Использование наружных дренажей и отказ от дренирования у пациентов с хронической субдуральной гематомой через трепанационное отверстие
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial, single‐centre (India) | |
| Participants | 51 participants (45 men and 6 women with an age range of 17‐85 years, mean 53 ± 15 years) with diagnosis of CSDH based on CT/MRI study of brain Exclusion criteria: children below 17 years of age; people who had already had surgery for CSDH (i.e. recurrence); people who had had a CSF diversion procedure who subsequently developed CSDH; and people with CSDH for whom burr‐hole evacuation was not the surgical approach used | |
| Interventions | All participants had 2 burr holes inserted over the maximum width of the haematoma Group 1: no drains Group 2: drains; continuous closed drainage for 48 h. A soft silicone drain (external diameter 2 mm and length of 20 cm) was inserted into the subdural space through the burr hole overlying the large part of the subdural cavity and tunnelled for a minimum of 5 cm away from the scalp incision | |
| Outcomes | Primary outcome: recurrence risk, defined as reappearance of clinical symptoms after a minimum period of 1 month after initial surgery with evidence of CSDH on the same site on plain CT head scan Secondary outcomes: GOS score, mortality at discharge and at 6 months, and complications of operation | |
| Notes | Duration of follow‐up 6 months. The primary outcome and complications of the operation were measured for all participants The study was conducted between September 2008 and March 2010 Quote: "The protocol was approved by the institutional ethics committee" p. 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization done using random allocation software (version 1.0)" p. 18 Review authors' comment: no reference to the software was provided |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome and complications of operation were measured for all participants. Mortality and clinical recovery measured by GOS score at discharge were recorded in all |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre (Turkey) | |
| Participants | 70 participants (54 men and 16 women) requiring surgery for CSDH Exclusion criteria: details not provided | |
| Interventions | Participants were treated with 1 or 2 burr holes at the surgeon's discretion Group 1: no drains; frontal or parietal burr holes on the side of the haematoma without drains Group 2: drains; burr hole with a 2.7 mm subdural drain in place for 48 h and then removed | |
| Outcomes | Primary outcome: recurrence risk Secondary outcome measures: clinical recovery, mortality, postoperative complications and length of hospital stay | |
| Notes | Duration of follow‐up: 1 month The study took place between 1994 and 2002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in text. Quote: "...were randomly assigned" p. 261 |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary and secondary outcome was measured for all participants |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre, parallel trial (Tehran, Iran) | |
| Participants | 40 participants (28 men and 12 women with an age range of 18‐96 years, mean = 67 years) were randomized with the balanced block method into 2 groups Exclusion criteria: people under 18 years of age; with images showing calcified or organised haematoma; severe comorbidity, end‐stage diseases or secondary haematoma after a shunting procedure, tumour infiltration, or a history of previous craniotomy | |
| Interventions | All participants had 2 burr holes inserted Group 1: no drains Group 2: drains; a blunt‐tip, side‐hole, 3‐mm internal diameter catheter was placed in the cavity after burr‐hole evacuation. The drain was connected to a sterile closed‐system bag adjusted at the level of patient's head without any negative pressure for 48 h and then removed | |
| Outcomes | Primary outcome measure was recurrence risk, which was defined as the risk of reoperation to treat recurrent chronic subdural haematoma. The recurrence of CSDH was defined as progressive neurological deficits or having no improvement apparently correlated with mass effect in the control CT scan Secondary outcome measures included GOS score, mortality and complications of acute subdural haematoma, cardiopulmonary complications, seizure, meningitis, and any significant surgical complications | |
| Notes | Every participant received follow‐up care for at least 6 months The study was conducted between June 2007 and July 2009 Ethics approval for the study was obtained from the Institutional Review Board p. 732 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomized with the balanced block method but we do not know how Quote: "...were randomized with the balanced block method into two groups..." p. 733 |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Participants were each examined by an independent observer. In the cases in which the independent observer confirmed the recurrence of CSDH, the outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary and secondary outcome was measured for all participants |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised control trial, single‐centre (India) | |
| Participants | 140 participants (113 men and 27 women between 32‐98 years of age, mean age = 64.86 years) with radiologically proven CSDH requiring burr‐hole evacuation Exclusion criteria: people under 18 years of age; those using oral anticoagulants; and those with bleeding diathesis | |
| Interventions | Group 1: no drains; 2 burr holes (frontal and parietal) on the site of the haematoma Group 2: drains; a single parietal burr hole with a subdural drain (sterile No. 8 infant feeding tubes) present for 48 h‐72 h and then removed | |
| Outcomes | Primary outcome: recurrence risk | |
| Notes | Duration of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients who qualified for the study were divided into two groups using a randomization chart. No matching was done for age or sex." p. 495 |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome was measured for all participants |
| Selective reporting (reporting bias) | Low risk | Important data (such as complications, outcome, and mortality) were not presented, but there was no evidence of selective reporting |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre (Germany) | |
| Participants | 144 adults with CSDG Exclusion criteria: details not provided | |
| Interventions | Participants in Group 1 all received a slightly enlarged frontoparietal burr hole. The trial report did not provide details about the number or location of holes for participants in Group 2 and Group 3 Group 1: internal drains; permanent subdural drain with subcutaneous reservoir after burr‐hole evacuation (not included in our analysis, as drain was internal, not external) Group 2: external drains; burr‐hole evacuation with external closed system drainage Group 3: no drains; burr‐hole evacuation without drainage | |
| Outcomes | Primary outcome: reoperation risk; reoperation defined as required when: 1) an initial neurological deficit was increasing, recurring, or not improving and the CT showed a corresponding space‐occupying lesion; 2) if there was an increase in the size of haematoma with or without neurological deterioration; 3) if there was permanent or recurrent severe headache with corresponding findings on CT scan Secondary outcome: complication risks of operation such as infection, seizures | |
| Notes | The follow up duration was only 3 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the sequence of the 3 possible operative procedures for all patients included in the study was randomly assigned" Quote: "were randomly divided into three treatment groups" (Abstract) |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome was measured for all participants |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre (Cambridge, UK) | |
| Participants | 215 participants (160 men and 55 women, age range 36‐95 years, mean age 76.8 years) who presented with symptomatic CSDH proven by CT scan requiring burr‐hole drainage Exclusion criteria: people treated for ipsilateral haematomas within 6 months of presentation; those with a CSF shunt in situ; those in whom surgery other than burr‐hole evacuation was indicated; and those for whom the operating surgeon judged drain insertion to be unsafe | |
| Interventions | All participants had 2 burr holes inserted Group 1: no drains Group 2: drains; a soft silicone drain inserted into the subdural space after burr‐hole evacuation. The drain was connected to a soft collection bag that was kept in a dependent position for 48 h and then removed | |
| Outcomes | Primary outcome measure was recurrence risk. Recurrence was defined as the occurrence of symptoms and signs attributable to an ipsilateral haematoma seen on a CT scan within 6 months of the original drainage procedure Secondary outcome measures were clinical outcome (mortality, MRS scores, GCS) at discharge and at 6 months, and length of hospital stay for neurosurgery | |
| Notes | The primary outcome and duration of neurosurgical hospital admission was measured for all participants. Mortality at 30 days and 6 months was recorded in all but 3 participants with drains and 2 without drains Study registration number: ISRCTN 97314294 The study was conducted between November 2004 and November 2007 Approved by the Cambridge Local Ethics Research Committee in October 2004, reference number: 04/Q0108/52 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation was used, with random sizes of blocks ranging from eight to 12 in an allocation ration of 1:1. Randomisation was done by the investigators with a web‐based randomisation software—Random Allocation Software version 1.0." p. 1069 |
| Allocation concealment (selection bias) | Low risk | Quote: "Instructions to use or not use drain were kept in sealed envelopes labelled with sequential study numbers, which were opened at surgery after drain insertion was judged to be safe." p. 1069 |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The nature of this intervention did not allow for masking of treatment allocation." p. 1069 |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Data were anonymised and clinicians were masked to outcomes when possible." p. 1069 |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome was measured for all participants. Analyses were done on an intention‐to‐treat basis for missing data for secondary outcome measures |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre (India) | |
| Participants | 200 adults with symptomatic CSDH proven by CT scan, and randomized into 2 groups once subdural haematoma was evacuated and drain placement was not contraindicated pre‐operatively Exclusion criteria: people treated for ipsilateral haematomas with diversion of CSF within 6 months of presentation; those in whom surgery other than burr‐hole evacuation was indicated; and those not needing surgical treatment because of size of CSDH or clinical status | |
| Interventions | Participants were treated with 1 or 2 burr holes at the surgeon's discretion Group 1: no drains; participants treated with burr‐hole craniostomy without closed‐system drainage Group 2: drains; participants treated by burr‐hole craniostomy with closed‐system drainage | |
| Outcomes | Primary outcome: recurrence risk; recurrence was defined as the occurrence of symptoms and signs attributable to an ipsilateral haematoma seen on a CT scan within 6 months of original drainage procedure All such symptomatic recurrences were re‐operated Secondary outcomes: complications of surgery, mortality, GCS at discharge and at 2 weeks | |
| Notes | Every participant received follow‐up care for at least 6 months. The primary outcome was measured for all participants The study was conducted between January 2011 and June 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were allocated randomly into two groups using random allocation software" |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Participants were followed up in the outpatient department, and the outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome was measured for all participants but 5 participants with drains and 4 without drains |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre (Japan) | |
| Participants | 118 participants (65 in Group 1, mean age 68 years; 53 in Group 2, mean age 69.7 years). Exclusion criteria: people with hygromas, infantile CSDH, calcified or ossified CSDH, and asymptomatic CSDH, because these conditions were considered to be different clinical entities | |
| Interventions | All participants had 1 burr hole inserted Group 1: no drains; burr‐hole craniostomy without drainage Group 2: drains; burr‐hole craniostomy with closed‐system drainage. The drain (SL‐C ventricular catheter; Sonne‐Ika Co, Tokyo, Japan) placed within the cavity was connected to a ventricular drainage bag with an antireflux valve (Hanaco Medical Co, Saitama, Japan), which was placed on the bed for approximately 1 day, in the longest instance for 3 days | |
| Outcomes | Primary outcome: recurrence risk; recurrence was defined as the reappearance of neurological symptoms within 6 months of surgery and an increase in the haematoma cavity volume on the operative side | |
| Notes | Every participant received follow‐up care for at least 6 months The study was conducted between July 1992 and June 1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomized selection of surgical methods (that is, whether to use drainage or not) was decided by the toss of a coin |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome was measured for all participants |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found, but the important data (such as complications, outcome, and mortality) were not present |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised controlled trial, single‐centre (Japan) 33 males and 5 females | |
| Participants | 38 participants (33 men and 5 women; Group 1 (n = 20): age range 52‐84 years, mean age 70.8 years; Group 2 (n = 18), age range 42‐84 years, mean 71.7 years) Exclusion criteria: people with severe disturbed states of consciousness or with coagulopathy at the time of admission | |
| Interventions | All participants had 1 burr hole inserted Group 1: no drains; burr‐hole craniostomy without drainage Group B: drains; burr‐hole craniostomy with closed‐system drainage. The drain (SL‐C ventricular catheter; Sonne‐Ika Co, Tokyo, Japan) placed within the cavity was connected to a ventricular drainage bag with an antireflux valve (Hanaco Medical Co, Saitama, Japan), which was placed on the bed for 2‐3 days | |
| Outcomes | Primary outcome: recurrence risk; recurrence was defined as reappearance of neurological symptoms, and increase in subdural cavity, and/or change in cavity density from low to iso‐/high‐density on CT scan Secondary outcomes: complications of surgery, mortality and clinical recovery | |
| Notes | Every participant received follow‐up care for 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants assigned sequentially according to admission to drains or no drains (using alternation) |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) | Low risk | The nature of this intervention did not allow for masking of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessment was objective |
| Incomplete outcome data (attrition bias) | Low risk | The primary and secondary outcomes were measured for all participants at 6 months |
| Selective reporting (reporting bias) | Low risk | All expected outcome data were reported and no selective reporting was found |
| Other bias | Low risk | No other bias detected |
Abbreviations
CSDH; chronic subdural haematoma
CSF; cerebro‐spinal fluid
CT; computed tomography
GCS; Glasgow Coma Scale
GOS; Glasgow Outcome Scale
MRI; magnetic resonance imaging
MRS; modified Rankin Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomized controlled trial | |
| Not a randomized controlled trial | |
| Not a randomized controlled trial, retrospective study | |
| Not a randomized controlled trial, retrospective study | |
| Not a randomized controlled trial, retrospective study | |
| Not a randomized controlled trial, retrospective study | |
| Not a randomized controlled trial | |
| Not a randomized controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial, single‐centre (Pakistan) |
| Participants | 50 participants (25 in each group, age range 20‐60 years, mean age 55.43 ± 5.16 years) with CSDH |
| Interventions | All participants had 1 burr hole inserted Group 1: no drains; participants treated by burr‐hole craniostomy without closed‐system drainage (one time drainage) Group 2: drains; participants treated by burr‐hole craniostomy with closed‐system drainage and the drain was removed after 48 h |
| Outcomes | Primary outcome: recurrence risk |
| Notes | Every participant received follow‐up care for 6 months |
Abbreviation
CSDH; chronic subdural haematoma
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of subdural drain placement after burr hole evacuation of chronic subdural haematomas on recurrence: a prospective randomised‐controlled multi‐centre study |
| Methods | Consecutive participants, 60 years old or above, diagnosed to have symptomatic CSDH and indicated for burr‐hole operative drainage will be randomly allocated into one of two groups: 1) for intraoperative subdural drain placement (intervention group); or 2) not for drain placement (control group). Using web‐based software, block randomisation with an allocation ratio of 1:1 will be conducted. Instructions to use or not to use a drain will be contained in sealed envelopes labelled with sequential study numbers |
| Participants | Inclusion criteria:
Exclusion criteria: patients for whom the operating surgeon judges that drain placement is unsafe will be excluded from the study |
| Interventions | Intraoperatively, if the surgeon‐in‐charge judges that after burr‐hole evacuation of the haematoma the participant's condition is unsafe for drain placement, the subject will be excluded from the study. Otherwise, randomisation will be performed at this juncture by the opening of the sealed envelope. The procedure involves placing a prefabricated silicone drain into the subdural space according to a standard protocol and will be removed on the second post‐operative day at the bedside. Drainage is undertaken passively by hanging the collection bag at the bedside in a dependent position. In addition to general demographic, clinical and radiological presentation data, potential risk factors for recurrence will be documented. Serial CT brain scans will be arranged (before discharge, at 4 weeks and 6 months) and the occurrence of significant subdural haematoma recurrence requiring repeat operative drainage at 6 months will be recorded. Other outcome measures to be determined at regular time intervals for a total follow‐up period of 6 months (upon discharge, at 4 weeks and 6 months) include: functional performance in terms of the extended GOS and MRS, added neurological deficit, death and other surgery‐related complications. All outcomes will be documented by the trial investigators or by the responsible clinician. The data obtained will be analyzed according to the principle of intention‐to‐treat |
| Outcomes | Primary outcomes: significant recurrent chronic subdural haematoma i.e. requiring repeat operative drainage at 6 months after the primary operation (time frame: 6 months after primary burr‐hole evacuation of chronic subdural haematoma) Secondary outcomes: functional performance in terms of the Extended GOS, added neurological deficit, surgery‐related complications, death, MRS |
| Starting date | June 2013 |
| Contact information | Peter YM Woo, FRCS (SN) 852+ 3517 2275 ext 2275 [email protected] |
| Notes | ClinicalTrials.gov Identifier: NCT01785797 The ethics committee approval detail is not provided |
Abbreviations
CSDH; chronic subdural haematoma
CT; computed tomography
GOS; Glasgow Outcome Scale
MRS; modified Rankin Scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence Show forest plot | 9 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.61] |
| Analysis 1.1  Comparison 1 Comparison of drains versus no drains, Outcome 1 Recurrence. | ||||
| 1.1 Two holes | 3 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.80] |
| 1.2 One hole | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.56] |
| 1.3 One or two holes | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.79] |
| 2 Complications Show forest plot | 7 | 710 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.72] |
| Analysis 1.2 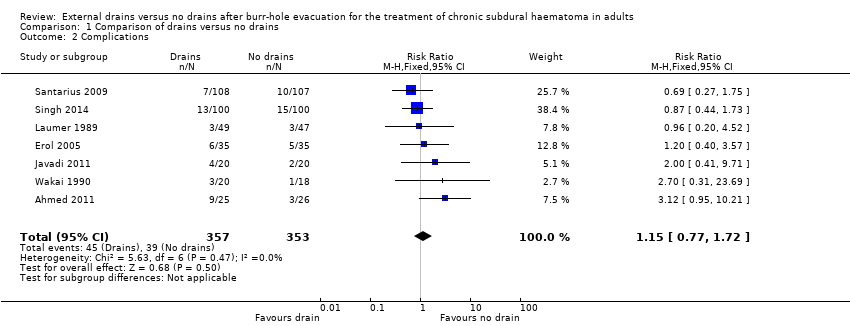 Comparison 1 Comparison of drains versus no drains, Outcome 2 Complications. | ||||
| 3 Mortality Show forest plot | 5 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.33] |
| Analysis 1.3 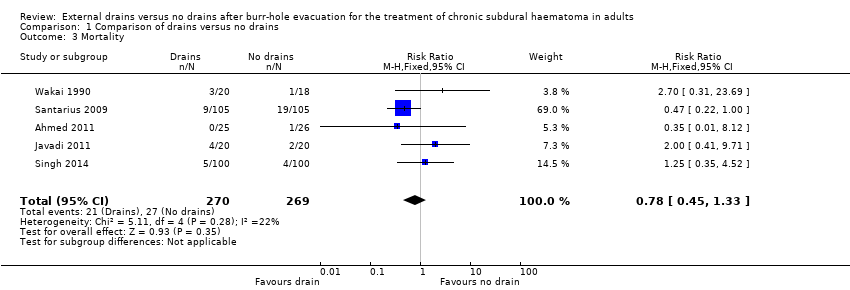 Comparison 1 Comparison of drains versus no drains, Outcome 3 Mortality. | ||||
| 4 Poor functional outcome Show forest plot | 5 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| Analysis 1.4 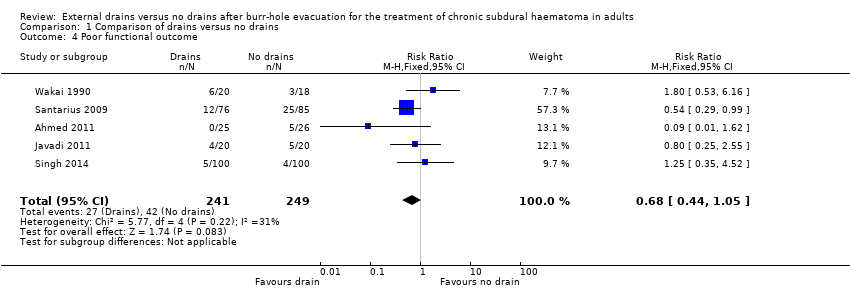 Comparison 1 Comparison of drains versus no drains, Outcome 4 Poor functional outcome. | ||||
| 5 Sensitivity analysis of recurrence Show forest plot | 7 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.22, 0.50] |
| Analysis 1.5  Comparison 1 Comparison of drains versus no drains, Outcome 5 Sensitivity analysis of recurrence. | ||||

Study flow diagram
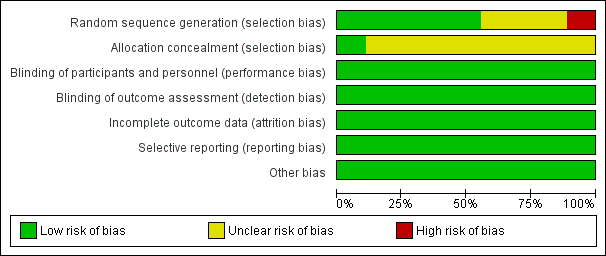
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Comparison of drains versus no drains, Outcome 1 Recurrence.
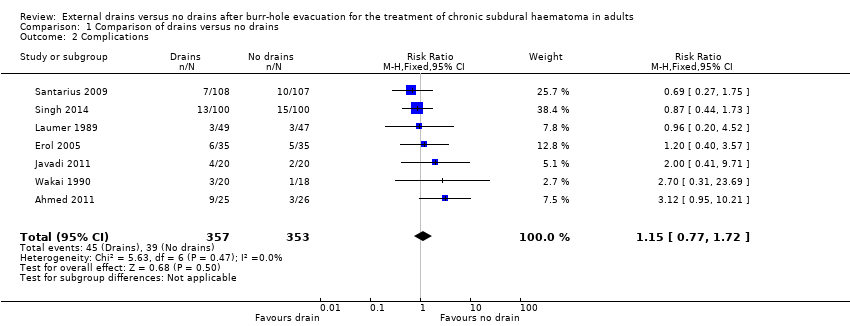
Comparison 1 Comparison of drains versus no drains, Outcome 2 Complications.
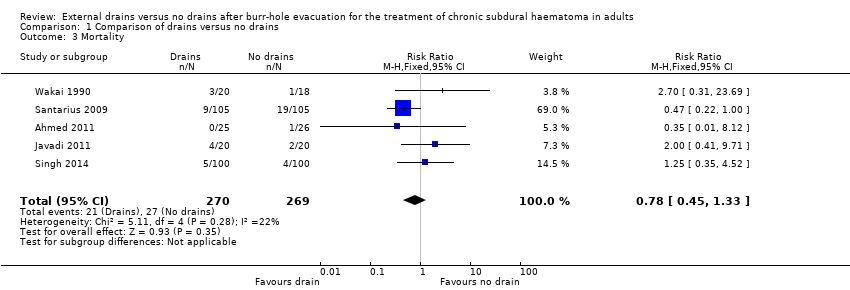
Comparison 1 Comparison of drains versus no drains, Outcome 3 Mortality.

Comparison 1 Comparison of drains versus no drains, Outcome 4 Poor functional outcome.
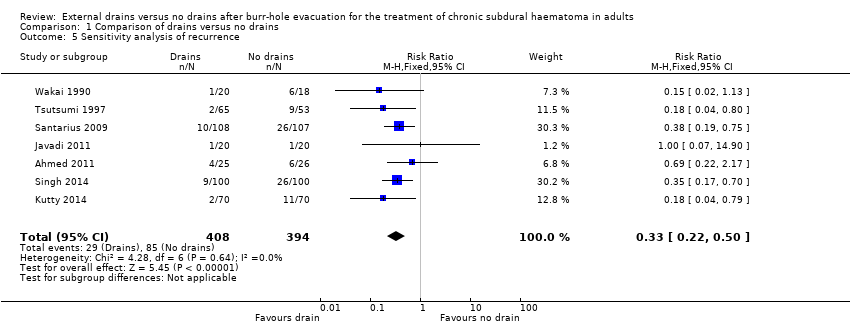
Comparison 1 Comparison of drains versus no drains, Outcome 5 Sensitivity analysis of recurrence.
| Drains compared to no drains for burr‐hole evacuation of CSDH in adults | ||||||
| Patient or population: adults with burr‐hole evacuation of CSDH Settings: hospital settings in India,Turkey, Iran, Germany, UK and Japan | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No drain | Drain | |||||
| Overall recurrence | Study population | RR 0.45 | 968 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 97 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 104 per 1000 | |||||
| Recurrence with 2 burr holes (subgroup) | Study population | RR 0.46 | 306 | ⊕⊕⊝⊝ | ||
| 216 per 1000 | 99 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 106 per 1000 | |||||
| Recurrence with 1 burr hole (subgroup) | Study population | RR 0.17 | 156 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 36 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 36 per 1000 | |||||
| Recurrence with 1 or 2 burr holes (subgroup) | Study population | RR 0.52 | 506 | ⊕⊕⊕⊝ | ||
| 218 per 1000 | 113 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 111 per 1000 | |||||
| Complications | Study population | RR 1.15 | 710 | ⊕⊕⊝⊝ | ||
| 110 per 1000 | 127 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 115 per 1000 | |||||
| Mortality | Study population | RR 0.78 | 539 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 44 per 1000 | |||||
| Poor functional outcome (includes death) | Study population | RR 0.68 | 490 | ⊕⊕⊝⊝ | ||
| 169 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 131 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one trial used adequate allocation concealment, and one trial used alternation as a randomisation method (Wakai 1990). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence Show forest plot | 9 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.61] |
| 1.1 Two holes | 3 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.80] |
| 1.2 One hole | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.56] |
| 1.3 One or two holes | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.79] |
| 2 Complications Show forest plot | 7 | 710 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.72] |
| 3 Mortality Show forest plot | 5 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.33] |
| 4 Poor functional outcome Show forest plot | 5 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 5 Sensitivity analysis of recurrence Show forest plot | 7 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.22, 0.50] |

