Tratamiento para la crioglobulinemia mixta asociada al virus de la hepatitis C
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Results are clearly stated |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation assignment was computer‐generated by an off‐site biostatistician using block sizes of 2 |
| Allocation concealment (selection bias) | Low risk | Patient assignments were sealed in opaque envelopes that were marked on the outside with a sequence number |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 37 patients completed the study". |
| Selective reporting (reporting bias) | Low risk | Outcomes well reported. |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control groups (non‐rituximab)
Both groups
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised at a ratio of 1:1"; method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis. 59 patients randomised. From non‐rituximab group, 23 patients were rescued with rituximab and from rituximab group, 3 patients lost to follow‐up but all data is presented |
| Selective reporting (reporting bias) | Low risk | All data referred in the "methods section" is presented |
| Other bias | Low risk | Unfunded study. Roche provided the study medication but, "Roche had no role in the study design or in the collection, analysis, or interpretation of the data" |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | In 'Table 2', there are 5 patients that drop‐out and the authors did not present these data |
| Selective reporting (reporting bias) | High risk | In 'Methods Section' the authors specify that "renal and neurological involvement" and they did not report these outcomes later |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or attrition reported |
| Selective reporting (reporting bias) | Low risk | Reported clearly the different outcomes |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Well specified. Quote: "All patients were followed for at least 12 months after the end of treatment". |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | There are also clinical variables that need blinding |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data balanced in number across intervention groups, but the authors did not impute it using appropriate methods |
| Selective reporting (reporting bias) | Low risk | Data well specified |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Dose changes within 25% of the dose specified in the protocol schedule were allowed | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation were performed centrally in a 1/1 ratio within balanced blocks of 8, stratified by the centre". |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Neurological impairment and extent of purpura was done by someone that is not well specified if was blinded or not. |
| Incomplete outcome data (attrition bias) | Low risk | Data well specified |
| Selective reporting (reporting bias) | High risk | No data at the end of the study of these items: purpura, joint pain, weakness, kidney impairment, neurological impairment, liver impairment |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned in a 1 to 1"; method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Central allocation: NIH Clinical Center Pharmacy performed the randomisation. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Data is detailed. It is indicated that "analyses were performed on an intention‐to‐treat basis". The authors also stated that they present a "selected adverse effects" but they refer to each outcome defined in the methods section |
| Selective reporting (reporting bias) | Low risk | Data well specified |
| Other bias | Low risk | Study appears free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Patients received immunosuppressive therapy for 12 weeks, then were randomly assigned to two groups Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomly assigned”; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Data well specified |
| Selective reporting (reporting bias) | Low risk | Data well specified |
| Other bias | Low risk | Although the immunoadsorption apheresis was interrupted before the scheduled therapy because of rapid relapse of symptomatology, the data of these patients is well reported |
ALT‐ alanine aminotransferase; BVAS ‐ Birmingham Vasculitis Activity Score; CMV ‐ cytomegalovirus; CrCl ‐ creatinine clearance; CSA ‐ cyclosporine; F/M ‐ female/male; IFN ‐ interferon; IV ‐ intravenous; Hb ‐ haemoglobin; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; PCR ‐ polymerase chain reaction; RCT ‐ randomised controlled trial; SC ‐ subcutaneous; SCr ‐ serum creatinine; SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Protocol of a RCT; no results identified | |
| Wrong population: mixed cryoglobulinaemia including different aetiologies with and without HCV | |
| Wrong population: II and III type mixed cryoglobulinaemia including different aetiologies with and without HCV | |
| Wrong population: II type mixed essential cryoglobulinaemia including different aetiologies with and without HCV | |
| Wrong population: II and III type mixed essential cryoglobulinaemia including different aetiologies with and without HCV |
HCV ‐ hepatitis C virus; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 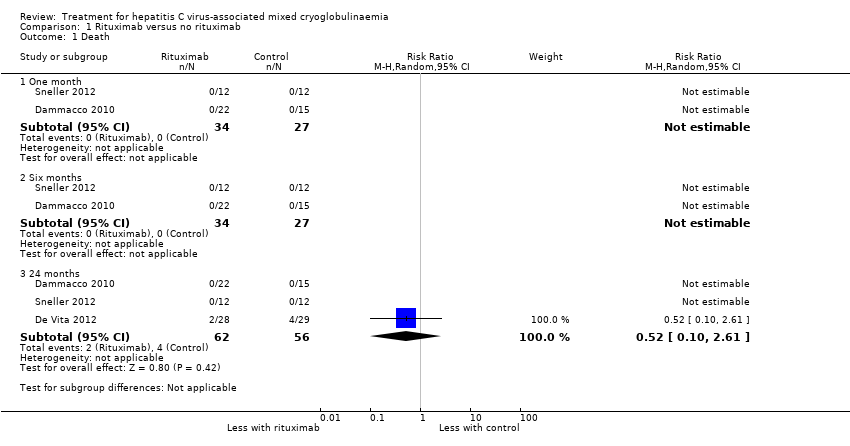 Comparison 1 Rituximab versus no rituximab, Outcome 1 Death. | ||||
| 1.1 One month | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Six months | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 24 months | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 2 Clinical manifestations Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Rituximab versus no rituximab, Outcome 2 Clinical manifestations. | ||||
| 2.1 Active urinary sediment at 1 month | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.65] |
| 2.2 Need for dialysis at 24 months | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Skin vasculitis (purpura, cutaneous ulcers or others) at 1 month | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.55, 12.27] |
| 2.4 Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 2.5 Skin vasculitis (purpura, cutaneous ulcers or others) at 36 months | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.08] |
| 3 Laboratory findings Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3 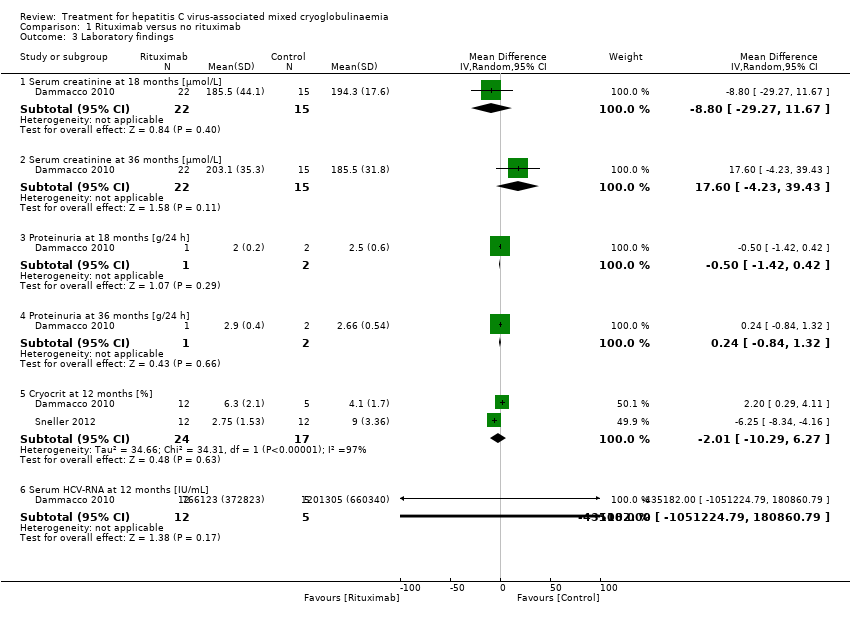 Comparison 1 Rituximab versus no rituximab, Outcome 3 Laboratory findings. | ||||
| 3.1 Serum creatinine at 18 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐8.80 [‐29.27, 11.67] |
| 3.2 Serum creatinine at 36 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 17.60 [‐4.23, 39.43] |
| 3.3 Proteinuria at 18 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.42, 0.42] |
| 3.4 Proteinuria at 36 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.84, 1.32] |
| 3.5 Cryocrit at 12 months [%] | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐10.29, 6.27] |
| 3.6 Serum HCV‐RNA at 12 months [IU/mL] | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐435182.0 [‐1051224.79, 180860.79] |
| 4 Adverse effects of the medication Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 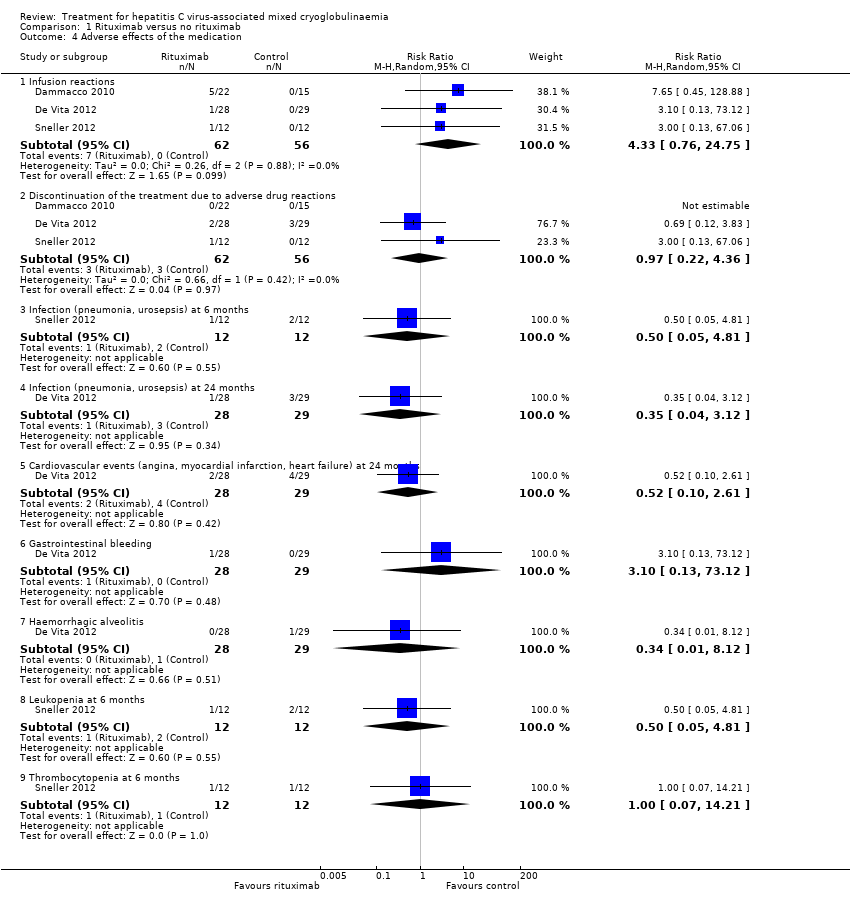 Comparison 1 Rituximab versus no rituximab, Outcome 4 Adverse effects of the medication. | ||||
| 4.1 Infusion reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [0.76, 24.75] |
| 4.2 Discontinuation of the treatment due to adverse drug reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.22, 4.36] |
| 4.3 Infection (pneumonia, urosepsis) at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.4 Infection (pneumonia, urosepsis) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.12] |
| 4.5 Cardiovascular events (angina, myocardial infarction, heart failure) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 4.6 Gastrointestinal bleeding | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.12] |
| 4.7 Haemorrhagic alveolitis | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.12] |
| 4.8 Leukopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.9 Thrombocytopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.21] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.77, 1.90] |
| Analysis 1.5 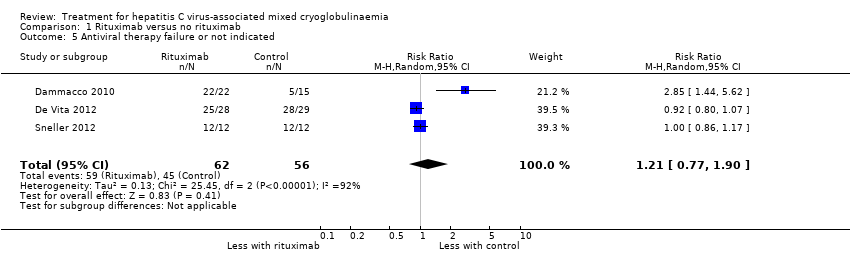 Comparison 1 Rituximab versus no rituximab, Outcome 5 Antiviral therapy failure or not indicated. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1 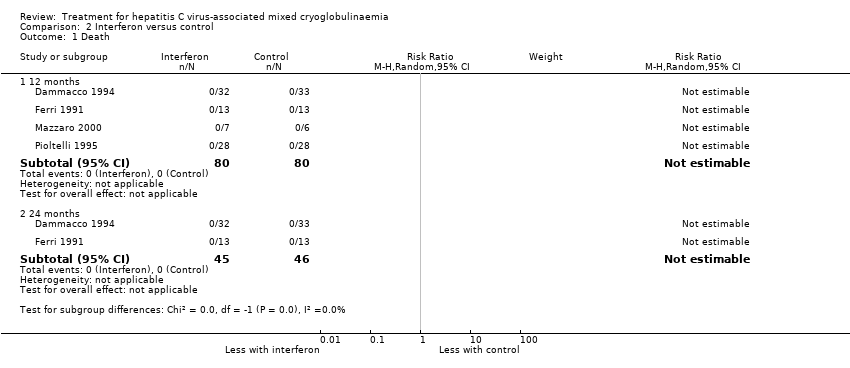 Comparison 2 Interferon versus control, Outcome 1 Death. | ||||
| 1.1 12 months | 4 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 24 months | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 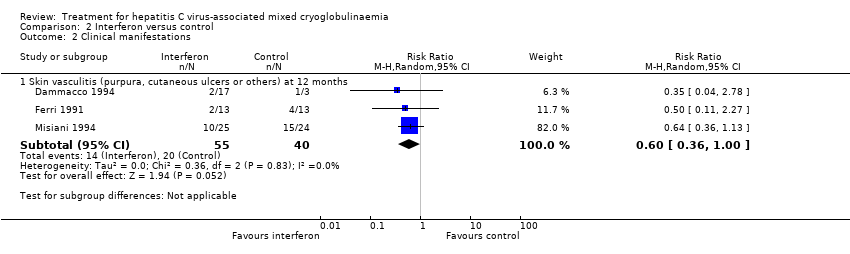 Comparison 2 Interferon versus control, Outcome 2 Clinical manifestations. | ||||
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 12 months | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| 3 Laboratory findings Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Interferon versus control, Outcome 3 Laboratory findings. | ||||
| 3.1 Serum creatinine at 18 months [µmol/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐30.32 [‐80.59, 19.95] |
| 3.2 Proteinuria at 18 months [g/24h] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐2.89, ‐1.07] |
| 3.3 ALT or GPT at 6 months [UI/L] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.89 [‐55.77, 43.99] |
| 3.4 ALT or GPT at 18 months [UI/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐28.28 [‐48.03, ‐8.54] |
| 3.5 Rheumatoid factor activity at 6 months [UI/mL] | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 97.0 [‐187.37, 381.37] |
| 3.6 C4 at 18 months [mg/dL] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.74, 2.67] |
| 3.7 IgM at 18 months [mg/dL] | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐595.75 [‐877.20, ‐314.30] |
| 3.8 Cryocrit at 6 months [%] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.38, ‐0.38] |
| 4 Adverse effects of the medication Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4 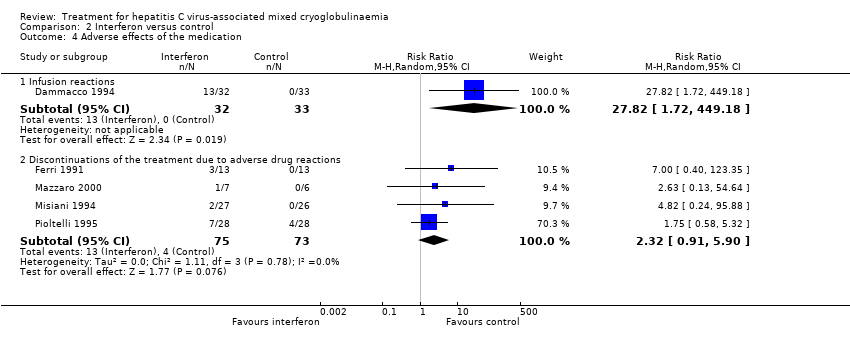 Comparison 2 Interferon versus control, Outcome 4 Adverse effects of the medication. | ||||
| 4.1 Infusion reactions | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 27.82 [1.72, 449.18] |
| 4.2 Discontinuations of the treatment due to adverse drug reactions | 4 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.91, 5.90] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.64] |
| Analysis 2.5 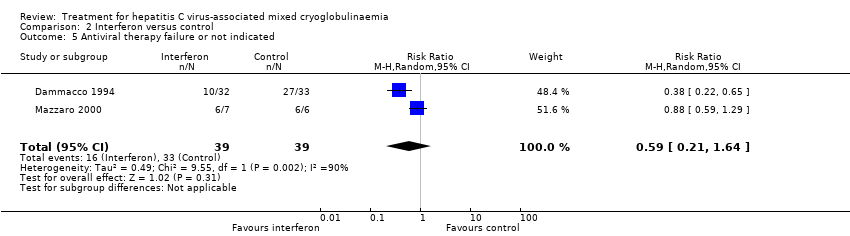 Comparison 2 Interferon versus control, Outcome 5 Antiviral therapy failure or not indicated. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.1  Comparison 3 Interferon for 6 months versus 1 year, Outcome 1 Death at 24 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.1  Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 1 Death at 24 months. | ||||
| 2 Clinical manifestations Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 2 Clinical manifestations. | ||||
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Peripheral neuropathies at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Peripheral joint arthralgia at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Laboratory findings Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 3 Laboratory findings. | ||||
| 3.1 Cryocrit [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects of the medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.4 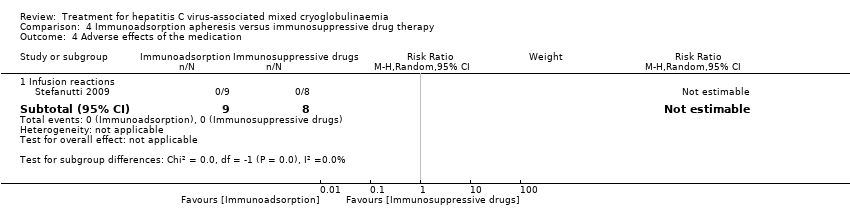 Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 4 Adverse effects of the medication. | ||||
| 4.1 Infusion reactions | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
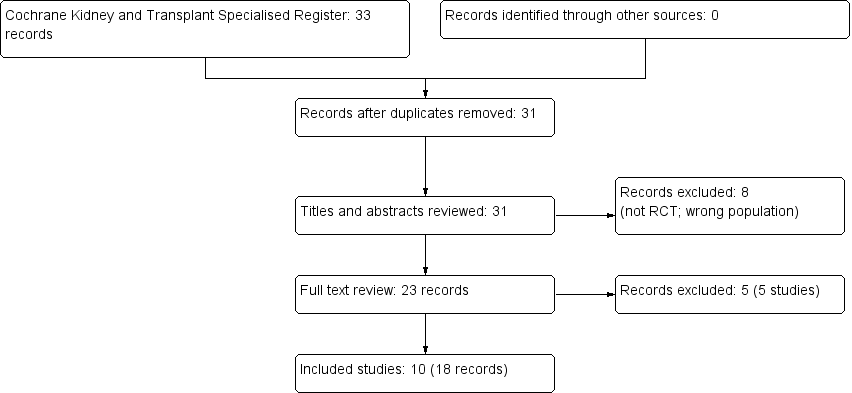
Flow chart showing source and identification of studies for inclusion.
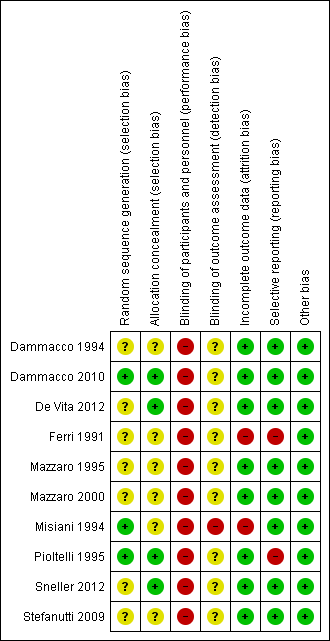
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
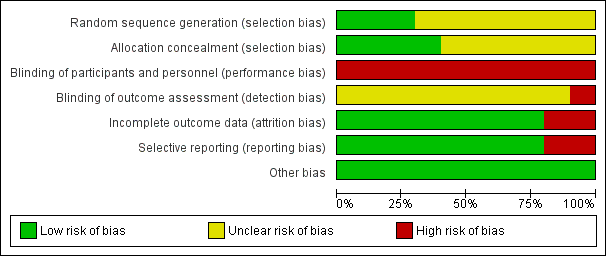
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Rituximab versus no rituximab, Outcome 1 Death.

Comparison 1 Rituximab versus no rituximab, Outcome 2 Clinical manifestations.

Comparison 1 Rituximab versus no rituximab, Outcome 3 Laboratory findings.

Comparison 1 Rituximab versus no rituximab, Outcome 4 Adverse effects of the medication.

Comparison 1 Rituximab versus no rituximab, Outcome 5 Antiviral therapy failure or not indicated.

Comparison 2 Interferon versus control, Outcome 1 Death.
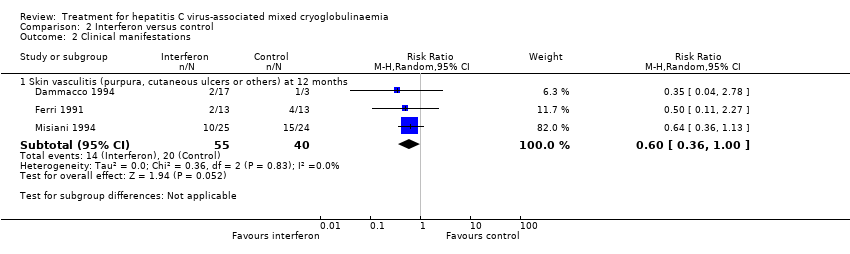
Comparison 2 Interferon versus control, Outcome 2 Clinical manifestations.
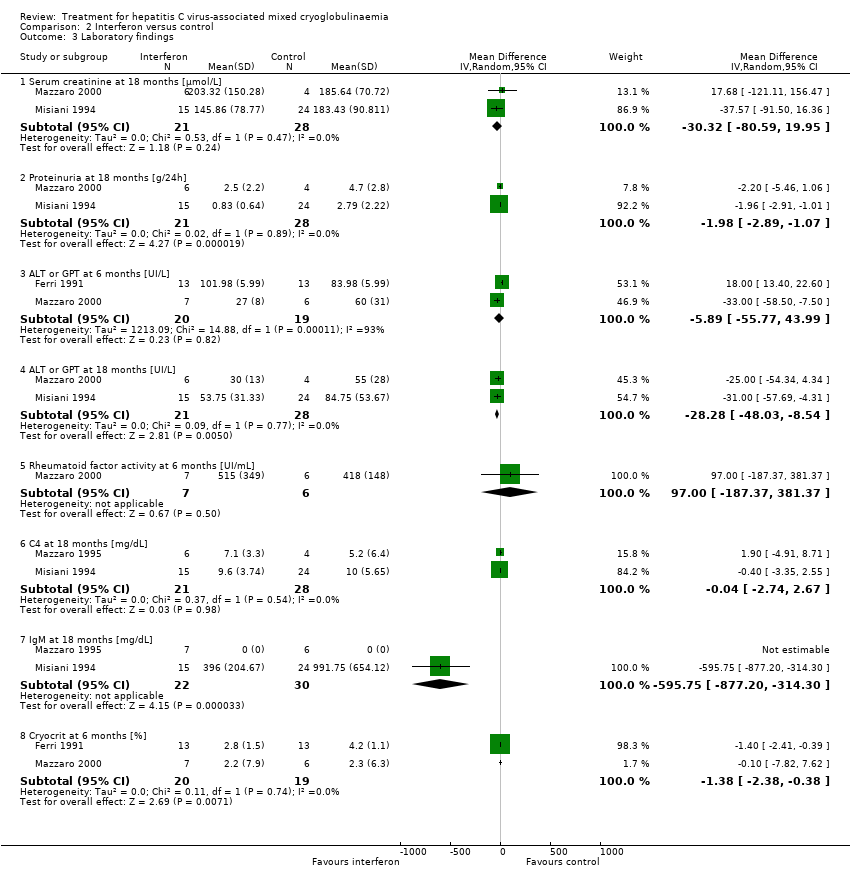
Comparison 2 Interferon versus control, Outcome 3 Laboratory findings.

Comparison 2 Interferon versus control, Outcome 4 Adverse effects of the medication.
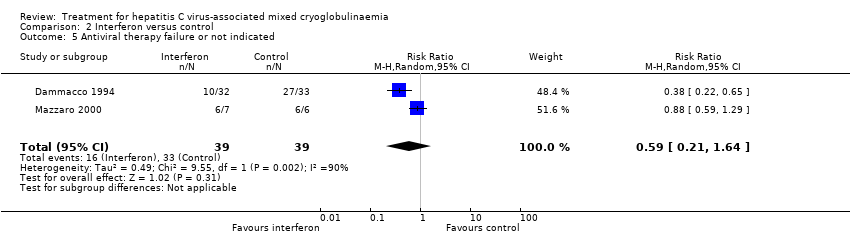
Comparison 2 Interferon versus control, Outcome 5 Antiviral therapy failure or not indicated.

Comparison 3 Interferon for 6 months versus 1 year, Outcome 1 Death at 24 months.
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 1 Death at 24 months.
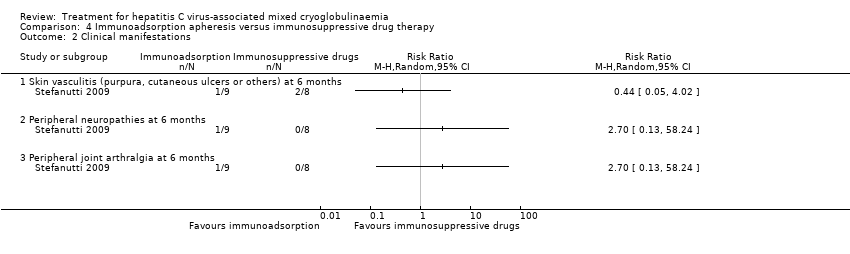
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 2 Clinical manifestations.
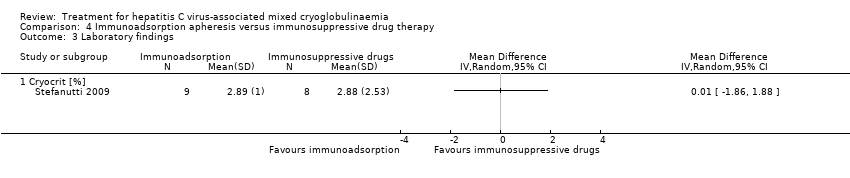
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 3 Laboratory findings.

Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 4 Adverse effects of the medication.
| Rituximab compared to no rituximab for hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Patient or population: hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no rituximab | Risk with rituximab | ||||
| Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months: number of affected patients | Study population | RR 0.57 | 78 (2) | ⊕⊕⊕⊝ | |
| 26 per 100 | 15 per 100 | ||||
| SCr at 18 months | The mean SCr at 18 months was 194.3 μmol/L | MD was 8.8 μmol/L lower | ‐ | 37 (1) | ⊕⊕⊕⊝ |
| Cryocrit at 12 months | The mean cryocrit at 12 months was 6.55% | MD was 2.01% lower | ‐ | 41 (2) | ⊕⊕⊝⊝ |
| Adverse effects ‐ infusion reactions: number of events | Study population | RR 4.33 | 118 (3) | ⊕⊕⊕⊝ | |
| 0 per 100 | 0 per 100 | ||||
| Activity outcomes | De Vita 2012 found a significant reduction in the BVAS at 2 months in the rituximab group (from mean ± SD: 11.9 ± 5.4 to 7.1 ± 5.7; P < 0.001), and this difference persisted at 6 months (6.9 ± 6.8; P < 0.001), 12 months (5.4 ± 6.2; P 0.0001), and 24 months (4.4 ± 4.6; P < 0.0001). Without differences in the control group. Sneller 2012 BVAS scores became significantly lower in the rituximab group at month 4 (from 10.2 ± 8.4, at 6 months: 0 ± 0; P < 0.02). Without differences in control group. | ‐ | 81 (2) | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 95% CI overlaps no effect, and the CI fails to exclude important benefit or important harm 2 Base on tests of heterogeneity which test the null hypothesis that all studies have the same underlying magnitude of effect, have a low P‐value (P = 0.00001), indicating to reject the null hypothesis I2 statistic, which quantifies the proportion of the variation in point estimates due to among‐study differences, is large (97%) 3 High risk of performance bias: non‐blinded participants and personnel | |||||
| Study ID | Study design | Patient profile | Objective | No. of patients | Treatment | Results | Adverse effects |
| Non‐randomised, non‐controlled prospective study | HCV RNA (+) + cryoglobulinaemic syndrome with organ damage and B‐cell lymphoproliferative syndrome | To assess the hepatovirological response, the clinical and immunological efficacy and the safety of using sofosbuvir‐based direct‐acting antiviral therapy in patients with HCV‐associated mixed cryoglobulinaemia(according to the latest guidelines) | 44 | 1) Sofosbuvir+ribavirin (18) 2) Sofosbuvir+simeprevir (12) ± ribavirin (6 of 12 patients) 3) Sofosbuvir+daclatasvir (4) ± ribavirin (1 patient) 4) Sofosbuvir+ledipasvir (10) ± ribavirin (3 patients) | Hepatovirological response ‐ Undetectable HCV RNA negative rate 100% at week 4; 12SVR and 24SVR remained 100% negative ‐ Decrease of ALT from 77.7 ± 10.3 IU/L at baseline to 27.3 ± 10.3 IU/L at 24SVR ‐ Decrease of AST from 55.2 ± 60.4 IU/L at baseline to 22.6 ± 8.3 IU/L at 24SVR (P < 0.001) Clinical efficacy ‐ Decrease of BVAS from 5.41 ± 3.53 at baseline to 1.27 ± 1.68 at 24SVR (P < 0.001) Immunological efficacy ‐ Decrease of cryocrit level from 7.2 ± 15.4% at baseline to 1.8 ± 5.1% at 24SVR (P < 0.001) | Total: 26/44 (59%) Withdrawals: 1 patient withdrew ribavirin while continuing sofosbuvir+simeprevir Death: none Relapse: none Most frequent AE: Anaemia (13, all receiving ribavirin); fatigue (15); nausea (7) | |
| Case series | Five patients with HVC RNA + with detectable cryoglobulins in plasma and symptomatic mixed cryoglobulinaemia Patient 1: bilateral foot neuropathy and purpura Patient 2: painful left foot drop and purpura Patient 3: purpura and MPGN Patient 4: MPGN Patient 5: MPGN + low grade lymphoma | Review of one centre's experience in treating patients with mixed cryoglobulinaemia with new oral antiviral agents and to assess common factors associated with persistence of mixed cryoglobulinaemia despite SVR | 5 | Patient 1: PEG‐IFN+ribavirin+boceprevir Patient 2: Firstly with rituximab (5 weeks); later with: PEG‐IFN+ribavirin+telaprevir for 4 weeks; PEG‐IFN+ribavirin for 12 weeks; and PEG‐IFN+ribavirin+sofosbuvir for 15 weeks (telaprevir discontinued for persistent viral load > 1000 IU/mL) Patient 3: PEG‐IFN+ribavirin+telaprevir for 47 weeks Patient 4: PEG‐IFN+ribavirin for 24 weeks; afterwards adding sofosbuvir until completing 12 weeks of triple therapy (total 36 weeks) Patient 5: 2 cycles of rituximab: weekly infusions 375mg/m2 during 4 weeks in 2010 then two extra doses of rituximab in 2013 ‐PEG‐IFN+ribavirin+sofosbuvir for 8 weeks in 2014 | Patient 1: complete clearance of virus at week 8; complete clearance of cryoglobulins at week 28. Persistence of neuropathy Patient 2: SVR and no detectable cryoglobulins at month 6 after last triple therapy. Persistence of neuropathy. Patient 3: clearance of HCV at week 4 after PEG‐IFN+ribavirin+telaprevir, with persistent cryoglobulins. Active MPGN and vasculitis after ending of previous treatment (responding to steroid therapy). Patient 4: SVR with persistence of cryoglobulinaemia. Kidney function remained stable. | ||
| Retrospective case series | HCV RNA > 1000 IU/mL + circulating purpura + cutaneous ulcers, Raynaud’s phenomenon, arthralgia, sicca syndrome, gastrointestinal vasculitis, neurologic involvement or renal involvement | Comparison of 2 historical cohorts: one treated with PEG‐IFN and ribavirin and the other sofosbuvir+simeprevir (8/12) or sofosbuvir+ribavirin (4/12). Evaluation of 12SVR, relapses, clinical, immunological (cryoglobulins) and biochemical (AST, Hb) response and adverse effects; without statistical comparison between them | 22 | 1) PEG‐IFN+ribavirin 2) IFN‐free regimens 2a) Sofosbuvir 400 mg/24 h + simeprevir 150 mg/24h 2b) Sofosbuvir 400 mg/24 h + ribavirin (adjusted to kidney function) | PEG‐IFN+ribavirin ‐ SVR12: 1/10 IFN‐free regimens ‐ SVR12: 10/12 (95%) ‐ ALT decreased from 42 U/L at baseline to 20 U/L after treatment ‐ Cryoglobulin levels decreased from 1.5% (0.5% to 4%) at baseline to 0.5% (0% to 2%) after treatment ‐ Decrease of proteinuria in all cases of kidney involvement (table IV) | Total PEG‐IFN+ribavirin: 10/10 IFN‐free regimens: 8/12 Withdrawals PEG‐IFN+ribavirin: 5/10 IFN‐free regimens: 1/12 (anxiety and insomnia) Deaths: none Relapses IFN‐free regimens: 2 (genotype 1 sofosbuvir+simeprevir; genotype 4 sofosbuvir+ribavirin) | |
| Open‐label, non‐controlled, prospective cohort study | Chronic active HCV infection with signs of mixed cryoglobulinaemia. All 23 patients had positive cryoglobulins in plasma at baseline or earlier | To analyse the safety and efficacy of Peg‐IFN‐alpha/ribavirin/protease inhibitor combination in HCV‐mixed cryoglobulinaemia | 23 | Peg‐IFN‐alpha+ribavirin a) + telaprevir (375 mg, 3 times/d for 12 weeks) for 48 weeks (15 patients) b) + boceprevir (800 mg, 3 times/d for 44 weeks) for 48 weeks (8 patients) | Complete clinical responders (improvement in all baseline clinical manifestations): 13 patients (56.5%) at week 24 Virological response (i.e., HCV RNA negative) was of 69.6% at week 24 (P = 0.005). Cryoglobulin level: decreased from 0.44 to 0.06 g/L(P = 0.0006) C4 level: increased from 0.09 to 0.15 g/L (P = 0.045) No significant difference was found between the two treatment regiments | Total: 105 Withdrawals: 8 patients (34.7%) ( virological non‐response (5); virological relapse (2); depression (1)) Death: none Relapse: 1 Most frequent AE: fatigue (87%); neutropenia (78.3%); thrombocytopenia (65.2%); infection (47.8%); pruritus (39.1%); depression (21.7%); nausea (21.7%) | |
| Open‐label, non‐controlled, prospective cohort study | Active HCV infection and active mixed cryoglobulinaemia. Excluded non‐active mixed cryoglobulinaemia, HIV or HBV active infection and current decompensated cirrhosis | To evaluate safety and efficacy of an oral IFN‐free regimen, sofosbuvir+ribavirin, in HCV‐mixed cryoglobulinaemia | 24 | Sofosbuvir (400 mg/d) + ribavirin (200 to 1400 mg/d) for 24 weeks “Rituximab was used in four cases, in addition to prednisone and plasmapheresis in two patients” | Complete response (improvement of ALL the affected organs involved at baseline) at week 24: 21 (87.5%) HCV RNA clearance at week 24: 22/24 (91.7%) SVR12: 74% Cryocrit: decrease from 0.35 g/L at baseline to 0 at 12 weeks after end‐of‐treatment. C4: increase from 0.1 g/L at baseline to 0.22 g/L at 12 week after end‐of treatment “No difference of outcome was found in patients who received immunosuppressive treatment or not” | Total: 14/24 (54%) Withdrawals: 2 (8%) (hallucination and irritability (1); grade 4 anaemia (1)) Death: 2 (severe pneumonia in the context of B cell lymphoma; pulmonary embolism in the context of hepatocellular carcinoma) Relapses: none Most frequent AE: (fatigue (25%); anaemia (25%); insomnia (21%); infection (17%); alopecia (8%)) | |
| 12SVR ‐ 12 week SVR; 24SVR ‐ 24 week SVR; AE ‐ adverse event; ALT ‐ alanine aminotransferase; AST‐ aspartate aminotransferase; BVAS ‐ Birmingham Vasculitis Activity Score; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; IFN ‐ interferon; MPGN ‐ membranoproliferative glomerulonephritis; PEG ‐ pegylated; SVR ‐ sustained viral response | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 One month | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Six months | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 24 months | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 2 Clinical manifestations Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Active urinary sediment at 1 month | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.65] |
| 2.2 Need for dialysis at 24 months | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Skin vasculitis (purpura, cutaneous ulcers or others) at 1 month | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.55, 12.27] |
| 2.4 Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 2.5 Skin vasculitis (purpura, cutaneous ulcers or others) at 36 months | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.08] |
| 3 Laboratory findings Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum creatinine at 18 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐8.80 [‐29.27, 11.67] |
| 3.2 Serum creatinine at 36 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 17.60 [‐4.23, 39.43] |
| 3.3 Proteinuria at 18 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.42, 0.42] |
| 3.4 Proteinuria at 36 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.84, 1.32] |
| 3.5 Cryocrit at 12 months [%] | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐10.29, 6.27] |
| 3.6 Serum HCV‐RNA at 12 months [IU/mL] | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐435182.0 [‐1051224.79, 180860.79] |
| 4 Adverse effects of the medication Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [0.76, 24.75] |
| 4.2 Discontinuation of the treatment due to adverse drug reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.22, 4.36] |
| 4.3 Infection (pneumonia, urosepsis) at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.4 Infection (pneumonia, urosepsis) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.12] |
| 4.5 Cardiovascular events (angina, myocardial infarction, heart failure) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 4.6 Gastrointestinal bleeding | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.12] |
| 4.7 Haemorrhagic alveolitis | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.12] |
| 4.8 Leukopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.9 Thrombocytopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.21] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.77, 1.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 12 months | 4 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 24 months | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 12 months | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| 3 Laboratory findings Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum creatinine at 18 months [µmol/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐30.32 [‐80.59, 19.95] |
| 3.2 Proteinuria at 18 months [g/24h] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐2.89, ‐1.07] |
| 3.3 ALT or GPT at 6 months [UI/L] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.89 [‐55.77, 43.99] |
| 3.4 ALT or GPT at 18 months [UI/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐28.28 [‐48.03, ‐8.54] |
| 3.5 Rheumatoid factor activity at 6 months [UI/mL] | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 97.0 [‐187.37, 381.37] |
| 3.6 C4 at 18 months [mg/dL] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.74, 2.67] |
| 3.7 IgM at 18 months [mg/dL] | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐595.75 [‐877.20, ‐314.30] |
| 3.8 Cryocrit at 6 months [%] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.38, ‐0.38] |
| 4 Adverse effects of the medication Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 27.82 [1.72, 449.18] |
| 4.2 Discontinuations of the treatment due to adverse drug reactions | 4 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.91, 5.90] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Peripheral neuropathies at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Peripheral joint arthralgia at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Laboratory findings Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Cryocrit [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects of the medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

