Haloperidol discontinuation for people with schizophrenia
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomly assigned. Blindness: double‐blind. Setting: inpatients and outpatients. | |
| Participants | Diagnosis: schizophrenia (Research Diagnostic Criteria). N = 43. History: "stable and requiring antipsychotic medication to prevent relapse"; "all participants were stable and given haloperidol decanoate injections 60 mg four weekly for 15 weeks before randomisation". | |
| Interventions | 1. Haloperidol discontinuation: replacement of haloperidol decanoate injections with placebo injections 4 weekly. N = 23. 2. Haloperidol continuation: continuation of haloperidol decanoate injections 60 mg/4 weeks. N = 20. | |
| Outcomes | Global state: relapse. Satisfaction with treatment: leaving the study early. Unable to use Mental state: Comprehensive Psychopathological Rating Scale (data not reported for both groups). Adverse effects: scales for evaluation of extrapyramidal side‐effects and tardive dyskinesia (data not reported for both groups). | |
| Notes | The authors did not report on conflict of interest. Funding was from the Swedish Medical Research Council and Janssen Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | Double using placebo injections. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No further details regarding blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis was used but the attrition rate was high: 42% left the study early. The attrition rate was higher in the placebo group and the reasons differed. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | Partially funded by Janssen Pharma. |
| Methods | Allocation: randomly assigned. Blindness: double‐blind using unmarked blind capsules. Setting: hospital‐based. Duration: 3 weeks. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐III‐R). N = 30 (21 available for analyses). Sex: men. History: stable on haloperidol, "participants were judged to be clinically stable with a mean Bunney‐Hamburg Psychosis rating of 4.3 ± 1.6". | |
| Interventions | 1. Haloperidol discontinuation: haloperidol discontinued and replaced with placebo. N = 9. 2. Haloperidol continuation: mean daily dose of 10.5 ± 4.2 mg, range 4 mg to 20 mg. N = 12. | |
| Outcomes | Cognitive functioning: recent memory (Wechsler Memory Scale‐Revised ‐‐ WMS‐R), remote memory (Boston Remote Memory Battery). Unable to use Leaving the study early: 9 participants were "unavailable for analysis". Not clear why. Mental state: Bunney‐Hamburg Psychosis Scale (Bunney 1963) (lack of data). Cognitive functioning: Trail Making A & B, and Visual Continuous Performance Test for attention (lack of data). | |
| Notes | The authors did not report on conflict of interest. They were funded by the National Institute of Mental Health, the Department of Veterans Affairs Research and Development Service, and the Bataan and Corregidor Medical Research Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind using unmarked blind capsules that remained equivalent in appearance and number throughout the study. |
| Blinding of outcome assessment (detection bias) | Low risk | "Assessment of the clinical status of subjects was conducted blindly on a daily basis throughout the duration of the study. Neuropsychological testing was administered to each subject and scored by a trained research assistant who was blind with regard to the clinical and medication status of the patient". |
| Incomplete outcome data (attrition bias) | Unclear risk | 30 participants were initially recruited and randomised. 9 participants (30%) were excluded from the outcome analysis for different reasons. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | No indication of other bias. |
| Methods | Allocation: randomly assigned. Duration: 1 year. | |
| Participants | Diagnosis: schizophrenia (DSM‐III). N = 87. | |
| Interventions | 1. Haloperidol discontinuation: replaced with placebo. N = 13. 2. Haloperidol continuation: fixed doses 1, 3 or 6 mg/day. N = 37. 3. Propericiazine: fixed dose 10, 30 or 60 mg/day. N = 37. All 3 groups also given biperidine and nitrazepam. Haloperidol discontinuation was the only randomised intervention. | |
| Outcomes | Global state: relapse. Satisfaction with treatment: "strong request to change the prescribed drug were indicators of dissatisfaction with treatment". | |
| Notes | For the purpose of this review, we have considered only the haloperidol continuation group and the placebo group. We combined all haloperidol doses in a single group. No information available about funding or conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | Double, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition rates in the haloperidol and the placebo groups. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | No indication of other bias. |
| Methods | Allocation: implied randomisation. | |
| Participants | Diagnosis: chronic schizophrenia maintained on haloperidol (mean dose 9.3 mg/day). | |
| Interventions | 1. Haloperidol discontinuation: haloperidol tablets replaced with placebo tablets and liquid. N = 16. (12 analysed) 2. Haloperidol continuation: haloperidol tablets, mean dose 8.8 mg/day, and placebo liquid. N = 17. (17 analysed) 3. Haloperidol continuation: haloperidol liquid, mean dose: 10.4 mg/day, and placebo tablets. N = 16. (15 analysed) We combined the two haloperidol continuation groups in a single group. N = 33 (32 analysed) | |
| Outcomes | Global state: improvement, relapse. Unable to use Mental state: Brief Psychiatric Rating Scale (no SD). Global state: Clinical Global Impression of Severity (no SD). Behaviour: Nurses Observation Scale for Inpatient Evalutation (no SD). | |
| Notes | No information available about funding or conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is implied ‒ assumed due to double‐blinding. |
| Allocation concealment (selection bias) | High risk | Implied randomisation ‒ no further details how allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Double, all participants received both tablets and liquid, active or placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double, all participants received both tablets and liquid, active or placebo. |
| Incomplete outcome data (attrition bias) | Low risk | Completer‐only analysis unlikely to be affected by low attrition rate (10%). |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | No indication of other bias. |
| Methods | Allocation: randomly assigned. Duration: 6 months. | |
| Participants | Diagnosis: schizophrenia (DSM‐III). N = 23. Age: mean 60 years. | |
| Interventions | 1. Haloperidol discontinuation: haloperidol dose reduced by 50% for 14 days, then changed to placebo. N = 12 (10 analysed). 2. Haloperidol continuation: at the same dose used at randomisation. N = 11 (8 analysed). | |
| Outcomes | Global state: relapse. Unable to use Mental state: BPRS (data not reported for both groups) Quality of life: data not reported for both groups. Leaving the study early: no details. | |
| Notes | No information available about funding or conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind: "participants and investigators were blind to treatment”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind: "participants and investigators were blind to treatment”. |
| Incomplete outcome data (attrition bias) | Low risk | Comparable low attrition rates in the two groups: 2/12 = 17% in the placebo group and 3/11 = 27% in the haloperidol group. |
| Selective reporting (reporting bias) | High risk | A planned outcome (quality of life) was not reported. |
| Other bias | Unclear risk | No indication of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: not randomised. | |
| Allocation: unclear. Participants: people with schizophrenia. Interventions: olanzapine versus haloperidol, not haloperidol discontinuation. | |
| Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: switching from haloperidol to olanzapine, not haloperidol discontinuation. | |
| Allocation: randomly assigned. Participants: people with schizophrenia, acute agitation. Interventions: risperidone oral solution versus intramuscular haloperidol shifting to oral, not haloperidol, discontinuation. | |
| Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: haloperidol versus quetiapine, not haloperidol discontinuation. | |
| Allocation: randomly assigned. Participants: people with tardive dyskinesia, not schizophrenia. Intervention: haloperidol versus molindone; not haloperidol discontinuation. | |
| Allocation: unclear. Participants: people with schizophrenia. Interventions: switching from haloperidol versus risperidone, not haloperidol discontinuation. | |
| Allocation: not randomised. | |
| Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: switching from long‐acting injectable fluphenazine or haloperidol decanoate to long‐acting injectable risperidone microspheres; not haloperidol discontinuation. | |
| Allocation: unclear. Participants: people with tardive dyskinesia not schizophrenia. Intervention: chlorprothixene versus perphenazine verus haloperidol versus haloperidol + biperiden; not haloperidol discontinuation. | |
| Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: haloperidol versus quetiapine, not haloperidol discontinuation. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: randomly assigned. Blindness: open. Duration: 8 weeks of acute treatment followed by a year of maintenance treatment, then people were randomised for a second year of continuing or discontinuing antipsychotic drug treatment. |
| Participants | Diagnosis: schizophrenia. N = 44. Age = 18 to 55 years. Sex: both. History: first episode. |
| Interventions | Year 1 1. Risperidone. N = 23. 2. Haloperidol: low‐dose. N = 21. Year 2 1. Risperidone: continuation. 2. Risperidone: discontinuation. 3. Haloperidol: continuation. 4. Haloperidol: discontinuation |
| Outcomes | Global state: Relapse, Clinical Global Impressions. Cognitive functioning. Functioning: Global Assessment of Functioning. |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.33, 3.20] |
| Analysis 1.1  Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term. | ||||
| 2 Global state: relapse Show forest plot | 4 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.18, 2.74] |
| Analysis 1.2  Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 2 Global state: relapse. | ||||
| 2.1 Short term | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.46] |
| 2.2 Medium term | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.58 [0.63, 33.36] |
| 2.3 Long term | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.13, 3.31] |
| 3 Satisfaction with treatment: various measures ‐ long term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 3 Satisfaction with treatment: various measures ‐ long term. | ||||
| 3.1 Leaving the study early | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.28] |
| 3.2 Switching drugs | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.59] |
| 4 Cognitive functioning: change in memory ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 4 Cognitive functioning: change in memory ‐ short term. | ||||
| 4.1 Recent (WMS‐R) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.21] |
| 4.2 Remote (Verbal Boston Battery) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.79, 5.04] |
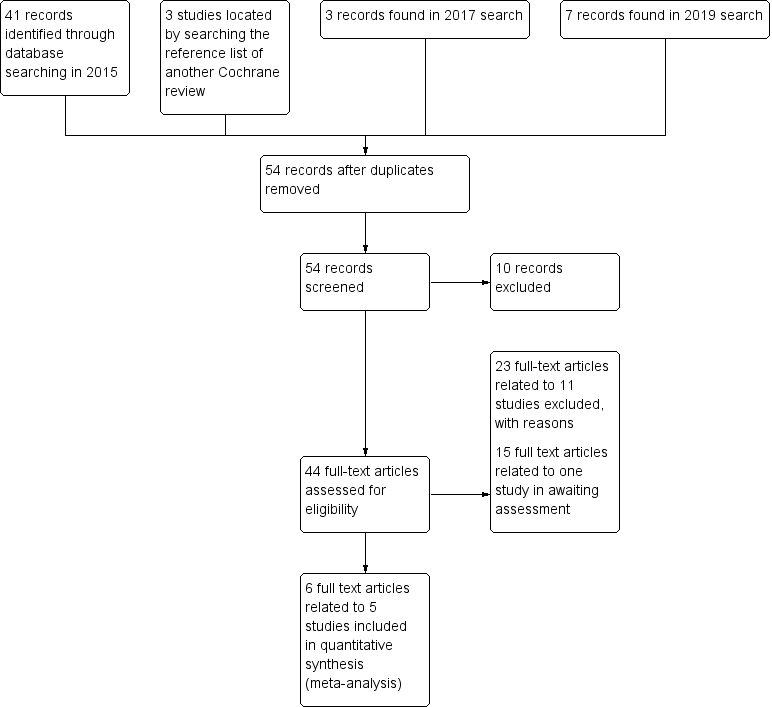
Study flow diagram (for searching up to January 2019)
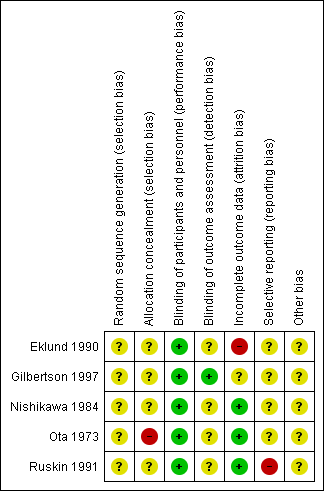
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term.

Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 2 Global state: relapse.

Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 3 Satisfaction with treatment: various measures ‐ long term.
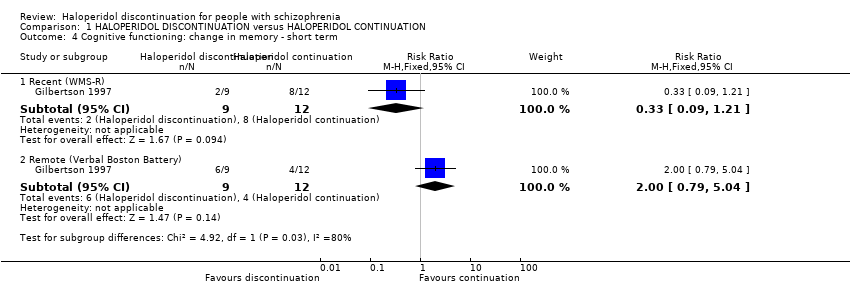
Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 4 Cognitive functioning: change in memory ‐ short term.
| Study | Particular focus of study | Intervention | Cochrane review | |
| #1 | #2 | |||
| Haloperidol | Olanzapine | |||
| Quetiapine | ||||
| Acute agitation | Haloperidol IM and then oral haloperidol | Risperidone oral solution | ||
| Eli 2003; NCT00191555 2005 | Haloperidol continuation | Switching to olanzapine | ||
| Switching to risperidone | ||||
| Fluphenazine decanoate or haloperidol decanoate continuation | Switching to long‐acting injectable risperidone microspheres | |||
| Tardive dyskinesia | Haloperidol | Haloperidol + biperiden | ||
| Chlorprothixene | ||||
| Perphenazine | ||||
| Haloperidol + biperiden | Chlorprothixene | |||
| Perphenazine | ||||
| Chlorprothixene | Perphenazine | ‐ | ||
| Haloperidol | Molindone | ‐ | ||
| Methods | Allocation: randomised ‒ clearly described generation of sequence and concealment of allocation Blinding: double ‐ described and tested Duration: 3 years |
| Participants | People with schizophrenia stable on haloperidol for at least 1 month N = 500 Age: any Sex: both |
| Interventions | 1. Haloperidol continuation 2. Haloperidol discontinuation (placebo after gradual withdrawal of haloperidol) |
| Outcomes | Relapse (primary outcome) Death ‒ suicide and natural causes Global state General functioning Behaviour Adverse effects ‒ general and specific Satisfaction with treatment (including subjective well‐being and family burden) Quality of life Economic outcomes Cognitive functioning |
| Haloperidol discontinuation compared to haloperidol continuation for schizophrenia | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with haloperidol continuation | Risk with haloperidol discontinuation | |||||
| Global state: clinically important change ‒ not improved (CGI) | Study population | RR 2.06 | 49 | ⊕⊝⊝⊝ | A single short term study. Placebo was compared to haloperidol tablets and haloperidol liquid. The two haloperidol groups were combined for this analysis. | |
| 42 per 100 | 20 per 100 | |||||
| Global state: relapse: | Study population | RR 1.80 | 165 | ⊝⊝⊝ | 1 short‐term, 1 medium‐term and 2 long‐term studies. The longest study was for 1 year. 3 studies used oral haloperidol and 1 used long‐acting intramuscular haloperidol. | |
| 36 per 100 | 68 per 100 | |||||
| Mental state: clinically important change in general mental state | No data were available for these important outcomes. | |||||
| General functioning: clinically important change in general functioning including working ability | ||||||
| General behaviour: clinically important change in general behaviour | ||||||
| Quality of life: clinically important change in quality of life | ||||||
| Satisfaction with treatment: leaving the study early | Study population | RR 0.13 | 43 | ⊕⊝⊝⊝ | ||
| 19 per 100 | 3 per 100 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 2 levels due to very serious risk of bias: randomisation is implied 2 Downgraded by 1 level due to imprecision: data from one trial, small sample size and wide confidence interval 3Downgraded by 1 level due to serious risk of bias: random assignment and allocation concealment unclear 4 Downgraded by 1 level due to inconsistency: high heterogenity I² > 70% 5 Downgraded by 1 level due to serious risk of attrition bias: high attrition rate | ||||||
| Comparison | Reference |
| Aripiprazole versus haloperidol for people with schizophrenia and schizophrenia‐like psychoses | |
| Depot haloperidol decanoate for schizophrenia | |
| Haloperidol (route of administration) for people with schizophrenia | |
| Haloperidol discontinuation for schizophrenia [Protocol of this review] | |
| Haloperidol dose for the acute phase of schizophrenia | |
| Haloperidol for long‐term aggression in psychosis | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Haloperidol versus chlorpromazine for schizophrenia | |
| Haloperidol versus first‐generation antipsychotics for the treatment of schizophrenia and other psychotic disorders | |
| Haloperidol versus low‐potency first‐generation antipsychotic drugs for schizophrenia | |
| Haloperidol versus placebo for schizophrenia | |
| Haloperidol versus risperidone for schizophrenia |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.33, 3.20] |
| 2 Global state: relapse Show forest plot | 4 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.18, 2.74] |
| 2.1 Short term | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.46] |
| 2.2 Medium term | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.58 [0.63, 33.36] |
| 2.3 Long term | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.13, 3.31] |
| 3 Satisfaction with treatment: various measures ‐ long term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Leaving the study early | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.28] |
| 3.2 Switching drugs | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.59] |
| 4 Cognitive functioning: change in memory ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Recent (WMS‐R) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.21] |
| 4.2 Remote (Verbal Boston Battery) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.79, 5.04] |

