | 1608 | 6296 |
| OXC | 978 | 623 | 1000 |
| TPM | 1898 | 920 | 6316 |
| GBP | 1209 | 506 | 2580 |
| LEV | 948 | 1441 | 4258 |
| ZNS | 282 | 182 | 606 |
| Total | 18,045 | 10,482 | 36,118 |
| CBZ: carbamazepine; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aAdverse event data were provided as detailed individual participant data for 23 trials and we extracted summary adverse event information from 36 trial publications. No adverse event data were reported in 18 trial publications. | |||
| Event (general description)a,b,c | CBZ | PHB | PHT | VPS | LTG | OXC | TPM | GBP | LEV | ZNS | Total |
| Accidental injury | 100 | 0 | 100 | 28 | 110 | 5 | 95 | 36 | 58 | 8 | 540 |
| Anorexia or weight loss | 126 | 0 | 126 | 24 | 116 | 6 | 394 | 58 | 62 | 25 | 937 |
| Anxiety/depression | 203 | 0 | 203 | 59 | 171 | 32 | 309 | 82 | 163 | 16 | 1238 |
| Aphasia | 59 | 7 | 66 | 11 | 26 | 4 | 106 | 22 | 11 | 2 | 314 |
| Asthenia | 59 | 1 | 60 | 26 | 41 | 1 | 31 | 33 | 37 | 10 | 299 |
| Ataxia | 172 | 37 | 209 | 32 | 55 | 17 | 61 | 40 | 32 | 8 | 663 |
| Cognitive (memory, concentration, confusion etc.) | 321 | 41 | 362 | 100 | 204 | 44 | 439 | 127 | 73 | 19 | 1730 |
| Dental problems | 93 | 0 | 93 | 28 | 62 | 5 | 61 | 24 | 70 | 7 | 443 |
| Dizziness/faintness | 617 | 0 | 617 | 171 | 348 | 140 | 269 | 160 | 394 | 23 | 2739 |
| Drowsiness/fatigue | 1270 | 1 | 1271 | 422 | 539 | 233 | 628 | 326 | 477 | 33 | 5200 |
| Fever or viral infection | 379 | 0 | 379 | 68 | 172 | 24 | 84 | 58 | 338 | 37 | 1539 |
| Gastrointestinal disturbances | 683 | 20 | 703 | 246 | 394 | 33 | 236 | 142 | 284 | 42 | 2783 |
| Hair loss | 47 | 0 | 47 | 130 | 22 | 15 | 39 | 8 | 16 | 3 | 327 |
| Headache or migraine | 843 | 0 | 843 | 264 | 556 | 137 | 315 | 171 | 596 | 47 | 3772 |
| Impotence | 90 | 24 | 114 | 13 | 17 | 0 | 27 | 32 | 11 | 3 | 331 |
| Increased/worsened seizures | 151 | 0 | 151 | 31 | 164 | 6 | 58 | 48 | 140 | 6 | 755 |
| Infection | 121 | 0 | 121 | 19 | 90 | 4 | 56 | 27 | 63 | 5 | 506 |
| Laboratory results abnormal | 367 | 0 | 367 | 103 | 117 | 8 | 47 | 19 | 90 | 32 | 1150 |
| Menstrual problems | 110 | 0 | 110 | 28 | 31 | 1 | 22 | 18 | 39 | 4 | 363 |
| Mood or behavioural change | 279 | 41 | 320 | 128 | 163 | 25 | 415 | 121 | 121 | 15 | 1628 |
| Nausea/vomiting | 413 | 1 | 414 | 167 | 233 | 53 | 132 | 92 | 142 | 20 | 1667 |
| Pain | 345 | 1 | 346 | 65 | 250 | 6 | 154 | 48 | 251 | 25 | 1491 |
| Paraesthesia or tingling | 56 | 0 | 56 | 22 | 33 | 2 | 708 | 34 | 28 | 7 | 946 |
| Problems sleeping/nightmares | 108 | 1 | 109 | 46 | 197 | 16 | 147 | 31 | 101 | 14 | 770 |
| Rash or skin disorder | 701 | 17 | 718 | 46 | 420 | 73 | 163 | 113 | 125 | 31 | 2407 |
| Renal/urinary disorder | 152 | 0 | 152 | 27 | 78 | 2 | 92 | 57 | 93 | 21 | 674 |
| Respiratory disorder | 233 | 0 | 233 | 53 | 124 | 4 | 190 | 23 | 131 | 17 | 1008 |
| Tremor or twitch | 171 | 1 | 172 | 258 | 219 | 19 | 56 | 23 | 51 | 2 | 972 |
| Visual disturbance/nystagmus | 199 | 0 | 199 | 53 | 96 | 33 | 86 | 59 | 33 | 8 | 766 |
| Weight gain | 259 | 0 | 259 | 347 | 167 | 22 | 71 | 258 | 70 | 1 | 1454 |
| CBZ: carbamazepine; GBP: gabapentin; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide aVerbatim or reported terms extracted from publications or provided in individual participant data were grouped under the definitions by one review author (SJN) and any uncertainties in definition were discussed with the senior clinical author (AGM). bAdverse event data were provided as detailed individual participant data for 23 trials and we extracted summary adverse event information from 36 trial publications. No adverse event data were reported in 18 trial publications. cSome trial publications reported only on the “most common” adverse events, the totals and frequencies are likely to be an underestimation of the true number of events and number of individuals experiencing events. Furthermore, detailed information was provided in the more recent trial publications and individual participant data requests of more recent trials, often involving newer antiepileptic drugs, such as LTG, LEV and TPM; which may indicate that these newer drugs are associated with more adverse events than older drugs such as PHB and PHT ,for which less detailed information was available. | |||||||||||

