Haloperidol discontinuation for people with schizophrenia
Appendices
Appendix 1. Previous searches
Search in Protocol
Electronic searches
1. Cochrane Schizophrenia Group’s Trials Register
The Trials Search Co‐ordinator of the Cochrane Schizophrenia Group will search the Group's Specialised Register (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/SCHIZ/frame.html) using the following search terms:
(haloperi* or R‐1625 or haldol* or alased* or aloperidi* or bioperido* or buterid* or ceree* or dozic* or duraperido* or fortuna* or serena* or serenel* or seviu* or sigaperid* or sylad* or zafri*) in Title or Abstract of REFERENCE or (haloperi* or R‐1625 or haldol* or alased* or aloperidi* or bioperido* or buterid* or ceree* or dozic* or duraperido* or fortuna* or serena* or serenel* or seviu* or sigaperid* or sylad* or zafri*) in Intervention of STUDY
The Cochrane Schizophrenia Group'ss Specialised Register is compiled by systematic searches of major resources (including AMED, BIOSIS, 95% CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of Clinical Trials) and their monthly updates, hand‐searches, grey literature, and conference proceedings.
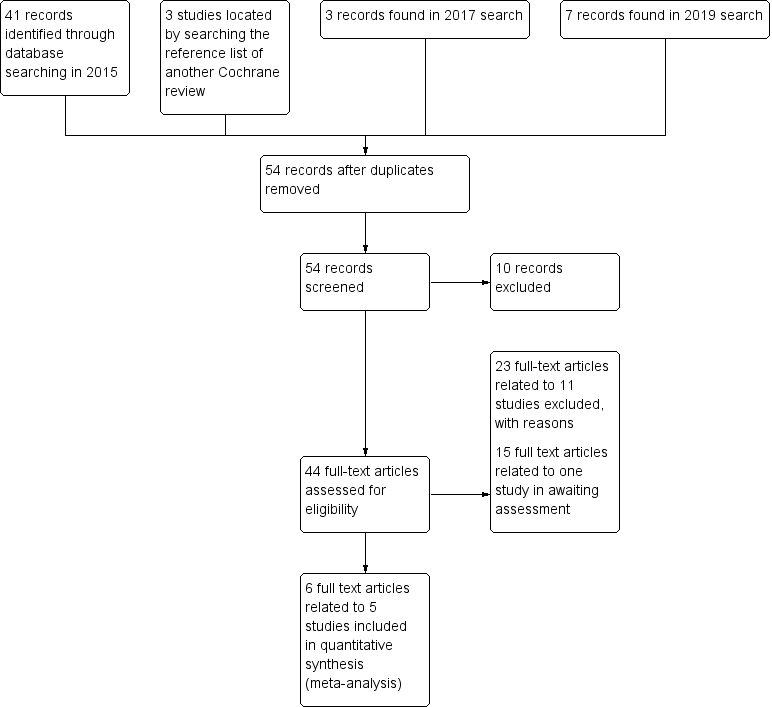
Study flow diagram (for searching up to January 2019)
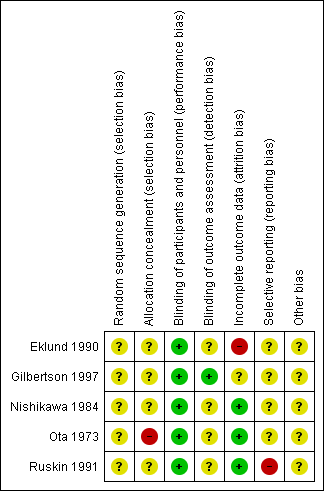
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term.

Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 2 Global state: relapse.

Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 3 Satisfaction with treatment: various measures ‐ long term.
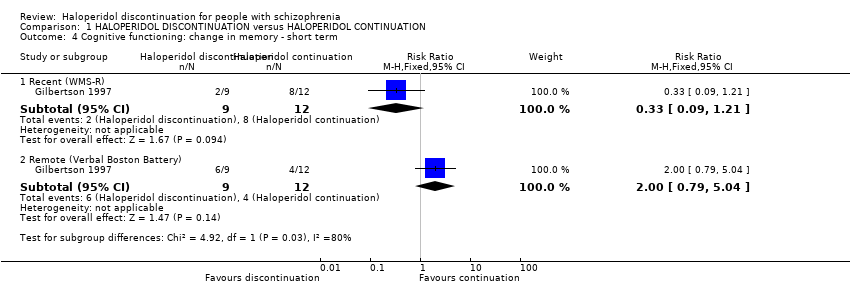
Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 4 Cognitive functioning: change in memory ‐ short term.
| Study | Particular focus of study | Intervention | Cochrane review | |
| #1 | #2 | |||
| Haloperidol | Olanzapine | |||
| Quetiapine | ||||
| Acute agitation | Haloperidol IM and then oral haloperidol | Risperidone oral solution | ||
| Eli 2003; NCT00191555 2005 | Haloperidol continuation | Switching to olanzapine | ||
| Switching to risperidone | ||||
| Fluphenazine decanoate or haloperidol decanoate continuation | Switching to long‐acting injectable risperidone microspheres | |||
| Tardive dyskinesia | Haloperidol | Haloperidol + biperiden | ||
| Chlorprothixene | ||||
| Perphenazine | ||||
| Haloperidol + biperiden | Chlorprothixene | |||
| Perphenazine | ||||
| Chlorprothixene | Perphenazine | ‐ | ||
| Haloperidol | Molindone | ‐ | ||
| Methods | Allocation: randomised ‒ clearly described generation of sequence and concealment of allocation Blinding: double ‐ described and tested Duration: 3 years |
| Participants | People with schizophrenia stable on haloperidol for at least 1 month N = 500 Age: any Sex: both |
| Interventions | 1. Haloperidol continuation 2. Haloperidol discontinuation (placebo after gradual withdrawal of haloperidol) |
| Outcomes | Relapse (primary outcome) Death ‒ suicide and natural causes Global state General functioning Behaviour Adverse effects ‒ general and specific Satisfaction with treatment (including subjective well‐being and family burden) Quality of life Economic outcomes Cognitive functioning |
| Haloperidol discontinuation compared to haloperidol continuation for schizophrenia | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with haloperidol continuation | Risk with haloperidol discontinuation | |||||
| Global state: clinically important change ‒ not improved (CGI) | Study population | RR 2.06 | 49 | ⊕⊝⊝⊝ | A single short term study. Placebo was compared to haloperidol tablets and haloperidol liquid. The two haloperidol groups were combined for this analysis. | |
| 42 per 100 | 20 per 100 | |||||
| Global state: relapse: | Study population | RR 1.80 | 165 | ⊝⊝⊝ | 1 short‐term, 1 medium‐term and 2 long‐term studies. The longest study was for 1 year. 3 studies used oral haloperidol and 1 used long‐acting intramuscular haloperidol. | |
| 36 per 100 | 68 per 100 | |||||
| Mental state: clinically important change in general mental state | No data were available for these important outcomes. | |||||
| General functioning: clinically important change in general functioning including working ability | ||||||
| General behaviour: clinically important change in general behaviour | ||||||
| Quality of life: clinically important change in quality of life | ||||||
| Satisfaction with treatment: leaving the study early | Study population | RR 0.13 | 43 | ⊕⊝⊝⊝ | ||
| 19 per 100 | 3 per 100 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 2 levels due to very serious risk of bias: randomisation is implied 2 Downgraded by 1 level due to imprecision: data from one trial, small sample size and wide confidence interval 3Downgraded by 1 level due to serious risk of bias: random assignment and allocation concealment unclear 4 Downgraded by 1 level due to inconsistency: high heterogenity I² > 70% 5 Downgraded by 1 level due to serious risk of attrition bias: high attrition rate | ||||||
| Comparison | Reference |
| Aripiprazole versus haloperidol for people with schizophrenia and schizophrenia‐like psychoses | |
| Depot haloperidol decanoate for schizophrenia | |
| Haloperidol (route of administration) for people with schizophrenia | |
| Haloperidol discontinuation for schizophrenia [Protocol of this review] | |
| Haloperidol dose for the acute phase of schizophrenia | |
| Haloperidol for long‐term aggression in psychosis | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Haloperidol versus chlorpromazine for schizophrenia | |
| Haloperidol versus first‐generation antipsychotics for the treatment of schizophrenia and other psychotic disorders | |
| Haloperidol versus low‐potency first‐generation antipsychotic drugs for schizophrenia | |
| Haloperidol versus placebo for schizophrenia | |
| Haloperidol versus risperidone for schizophrenia |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.33, 3.20] |
| 2 Global state: relapse Show forest plot | 4 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.18, 2.74] |
| 2.1 Short term | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.46] |
| 2.2 Medium term | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.58 [0.63, 33.36] |
| 2.3 Long term | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.13, 3.31] |
| 3 Satisfaction with treatment: various measures ‐ long term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Leaving the study early | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.28] |
| 3.2 Switching drugs | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.59] |
| 4 Cognitive functioning: change in memory ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Recent (WMS‐R) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.21] |
| 4.2 Remote (Verbal Boston Battery) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.79, 5.04] |

