Использование наружных дренажей и отказ от дренирования у пациентов с хронической субдуральной гематомой через трепанационное отверстие
Appendices
Appendix 1. Search strategies
Cochrane Injuries Group Specialised Register
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library)
#1MESH DESCRIPTOR Hematoma, Subdural, Intracranial
#2MESH DESCRIPTOR Intracranial Hemorrhage, Traumatic
#3(chronic near1 sub?dural):TI,AB,KY
#4(haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*):TI,AB,KY
#5(subepidural or pachymening* or extracran*):TI,AB,KY
#6MESH DESCRIPTOR hematoma, subdural
#7MESH DESCRIPTOR hematoma, subdural, chronic
#8#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
#9MESH DESCRIPTOR Drainage
#10(burr hole drainage):TI,AB,KY
#11(double burr hole drainage):TI,AB,KY
#12(drainage adj1 system*):TI,AB,KY
#13(gravity adj1 drainage):TI,AB,KY
#14(closed‐system drainage):TI,AB,KY
#15(closed‐suction drainage):TI,AB,KY
#16#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#17#8 AND #16
#18* NOT INMEDLINE NOT INEMBASE
#19#17 AND #18
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R)
1. randomi?ed.ab,ti.
2. randomized controlled trial.pt.
3. controlled clinical trial.pt.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. Comparative Study/
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. (animals not (humans and animals)).sh.
11. 9 not 10
12. hematoma, subdural/ or hematoma, subdural, chronic/
13. (chronic adj1 sub?dural).mp.
14. (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*).ab,ti.
15. (subepidural or pachymening* or extracran*).mp.
16. Intracranial Hemorrhage, Traumatic/
17. Hematoma, Subdural, Intracranial/
18. 12 or 13 or 14 or 15 or 16 or 17
19. Drainage/
20. burr hole drainage.mp.
21. (drainage adj1 (procedure* or pressure)).mp.
22. drain*.mp.
23. single burr hole drainage.mp.
24. double burr hole drainage.mp.
25. (drainage adj1 system*).mp.
26. (gravity adj1 drainage).mp.
27. closed‐system drainage.mp.
28. closed‐suction drainage.mp.
29. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30. 18 and 29
31. 11 and 30
Embase Classic + Embase
1. hematoma, subdural/ or hematoma, subdural, chronic/
2. (chronic adj1 sub?dural).ab,ti.
3. (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*).ab,ti.
4. (subepidural or pachymening* or extracran*).ab,ti.
5. Intracranial Hemorrhage, Traumatic/
6. Hematoma, Subdural, Intracranial/
7. 1 or 2 or 3 or 4 or 5 or 6
8. Drainage/
9. burr hole drainage.mp.
10. (drainage adj1 (procedure* or pressure)).mp.
11. drain*.ab,ti.
12. single burr hole drainage.mp.
13. double burr hole drainage.mp.
14. (drainage adj1 system*).mp.
15. (gravity adj1 drainage).mp.
16. closed‐system drainage.mp.
17. closed‐suction drainage.mp.
18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19. 7 and 18
20. exp Randomized Controlled Trial/
21. exp controlled clinical trial/
22. exp controlled study/
23. comparative study/
24. randomi?ed.ab,ti.
25. placebo.ab.
26. *Clinical Trial/
27. exp major clinical study/
28. randomly.ab.
29. (trial or study).ti.
30. 20 or 21 or 22 or 24 or 25 or 26 or 27 or 28 or 29
31. exp animal/ not (exp human/ and exp animal/)
32. 30 not 31
33. 19 and 32
34. remove duplicates from 33
PubMed
(((publisher[sb] NOT pubstatusnihms))) AND ((((((((("Comparative Study"[Publication Type]) OR "Randomized Controlled Trial"[Publication Type]) OR "Controlled Clinical Trial"[Publication Type])) OR (((((((randomized[Title/Abstract]) OR randomised[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract]) OR group[Title/Abstract]))) NOT (("Animals"[Mesh]) NOT ("Animals"[Mesh] AND "Humans"[Mesh])))) AND (((((((((((((burr hole drainage[Title/Abstract]) OR drainage procedure*[Title/Abstract]) OR drainage pressure[Title/Abstract]) OR single burr hole drainage[Title/Abstract]) OR double burr hole drainage[Title/Abstract]) OR drainage system[Title/Abstract]) OR gravity drainage[Title/Abstract]) OR "closed system drainage"[Title/Abstract]) OR "closed suction drainage"[Title/Abstract])) OR "Drainage"[Mesh:noexp])) AND ((((((("Intracranial Hemorrhage, Traumatic"[Mesh:noexp]) OR "Hematoma, Subdural, Intracranial"[Mesh])) OR subepidural[Title/Abstract] OR pachymening*[Title/Abstract] OR extracran*[Title/Abstract]) OR (haematoma*[title/abstract] OR hematoma*[title/abstract] OR haemorrhag*[title/abstract] OR hemorrhag*[title/abstract] OR bleed*[title/abstract])) OR ((chronic subdural[Title/Abstract]) OR chronic sub‐dural[Title/Abstract])) OR (((("Hematoma, Subdural"[Mesh:noexp]) OR "Hematoma, Subdural, Chronic"[Mesh]) OR "Intracranial Hemorrhage, Traumatic"[Mesh:noexp]) OR "Hematoma, Subdural, Intracranial"[Mesh]))))
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐ Science (CPCI‐S)
#12#11 AND #10 AND #6
# 11TS=(drainage) OR TS=("burr hole drainage") OR TS=("closed‐system drainage") OR TS=("closed‐suction drainage")
# 10#9 OR #8 OR #7
# 9TS=(subepidural or pachymening* or extracran*)
# 8TS=(haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*)
# 7TS=("subdural hematoma") OR TS=("subdural haematoma")
# 6#5 AND #4
# 5TS=(human*)
# 4#3 OR #2 OR #1
# 3TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*))
# 2TS=(controlled clinical trial OR controlled trial OR clinical trial OR placebo)
# 1TS=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial)
Clinicaltrials.gov
( chronic subdural haematoma OR CSDH ) [DISEASE] AND ( burr‐hole OR burr hole ) [TREATMENT]
WHO International Clinical Trials Registry Platform
Condition: chronic AND subdural AND haematoma
Appendix 2. Chinese database search strategies
The Chinese Biomedical Database (CBM)
#1 (("血肿, 硬膜下, 慢性"[不加权:扩展]) OR "血肿, 硬膜下"[不加权:扩展]) OR "颅内出血, 创伤性"[不加权:扩展]
#2 ((("慢性硬膜下血肿"[中文标题:智能]) OR "硬膜下血肿"[中文标题:智能]) OR "硬膜下"[中文标题:智能]) OR "血肿"[中文标题:智能]
#3 "引流术"[不加权:扩展]
#4 ((((((("钻孔"[摘要:智能]) OR "钻孔引流"[摘要:智能]) OR "锥孔"[摘要:智能]) OR "引流"[摘要:智能]) OR "冲洗"[摘要:智能]) OR "负压引流"[摘要:智能]) OR "闭式引流"[摘要:智能]) OR "外引流"[摘要:智能]
#5 (("随机对照试验"[不加权:扩展]) OR "随机对照试验(主题)"[不加权:扩展]) OR "随机对照试验(主题)"[不加权:扩展]
#6 ((("随机对照"[摘要:智能]) OR "随机"[摘要:智能]) OR "对照研究"[摘要:智能]) OR "对照"[摘要:智能]
#7 ((#1) OR (#2))
#8 ((#3) OR (#4))
#9 (#5) OR (#6))
#10 ((((#7) AND (#8))) AND (#9))
The Chinese National Knowledge Infrastructure (CNKI)
((TI='慢性硬膜下血肿' OR TI='硬膜下血肿' OR TI='硬膜下出血' OR TI='硬膜下') AND (TI='单孔' OR TI='双孔' OR TI='钻孔' OR AB='负压引流' OR AB='负压封闭引流' OR AB='钻孔引流' OR TI='锥孔' OR TI='引流' OR TI='冲洗' OR TI='闭式引流')) AND (AB='随机对照试验' OR AB='随机对照' OR AB='随机' OR AB='对照' OR AB='对照研究')
China Online Journals (WanFang Medical Online)
((Title=慢性硬膜下血肿 or Title=硬膜下血肿 or Title=硬膜下出血 or Title=硬膜下) and (单孔 or 双孔 or 钻孔 or 锥孔 or 引流 or 冲洗 or 闭式引流)) and (随机 or 对照)

Study flow diagram
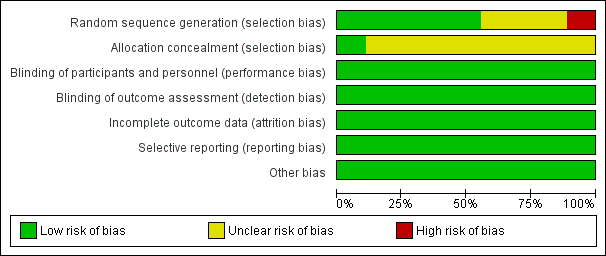
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Comparison of drains versus no drains, Outcome 1 Recurrence.
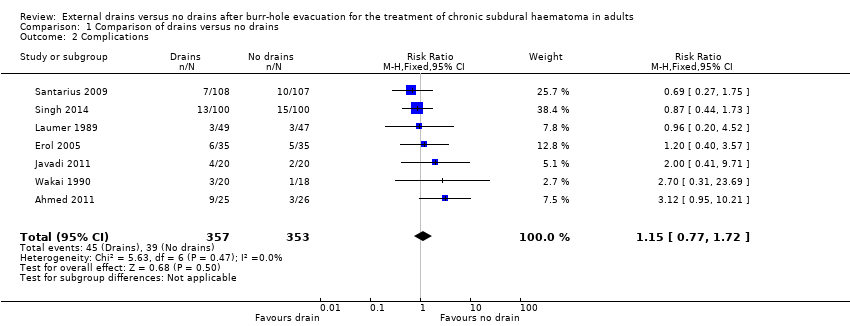
Comparison 1 Comparison of drains versus no drains, Outcome 2 Complications.
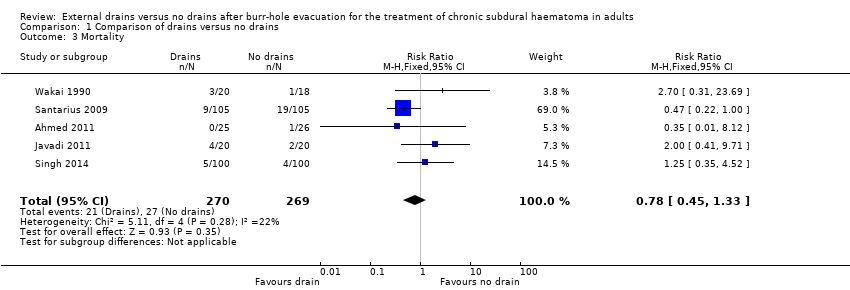
Comparison 1 Comparison of drains versus no drains, Outcome 3 Mortality.

Comparison 1 Comparison of drains versus no drains, Outcome 4 Poor functional outcome.
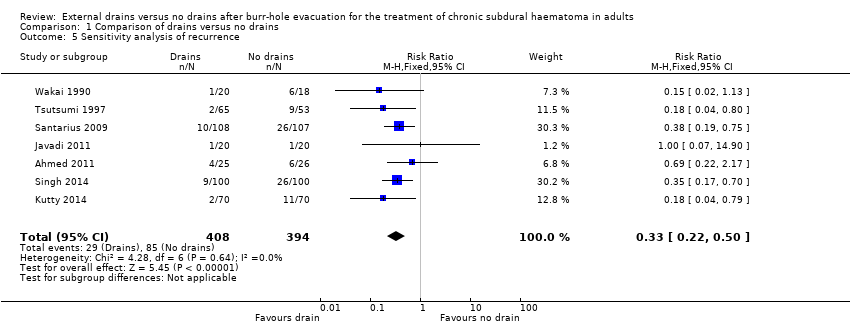
Comparison 1 Comparison of drains versus no drains, Outcome 5 Sensitivity analysis of recurrence.
| Drains compared to no drains for burr‐hole evacuation of CSDH in adults | ||||||
| Patient or population: adults with burr‐hole evacuation of CSDH Settings: hospital settings in India,Turkey, Iran, Germany, UK and Japan | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No drain | Drain | |||||
| Overall recurrence | Study population | RR 0.45 | 968 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 97 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 104 per 1000 | |||||
| Recurrence with 2 burr holes (subgroup) | Study population | RR 0.46 | 306 | ⊕⊕⊝⊝ | ||
| 216 per 1000 | 99 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 106 per 1000 | |||||
| Recurrence with 1 burr hole (subgroup) | Study population | RR 0.17 | 156 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 36 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 36 per 1000 | |||||
| Recurrence with 1 or 2 burr holes (subgroup) | Study population | RR 0.52 | 506 | ⊕⊕⊕⊝ | ||
| 218 per 1000 | 113 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 111 per 1000 | |||||
| Complications | Study population | RR 1.15 | 710 | ⊕⊕⊝⊝ | ||
| 110 per 1000 | 127 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 115 per 1000 | |||||
| Mortality | Study population | RR 0.78 | 539 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 44 per 1000 | |||||
| Poor functional outcome (includes death) | Study population | RR 0.68 | 490 | ⊕⊕⊝⊝ | ||
| 169 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 131 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one trial used adequate allocation concealment, and one trial used alternation as a randomisation method (Wakai 1990). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence Show forest plot | 9 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.61] |
| 1.1 Two holes | 3 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.80] |
| 1.2 One hole | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.56] |
| 1.3 One or two holes | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.79] |
| 2 Complications Show forest plot | 7 | 710 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.72] |
| 3 Mortality Show forest plot | 5 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.33] |
| 4 Poor functional outcome Show forest plot | 5 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 5 Sensitivity analysis of recurrence Show forest plot | 7 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.22, 0.50] |

