Tratamiento para la crioglobulinemia mixta asociada al virus de la hepatitis C
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
| Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
| Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
| Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
| Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. | Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
| Selective reporting Reporting bias due to selective outcome reporting | Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
| Other bias Bias due to problems not covered elsewhere in the table | Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
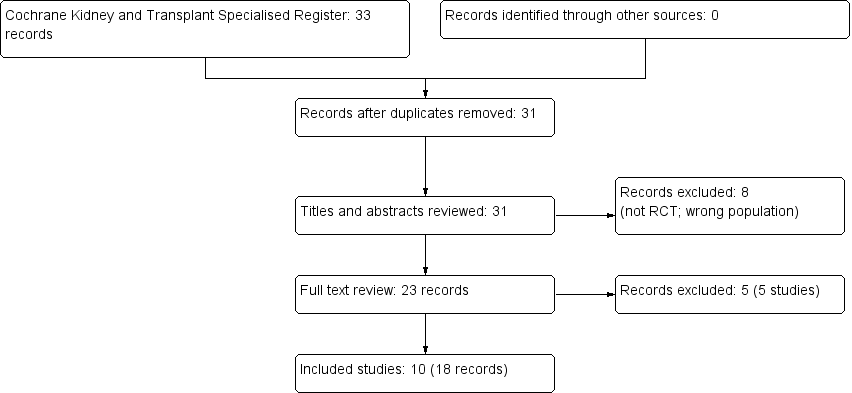
Flow chart showing source and identification of studies for inclusion.
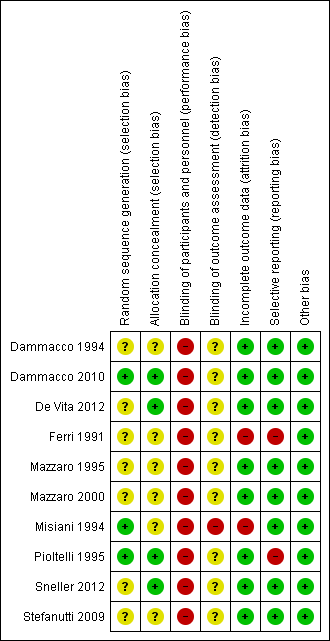
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
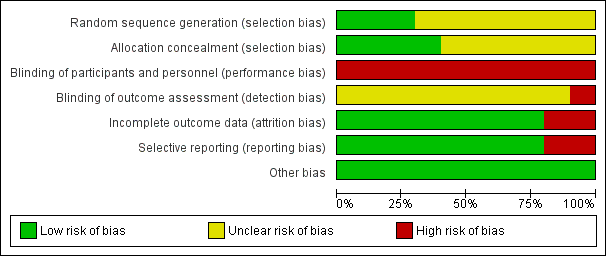
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Rituximab versus no rituximab, Outcome 1 Death.

Comparison 1 Rituximab versus no rituximab, Outcome 2 Clinical manifestations.

Comparison 1 Rituximab versus no rituximab, Outcome 3 Laboratory findings.

Comparison 1 Rituximab versus no rituximab, Outcome 4 Adverse effects of the medication.

Comparison 1 Rituximab versus no rituximab, Outcome 5 Antiviral therapy failure or not indicated.

Comparison 2 Interferon versus control, Outcome 1 Death.
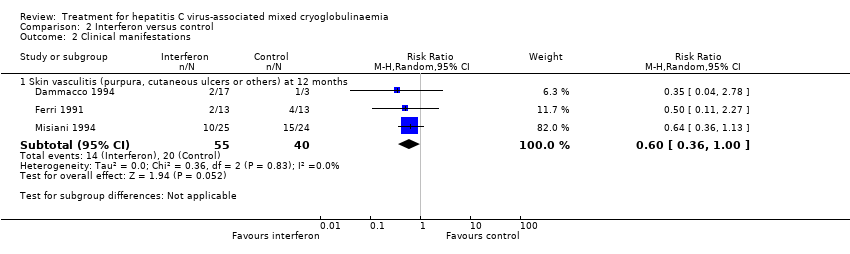
Comparison 2 Interferon versus control, Outcome 2 Clinical manifestations.
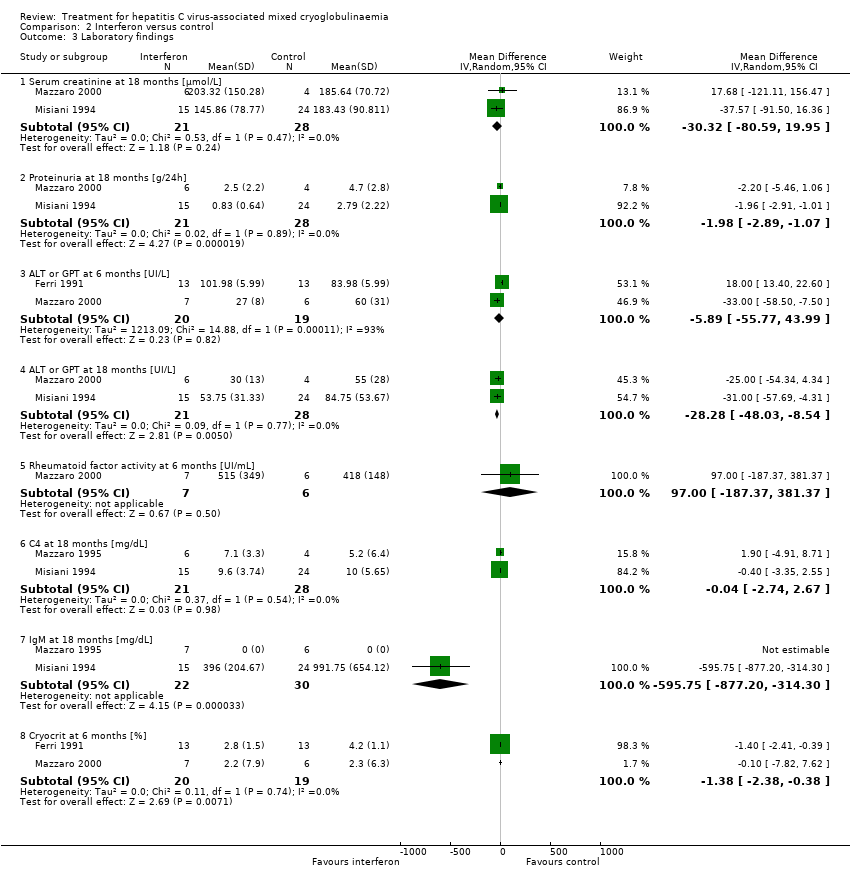
Comparison 2 Interferon versus control, Outcome 3 Laboratory findings.

Comparison 2 Interferon versus control, Outcome 4 Adverse effects of the medication.
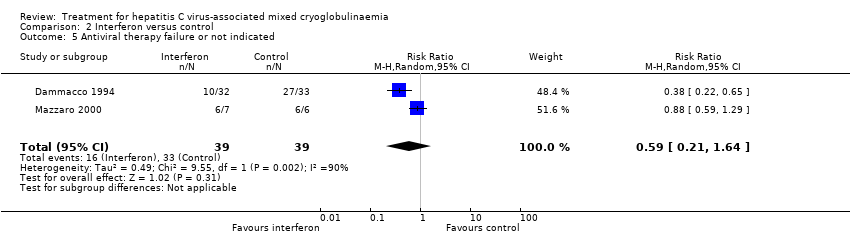
Comparison 2 Interferon versus control, Outcome 5 Antiviral therapy failure or not indicated.

Comparison 3 Interferon for 6 months versus 1 year, Outcome 1 Death at 24 months.
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 1 Death at 24 months.
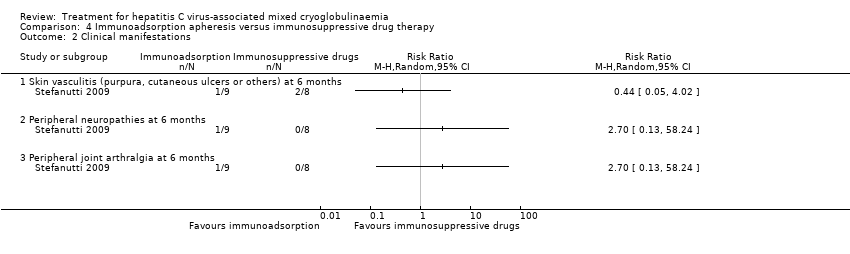
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 2 Clinical manifestations.
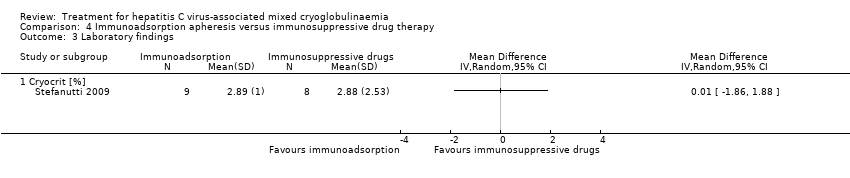
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 3 Laboratory findings.

Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 4 Adverse effects of the medication.
| Rituximab compared to no rituximab for hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Patient or population: hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no rituximab | Risk with rituximab | ||||
| Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months: number of affected patients | Study population | RR 0.57 | 78 (2) | ⊕⊕⊕⊝ | |
| 26 per 100 | 15 per 100 | ||||
| SCr at 18 months | The mean SCr at 18 months was 194.3 μmol/L | MD was 8.8 μmol/L lower | ‐ | 37 (1) | ⊕⊕⊕⊝ |
| Cryocrit at 12 months | The mean cryocrit at 12 months was 6.55% | MD was 2.01% lower | ‐ | 41 (2) | ⊕⊕⊝⊝ |
| Adverse effects ‐ infusion reactions: number of events | Study population | RR 4.33 | 118 (3) | ⊕⊕⊕⊝ | |
| 0 per 100 | 0 per 100 | ||||
| Activity outcomes | De Vita 2012 found a significant reduction in the BVAS at 2 months in the rituximab group (from mean ± SD: 11.9 ± 5.4 to 7.1 ± 5.7; P < 0.001), and this difference persisted at 6 months (6.9 ± 6.8; P < 0.001), 12 months (5.4 ± 6.2; P 0.0001), and 24 months (4.4 ± 4.6; P < 0.0001). Without differences in the control group. Sneller 2012 BVAS scores became significantly lower in the rituximab group at month 4 (from 10.2 ± 8.4, at 6 months: 0 ± 0; P < 0.02). Without differences in control group. | ‐ | 81 (2) | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 95% CI overlaps no effect, and the CI fails to exclude important benefit or important harm 2 Base on tests of heterogeneity which test the null hypothesis that all studies have the same underlying magnitude of effect, have a low P‐value (P = 0.00001), indicating to reject the null hypothesis I2 statistic, which quantifies the proportion of the variation in point estimates due to among‐study differences, is large (97%) 3 High risk of performance bias: non‐blinded participants and personnel | |||||
| Study ID | Study design | Patient profile | Objective | No. of patients | Treatment | Results | Adverse effects |
| Non‐randomised, non‐controlled prospective study | HCV RNA (+) + cryoglobulinaemic syndrome with organ damage and B‐cell lymphoproliferative syndrome | To assess the hepatovirological response, the clinical and immunological efficacy and the safety of using sofosbuvir‐based direct‐acting antiviral therapy in patients with HCV‐associated mixed cryoglobulinaemia(according to the latest guidelines) | 44 | 1) Sofosbuvir+ribavirin (18) 2) Sofosbuvir+simeprevir (12) ± ribavirin (6 of 12 patients) 3) Sofosbuvir+daclatasvir (4) ± ribavirin (1 patient) 4) Sofosbuvir+ledipasvir (10) ± ribavirin (3 patients) | Hepatovirological response ‐ Undetectable HCV RNA negative rate 100% at week 4; 12SVR and 24SVR remained 100% negative ‐ Decrease of ALT from 77.7 ± 10.3 IU/L at baseline to 27.3 ± 10.3 IU/L at 24SVR ‐ Decrease of AST from 55.2 ± 60.4 IU/L at baseline to 22.6 ± 8.3 IU/L at 24SVR (P < 0.001) Clinical efficacy ‐ Decrease of BVAS from 5.41 ± 3.53 at baseline to 1.27 ± 1.68 at 24SVR (P < 0.001) Immunological efficacy ‐ Decrease of cryocrit level from 7.2 ± 15.4% at baseline to 1.8 ± 5.1% at 24SVR (P < 0.001) | Total: 26/44 (59%) Withdrawals: 1 patient withdrew ribavirin while continuing sofosbuvir+simeprevir Death: none Relapse: none Most frequent AE: Anaemia (13, all receiving ribavirin); fatigue (15); nausea (7) | |
| Case series | Five patients with HVC RNA + with detectable cryoglobulins in plasma and symptomatic mixed cryoglobulinaemia Patient 1: bilateral foot neuropathy and purpura Patient 2: painful left foot drop and purpura Patient 3: purpura and MPGN Patient 4: MPGN Patient 5: MPGN + low grade lymphoma | Review of one centre's experience in treating patients with mixed cryoglobulinaemia with new oral antiviral agents and to assess common factors associated with persistence of mixed cryoglobulinaemia despite SVR | 5 | Patient 1: PEG‐IFN+ribavirin+boceprevir Patient 2: Firstly with rituximab (5 weeks); later with: PEG‐IFN+ribavirin+telaprevir for 4 weeks; PEG‐IFN+ribavirin for 12 weeks; and PEG‐IFN+ribavirin+sofosbuvir for 15 weeks (telaprevir discontinued for persistent viral load > 1000 IU/mL) Patient 3: PEG‐IFN+ribavirin+telaprevir for 47 weeks Patient 4: PEG‐IFN+ribavirin for 24 weeks; afterwards adding sofosbuvir until completing 12 weeks of triple therapy (total 36 weeks) Patient 5: 2 cycles of rituximab: weekly infusions 375mg/m2 during 4 weeks in 2010 then two extra doses of rituximab in 2013 ‐PEG‐IFN+ribavirin+sofosbuvir for 8 weeks in 2014 | Patient 1: complete clearance of virus at week 8; complete clearance of cryoglobulins at week 28. Persistence of neuropathy Patient 2: SVR and no detectable cryoglobulins at month 6 after last triple therapy. Persistence of neuropathy. Patient 3: clearance of HCV at week 4 after PEG‐IFN+ribavirin+telaprevir, with persistent cryoglobulins. Active MPGN and vasculitis after ending of previous treatment (responding to steroid therapy). Patient 4: SVR with persistence of cryoglobulinaemia. Kidney function remained stable. | ||
| Retrospective case series | HCV RNA > 1000 IU/mL + circulating purpura + cutaneous ulcers, Raynaud’s phenomenon, arthralgia, sicca syndrome, gastrointestinal vasculitis, neurologic involvement or renal involvement | Comparison of 2 historical cohorts: one treated with PEG‐IFN and ribavirin and the other sofosbuvir+simeprevir (8/12) or sofosbuvir+ribavirin (4/12). Evaluation of 12SVR, relapses, clinical, immunological (cryoglobulins) and biochemical (AST, Hb) response and adverse effects; without statistical comparison between them | 22 | 1) PEG‐IFN+ribavirin 2) IFN‐free regimens 2a) Sofosbuvir 400 mg/24 h + simeprevir 150 mg/24h 2b) Sofosbuvir 400 mg/24 h + ribavirin (adjusted to kidney function) | PEG‐IFN+ribavirin ‐ SVR12: 1/10 IFN‐free regimens ‐ SVR12: 10/12 (95%) ‐ ALT decreased from 42 U/L at baseline to 20 U/L after treatment ‐ Cryoglobulin levels decreased from 1.5% (0.5% to 4%) at baseline to 0.5% (0% to 2%) after treatment ‐ Decrease of proteinuria in all cases of kidney involvement (table IV) | Total PEG‐IFN+ribavirin: 10/10 IFN‐free regimens: 8/12 Withdrawals PEG‐IFN+ribavirin: 5/10 IFN‐free regimens: 1/12 (anxiety and insomnia) Deaths: none Relapses IFN‐free regimens: 2 (genotype 1 sofosbuvir+simeprevir; genotype 4 sofosbuvir+ribavirin) | |
| Open‐label, non‐controlled, prospective cohort study | Chronic active HCV infection with signs of mixed cryoglobulinaemia. All 23 patients had positive cryoglobulins in plasma at baseline or earlier | To analyse the safety and efficacy of Peg‐IFN‐alpha/ribavirin/protease inhibitor combination in HCV‐mixed cryoglobulinaemia | 23 | Peg‐IFN‐alpha+ribavirin a) + telaprevir (375 mg, 3 times/d for 12 weeks) for 48 weeks (15 patients) b) + boceprevir (800 mg, 3 times/d for 44 weeks) for 48 weeks (8 patients) | Complete clinical responders (improvement in all baseline clinical manifestations): 13 patients (56.5%) at week 24 Virological response (i.e., HCV RNA negative) was of 69.6% at week 24 (P = 0.005). Cryoglobulin level: decreased from 0.44 to 0.06 g/L(P = 0.0006) C4 level: increased from 0.09 to 0.15 g/L (P = 0.045) No significant difference was found between the two treatment regiments | Total: 105 Withdrawals: 8 patients (34.7%) ( virological non‐response (5); virological relapse (2); depression (1)) Death: none Relapse: 1 Most frequent AE: fatigue (87%); neutropenia (78.3%); thrombocytopenia (65.2%); infection (47.8%); pruritus (39.1%); depression (21.7%); nausea (21.7%) | |
| Open‐label, non‐controlled, prospective cohort study | Active HCV infection and active mixed cryoglobulinaemia. Excluded non‐active mixed cryoglobulinaemia, HIV or HBV active infection and current decompensated cirrhosis | To evaluate safety and efficacy of an oral IFN‐free regimen, sofosbuvir+ribavirin, in HCV‐mixed cryoglobulinaemia | 24 | Sofosbuvir (400 mg/d) + ribavirin (200 to 1400 mg/d) for 24 weeks “Rituximab was used in four cases, in addition to prednisone and plasmapheresis in two patients” | Complete response (improvement of ALL the affected organs involved at baseline) at week 24: 21 (87.5%) HCV RNA clearance at week 24: 22/24 (91.7%) SVR12: 74% Cryocrit: decrease from 0.35 g/L at baseline to 0 at 12 weeks after end‐of‐treatment. C4: increase from 0.1 g/L at baseline to 0.22 g/L at 12 week after end‐of treatment “No difference of outcome was found in patients who received immunosuppressive treatment or not” | Total: 14/24 (54%) Withdrawals: 2 (8%) (hallucination and irritability (1); grade 4 anaemia (1)) Death: 2 (severe pneumonia in the context of B cell lymphoma; pulmonary embolism in the context of hepatocellular carcinoma) Relapses: none Most frequent AE: (fatigue (25%); anaemia (25%); insomnia (21%); infection (17%); alopecia (8%)) | |
| 12SVR ‐ 12 week SVR; 24SVR ‐ 24 week SVR; AE ‐ adverse event; ALT ‐ alanine aminotransferase; AST‐ aspartate aminotransferase; BVAS ‐ Birmingham Vasculitis Activity Score; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; IFN ‐ interferon; MPGN ‐ membranoproliferative glomerulonephritis; PEG ‐ pegylated; SVR ‐ sustained viral response | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 One month | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Six months | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 24 months | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 2 Clinical manifestations Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Active urinary sediment at 1 month | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.65] |
| 2.2 Need for dialysis at 24 months | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Skin vasculitis (purpura, cutaneous ulcers or others) at 1 month | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.55, 12.27] |
| 2.4 Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 2.5 Skin vasculitis (purpura, cutaneous ulcers or others) at 36 months | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.08] |
| 3 Laboratory findings Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum creatinine at 18 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐8.80 [‐29.27, 11.67] |
| 3.2 Serum creatinine at 36 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 17.60 [‐4.23, 39.43] |
| 3.3 Proteinuria at 18 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.42, 0.42] |
| 3.4 Proteinuria at 36 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.84, 1.32] |
| 3.5 Cryocrit at 12 months [%] | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐10.29, 6.27] |
| 3.6 Serum HCV‐RNA at 12 months [IU/mL] | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐435182.0 [‐1051224.79, 180860.79] |
| 4 Adverse effects of the medication Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [0.76, 24.75] |
| 4.2 Discontinuation of the treatment due to adverse drug reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.22, 4.36] |
| 4.3 Infection (pneumonia, urosepsis) at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.4 Infection (pneumonia, urosepsis) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.12] |
| 4.5 Cardiovascular events (angina, myocardial infarction, heart failure) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 4.6 Gastrointestinal bleeding | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.12] |
| 4.7 Haemorrhagic alveolitis | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.12] |
| 4.8 Leukopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.9 Thrombocytopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.21] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.77, 1.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 12 months | 4 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 24 months | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 12 months | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| 3 Laboratory findings Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum creatinine at 18 months [µmol/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐30.32 [‐80.59, 19.95] |
| 3.2 Proteinuria at 18 months [g/24h] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐2.89, ‐1.07] |
| 3.3 ALT or GPT at 6 months [UI/L] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.89 [‐55.77, 43.99] |
| 3.4 ALT or GPT at 18 months [UI/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐28.28 [‐48.03, ‐8.54] |
| 3.5 Rheumatoid factor activity at 6 months [UI/mL] | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 97.0 [‐187.37, 381.37] |
| 3.6 C4 at 18 months [mg/dL] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.74, 2.67] |
| 3.7 IgM at 18 months [mg/dL] | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐595.75 [‐877.20, ‐314.30] |
| 3.8 Cryocrit at 6 months [%] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.38, ‐0.38] |
| 4 Adverse effects of the medication Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 27.82 [1.72, 449.18] |
| 4.2 Discontinuations of the treatment due to adverse drug reactions | 4 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.91, 5.90] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Peripheral neuropathies at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Peripheral joint arthralgia at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Laboratory findings Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Cryocrit [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects of the medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

