Immediate versus delayed treatment for recently symptomatic carotid artery stenosis
Abstract
Background
The timing of surgery for recently symptomatic carotid artery stenosis remains controversial. Early cerebral revascularization may prevent a disabling or fatal ischemic recurrence, but it may also increase the risk of hemorrhagic transformation, or of dislodging a thrombus. This review examined the randomized controlled evidence that addressed whether the increased risk of recurrent events outweighed the increased benefit of an earlier intervention.
Objectives
To assess the risks and benefits of performing very early cerebral revascularization (within two days) compared with delayed treatment (after two days) for people with recently symptomatic carotid artery stenosis.
Search methods
We searched the Cochrane Stroke Group Trials Register in January 2016, the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 1), MEDLINE (1948 to 26 January 2016), EMBASE (1974 to 26 January 2016), LILACS (1982 to 26 January 2016), and trial registers (from inception to 26 January 2016). We also handsearched conference proceedings and journals, and searched reference lists. There were no language restrictions. We contacted colleagues and pharmaceutical companies to identify further studies and unpublished trials.
Selection criteria
All completed, truly randomized trials (RCT) that compared very early cerebral revascularization (within two days) with delayed treatment (after two days) for people with recently symptomatic carotid artery stenosis.
Data collection and analysis
We independently selected trials for inclusion according to the above criteria, assessed risk of bias for each trial, and performed data extraction. We utilized an intention‐to‐treat analysis strategy.
Main results
We identified one RCT that involved 40 participants, and addressed the timing of surgery for people with recently symptomatic carotid artery stenosis. It compared very early surgery with surgery performed after 14 days of the last symptomatic event. The overall quality of the evidence was very low, due to the small number of participants from only one trial, and missing outcome data. We found no statistically significant difference between the effects of very early or delayed surgery in reducing the combined risk of stroke and death within 30 days of surgery (risk ratio (RR) 3.32; confidence interval (CI) 0.38 to 29.23; very low‐quality evidence), or the combined risk of perioperative death and stroke (RR 0.47; CI 0.14 to 1.58; very low‐quality evidence). To date, no results are available to confirm the optimal timing for surgery.
Authors' conclusions
There is currently no high‐quality evidence available to support either very early or delayed cerebral revascularization after a recent ischemic stroke. Hence, further randomized trials to identify which patients should undergo very urgent revascularization are needed. Future studies should stratify participants by age group, sex, grade of ischemia, and degree of stenosis. Currently, there is one ongoing RCT that is examining the timing of cerebral revascularization.
PICO
Plain language summary
Timing of treatment for people with recent symptoms from neck artery narrowing
Background
Ischemic stroke occurs when blood flow is blocked from part of the brain. This can be caused by a disease in the neck (carotid) artery that can cause a severe narrowing of the artery, leading to blood clot formation and blockage of a smaller blood vessel downstream. Opening up the carotid artery can reestablish adequate blood flow by surgical removal of the diseased area, or by inserting a tube (stent) to open the artery.
There is uncertainty about whether to perform the treatment immediately, or to wait a few days. Early treatment can improve blood flow, and prevent new strokes. However, early treatment may carry a higher risk of causing a stroke or associated bleeding.
Review question
We reviewed the effectiveness of performing very early treatment (within two days) compared with delayed treatment (after two days) for individuals with recent symptoms from neck (carotid) artery narrowing.
Study characteristics
The searches are up‐to‐date to 26 January 2016. We found only one randomized trial that assessed the effect of the timing of surgery. It included a total of 40 participants, ranging in age from 47 to 84 years.
Key results
From the limited evidence available, we cannot tell if the timing of surgery is an important factor in determining the outcome for individuals with recent symptoms from carotid artery narrowing.
Quality of the evidence
There is not enough evidence on the best time for surgical treatment for people with recent symptoms from carotid artery narrowing. The overall quality of the evidence was very low, due to the small number of participants from only one trial and missing outcome data. Further studies with a larger number of patients are needed.
Authors' conclusions
Summary of findings
| Very early cerebral revascularization compared with delayed treatment for recently symptomatic carotid artery stenosis | ||||||
| Patient or population: people with recently symptomatic carotid artery stenosis Settings: hospital Intervention: very early cerebral revascularization (within two days) Comparison: delayed treatment (after two days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Very early cerebral revascularization | Delayed Treatment | |||||
| Stroke and Death < 30 days | This outcome was poorly reported | RR 3.3 (0.4 to 29.2) | 40 (1 study) | ⊝⊝⊝ | ||
| Perioperative death and strokes | This outcome was poorly reported | RR 0.5 (0.1 to 1.6) | 40 (1 study) | ⊝⊝⊝ | ||
| Lenght of hospital stay | This outcome was poorly reported: One study reported no difference between groups. No standard deviation was reported. | |||||
| Myocardial infarction < 30 days | This outcome was not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level: one study with small sample size 2Downgraded one level due to risk of bias: incomplete outcome data 3Downgraded one level due to uncertainty in outcome measurement | ||||||
Background
Description of the condition
Annually, approximately 15 million people have a stroke worldwide, and 5.5 million of them die as a result of the event (Mackay 2004). Approximately 88% of all strokes are ischemic (Park 2012), and thromboembolism from an atheromatous plaque in the extracranial carotid artery has been determined to be the sole cause of stroke or a transient ischemic attack (TIA) in about 8% (Flaherty 2013; Park 2012). The risk of developing a future cerebrovascular ischemic event is related to inadequate cerebral blood perfusion as a result of hemodynamic stenosis, or plaque rupture and obstruction of smaller, more distally located blood vessels (Mokin 2013). In people with a recent cerebral or ocular ischemic episode, in whom the relevant (i.e. symptomatic) carotid artery is patent but the atheroma has caused a stenosis, some type of revascularization procedure may be required. According to different guidelines, surgical revascularization is recommended for people with symptomatic 50% to 99% carotid artery stenosis (Brott 2011; Kakisis 2012; Ricotta 2011). Carotid revascularization can be achieved by carotid endarterectomy or carotid artery stenting. The effectiveness of carotid endarterectomy for stroke prevention in people with symptomatic extracranial carotid disease has been established in randomized controlled trials (RCTs; Rothwell 2003). Angioplasty with stenting has emerged as an alternative, less invasive therapeutic method of treating all patients with carotid disease, with the increased risk of periprocedural stroke or death limited to older patients (Bonati 2012).
Description of the intervention
Since the 1950s, when carotid endarterectomy was introduced as an option for treatment and prevention of stroke, the number of procedures has increased (Rerkasem 2011). Carotid revascularization can reestablish adequate blood flow by removal of a critical carotid stenosis; this can be accomplished by surgical or endovascular treatment. Surgical options comprise conventional and eversion carotid endarterectomy. Conventional carotid endarterectomy is achieved by a longitudinal carotid arteriotomy with patch angioplasty, whereas eversion carotid endarterectomy involves a transection of the internal carotid artery and reimplantation into the common carotid artery (Antonopoulos 2011; Ricotta 2011). Clamping of the carotid arteries is required for removal of the plaque in both techniques (Kakisis 2012).
Furthermore, endovascular treatment may be a useful alternative to carotid endarterectomy. Carotid artery stenosis can be treated after a catheter inserted into the femoral artery is advanced, and after the target vessel lesion has been crossed, by deploying a self‐expanding stent to cover the entire lesion (Kakisis 2012). Several embolic protection systems, including filters and flow arrest and reversal devices, have been developed in an attempt to reduce the chances of cerebral embolization (Paraskevas 2016). In addition, a transcervical approach carotid artery stenting using flow reversal protection has also been described (EMPIRE 2011).
How the intervention might work
The timing of revascularization of symptomatic internal carotid artery stenosis has changed over the years. Rothwell 2003 showed greater benefit of surgery when performed within 14 days of the last symptomatic event. However, no definite time lag has been observed between occurrence of the neurologic event and surgery. Early revascularization is beneficial for symptomatic patients because this intervention may prevent a disabling or fatal stroke, but an early intervention may increase perioperative risk of stroke and death from carotid revascularization (Capoccia 2012; Imai 2005; Rantner 2011).
An ischemic stroke recurrence and perioperative risks of revascularization have very different mechanisms (Rerkasem 2011). The presumption is that early revascularization will improve perfusion to a large territory at risk surrounding an infarct core (i.e. the penumbra), will recruit brain areas before cell death occurs, and will prevent new embolic events (Capoccia 2011; Ricotta 2011). Major concerns about early revascularization after TIA or stroke include the following:
-
Transformation of the preexisting infarct core into a hemorrhagic lesion as a result of reperfusion or hyperperfusion injury (Ferrero 2014; Tsivgoulis 2014);
-
Increased vulnerability of recently infarcted brain tissue to periprocedural cerebral ischemia induced by carotid clamping or profoundly decreased blood pressure (Tsivgoulis 2014);
-
Increased vulnerability of carotid plaques in the early phase of cerebral ischemia and thus hazardous removal without distal embolization (Strömberg 2012; Tsivgoulis 2014).
On one hand, the danger of delaying investigation and treatment after a TIA or a nondisabling stroke depends on the early risk of ischemic recurrence (Lovett 2003; Rantner 2011). Early risk of stroke after a TIA or a non‐disabling stroke is around 3.1% to 5.2% within 48 hours; this increases to 8.3% to 11.2% at 14 days (Giles 2007; Johansson 2013; Marnane 2011). On the other hand, the risk of hemorrhagic transformation or of dislodging a thrombus is a matter of major concern during the early intervention. All major adverse events and vascular complications that occur within 30 days of the procedure are attributed to the procedure. Within 30 days of endarterectomy, the risk of death or stroke is 2.4% to 7.6% within 48 hours, and 0.8% to 6.9% at 14 days (Halm 2009; Johansson 2013; Rantner 2013; Sharpe 2013).
In addition, carotid artery stenting has a periprocedural stroke and death risk of 7.1% within 48 hours of a neurologic event, and 2.8% to 8.1% at 14 days (Rantner 2013; Wach 2014). The theoretical benefit of early carotid artery stenting is that it provides the possibility of reopening a critical stenosis with rare reduction in cerebral blood flow (Imai 2005). However, carotid artery stenting within the first few days of a neurologic event may carry excessive risk of mobilization of a thrombus (Rantner 2013).
Why it is important to do this review
The inability to predict which patients may have neurologic worsening during the first 24 to 48 hours after stroke may explain the variation in management of acute stroke among physicians and institutions (Battocchio 2012; Ferrero 2014).
In the UK, current guidelines recommend that carotid intervention should ideally be performed within two days for symptomatic severe carotid stenosis in stable patients (as cited in Capoccia 2011; Strömberg 2012). In the USA, it is recommended that carotid endarterectomy should be performed within two weeks of the neurologic event, and urgent revascularization may be considered for stable patients who have limited areas of infarct with a large penumbra (Ricotta 2011).
Analysis of RCTs is necessary to determine whether increased risk of recurrent events outweighs the increased risk of an earlier intervention. Reliable data on risk of surgery or carotid artery stenting in relation to timing of the intervention are necessary if clinicians are to plan surgery or carotid artery stenting most effectively, to adjust risks for case‐mix, and to understand the mechanisms of operative stroke.
Objectives
To assess the risks and benefits of performing very early cerebral revascularization (within two days) compared with delayed treatment (after two days) for people with recently symptomatic carotid artery stenosis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that compared early intervention versus delayed revascularization in people with recently symptomatic carotid artery stenosis (up to 14 days from onset of symptoms). We included studies only if data on clinically significant endpoints, such as ischemic stroke, hemorrhagic stroke, or death, were available.
Types of participants
We included people who had suffered a recent neurologic (TIA or stroke) event ipsilateral to stenosis of 50% to 99% in the carotid artery. We defined a stroke as any cerebrovascular or retinal event with symptoms lasting longer than 24 hours (Rerkasem 2011; Rothwell 2003), and a TIA as a focal neurologic deficit lasting 24 hours (Ferrero 2014; Giles 2007). We defined a nondisabling stroke as a stroke that resulted in no disability of functional significance (modified Rankin score < 3; Brott 2011; Rerkasem 2011; Rothwell 2003).
Brain imaging that demonstrated a new lesion involving a different anatomic site or vascular territory from the index event could be used to support the diagnosis of perioperative stroke. We classified strokes as disabling or nondisabling (as defined by trial authors), fatal or nonfatal, or contralateral, ipsilateral, hemorrhagic, or ischemic.
Types of interventions
All techniques aimed at revascularization in symptomatic carotid artery stenosis, including but not confined to:
-
percutaneous transluminal balloon angioplasty and stenting;
-
carotid endarterectomy; and
-
carotid eversion.
We included randomized studies comparing early intervention (within two days) versus delayed treatment (after two days).
Types of outcome measures
Primary outcomes
-
The combined outcome of any stroke or death occurring within 30 days of surgery or endovascular treatment (postoperative).
Secondary outcomes
-
Myocardial infarction within 30 days of surgery.
-
Myocardial infarction within 30 days of endovascular treatment.
-
Strokes or deaths, or both, during the perioperative period. For the purposes of this review the term perioperative refers to the three phases of surgery: preoperative, intraoperative, and postoperative, and commonly includes ward admission, anesthesia, surgery, and recovery.
-
Duration of procedures, length of hospital stay, and procedure‐related costs (if data are available).
-
Significant local complications related to revascularization, such as access site hematoma, infection, cranial nerve palsy, or pseudoaneurysm formation.
Myocardial infarction is defined by the presence of two of the following criteria:
-
Specific cardiac enzymes more than twice the upper limit of normal;
-
History of chest discomfort lasting at least 30 minutes;
-
Development of specific abnormalities on a standard 12‐lead electrocardiogram (ECG).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for relevant trials published in all languages and arranged translation of trial reports if required.
Electronic searches
We searched the Cochrane Stroke Group's Trials Register (strokecenter.org/trials) and the following electronic databases and trials registers:
-
The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 1; Appendix 1);
-
MEDLINE (Ovid; 1948 to 26 January 2016; Appendix 2);
-
EMBASE (Ovid; 1948 to 26 January 2016; Appendix 3);
-
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS; 1948 to 26 January 2016; Appendix 4);
-
ClinicalTrials.gov (clinicaltrials.gov)
-
Current Controlled Trials (controlled‐trials.com)
-
European Union (EU) Clinical Trials Register (clinicaltrialsregister.eu)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch)
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and modified it for use with the other databases.
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials, we:
-
searched the reference lists of identified studies and reviews;
-
contacted study authors and experts in the field;
-
contacted relevant pharmaceutical companies; and
-
used Science Citation Index Cited Reference Search for forward tracking of important articles.
Data collection and analysis
Selection of studies
Two review authors (VV and NC) independently screened titles and abstracts of the references obtained as a result of our searching activities and excluded obviously irrelevant reports. We retrieved full‐text articles for the remaining references, and two review authors (VV and NC) independently screened the full‐text articles, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion; if required, we consulted a third review author (JCCBS) for resolution. We collated multiple reports on the same study, so that each study, not each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 1).
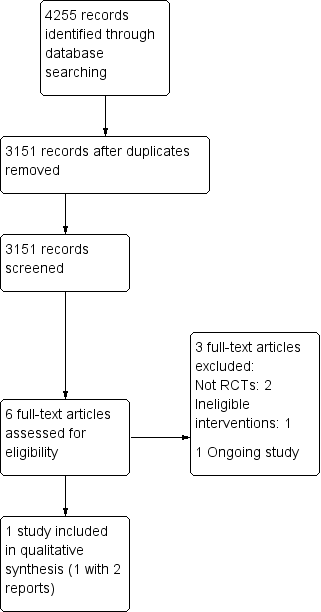
Study flow diagram.
Data extraction and management
Two review authors (VV and NC) independently extracted the following data from eligible studies and recorded this information on standard data extraction forms:
-
Participants: sample size, age, sex, number of participants originally allocated to each treatment group, diagnostic criteria used for carotid stenosis, number of participants in each group with early or delayed intervention.
-
Intervention: time interval from onset of symptoms of TIA or stroke to randomization, and from randomization to surgery, type of anesthesia, technique of carotid endarterectomy, technique of carotid artery stenting, any other major vascular surgery.
-
Outcomes: number of participants in each group with outcome events, including stroke, myocardial infarction, and death.
-
Withdrawals and adverse effects.
-
Length of follow‐up.
-
Additional important information.
We resolved disagreements between review authors by discussion.
Assessment of risk of bias in included studies
Two review authors (VV and NC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by involving another review author (JCCBS). We assessed the following domains as 'Yes' (low risk of bias), 'Unclear' (uncertain risk of bias) or 'No' (high risk of bias):
-
Random sequence generation: was the sequence generation adequate?
-
Allocation concealment: was allocation adequately concealed?
-
Blinding of participants and personnel: were participants and personnel blinded to the allocated interventions?
-
Blinding of outcome assessment: were the outcome assessors blinded to the allocated interventions?
-
Incomplete outcome data: were incomplete outcome data adequately addressed and similar across intervention groups?
-
Selective outcome reporting: are reports of the study free of the suggestion of selective outcome reporting?
-
Other bias: was the study apparently free of other problems that could put it at a risk of bias?
We graded the risk of bias for each domain as high, low, or unclear and provided information from the study report together with a justification for our judgment in the 'Risk of bias' tables.
Measures of treatment effect
For dichotomous variables, we calculated risk ratios (RRs) and 95% confidence intervals (CIs). For continuous data, we would have calculated mean differences (MDs) and 95% CIs between treatment groups if studies reported exactly the same outcomes. If similar outcomes were reported on different scales, we would have calculated the standardized mean difference (SMD) and the 95% CI. The most appropriate way of summarizing time‐to‐event data would have been to use methods of survival analysis while expressing the intervention effect as a hazard ratio; we extracted these data directly from the results of studies (Higgins 2011).
Unit of analysis issues
We based the unit of analysis on the individual participant (unit randomized for interventions to be compared; i.e. the number of observations in the analysis should have matched the number of individuals randomly assigned).
Dealing with missing data
For missing or unavailable data, we contacted study authors to request additional information. If we received no response, regardless of the type of data, we had planned to report dropout rates in the 'Characteristics of included studies' tables of the review, and use intention‐to‐treat analysis (Higgins 2011). Therefore, we included all randomized participants in the groups to which they were allocated.
Assessment of heterogeneity
We would have assessed clinical and methodological diversity in terms of participants, interventions, outcomes, and study characteristics for the included studies in order to determine whether a meta‐analysis was appropriate. We would have conducted this by observing these data from the data extraction tables. We would have assessed statistical heterogeneity by visual inspection of the forest plot for obvious differences in results between the studies, and by using the I² and Chi² statistical tests.
We would have qualified inconsistency among pooled estimates by using the I² statistic: ((Q ‐ df)/Q) × 100% test, where Q was the Chi² statistic and df represented the degree of freedom). This would have examined the percentage of total variation across studies due to heterogeneity rather than to chance (Higgins 2003; Higgins 2011).
We would have used these thresholds for interpretation of I²:
-
0% to 25%: low heterogeneity.
-
25% to 75%: moderate heterogeneity.
-
Greater than 75%: significant heterogeneity (Higgins 2003).
Assessment of reporting biases
Given the limited number of eligible trials available, we did not create funnel plots to assess reporting biases. In future updates of this review, we will interpret any funnel plot asymmetry with caution.
Data synthesis
Methods of synthesizing studies depend on quality, design, and heterogeneity. We would have explored both clinical and statistical heterogeneity. Where we considered studies to be sufficiently similar, we would have conducted a meta‐analysis by pooling appropriate data using RevMan (RevMan 2014).
We assessed the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE 2015) approach and constructed 'summary of findings Table for the main comparison for the outcomes.
Subgroup analysis and investigation of heterogeneity
If we had found sources of heterogeneity, and when data were sufficient, we had planned to conduct meta‐analyses by subgroups. If we had identified an adequate number of studies, we had planned to performed subgroup analyses according to participant age, participant sex, type of revascularization, degree of stenosis, and initial neurologic severity.
If we had identified no significant heterogeneity, we had planned to compute pooled estimates of the treatment effect for each outcome using a fixed‐effect model. When we detected significant heterogeneity despite subgroup analyses, we would have calculated the pooled estimate of treatment effects using random‐effects models.
Sensitivity analysis
If an adequate number of studies had been identified, we had planned to perform sensitivity analyses based on separation of studies according to risk of bias. We would have done this by excluding trials that were most susceptible to bias, based on our risk of bias assessment: those with inadequate allocation concealment, high levels of post‐randomization losses or exclusions, and uncertain or unblinded outcome assessment (Deeks 2011).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We identified a total of 4255 articles, and after removing duplicates, we manually screened 3151 records. We identified six articles from the search of the Cochrane Stroke Group's Trials Register; two reports of one study fulfilled the entry criteria (McCollum 2004). There were no randomized clinical trials involving carotid percutaneous transluminal balloon angioplasty and stenting. Figure 1 depicts the process of study identification and selection.
Included studies
To date we have identified one completed randomized controlled trial comparing very early cerebral revascularization with delayed treatment for individuals with recently symptomatic carotid artery stenosis (McCollum 2004). However, it compared very early surgery with surgery performed after 14 days of the last symptomatic event. Forty participants fit for surgery with partial anterior circulation infarction and a Barthel score greater than 18 within the preceding seven days and with 70% ipsilateral internal carotid stenosis were randomized into two surgical treatment groups:
-
very early surgery (within 24 hours); and
-
late surgery (six to eight weeks).
Participants in the late surgery group received the best medical care at the time, which consisted of antiplatelet therapy and rigorous attention to the control of risk factors. Outcomes were assessed at seven days, and two, six and 12 months following the surgery.
Excluded studies
Excluded studies were those that did not meet the inclusion criteria after we had reviewed the full text. We excluded three studies because they were:
-
confounded with no control group (Keunen 2012); or
-
non‐acute study (Ballota 2002, Sbatigia 2003); or
-
not randomized (Keunen 2012, Sbatigia 2003).
Ongoing studies
There is one ongoing randomized trial that involves the timing of cerebral revascularization (SPREAD‐STACI). Correspondence with the principal investigator indicated that a full publication is planned in a few years.
Risk of bias in included studies
The included study was of very low methodological quality (McCollum 2004). This study was classified as having a low risk of bias for randomization, performance and reporting. It was also classified as having an unclear risk of bias for detection and allocation concealment because the study did not provide any information on this. We judged that the included study was at high risk for attrition and small sample size biases (Figure 2).
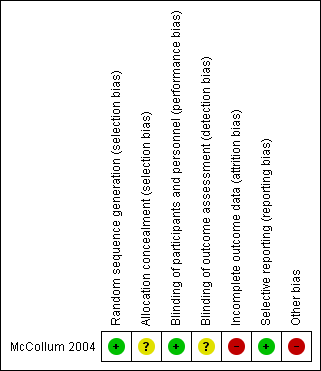
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomization of 40 participants with recently symptomatic carotid artery stenosis was undertaken by computer. Although the participants in the early group underwent surgery soon after randomization, the time between the neurological event and the operation was not clear. According to the author, most of the participants in the trial were operated on within a day or two of cerebral infarction. However, there were no available data on the proportion who underwent surgery within 48 hours of the onset of symptoms (personal communication).
Blinding
Due to the study design and the nature of the intervention and outcomes, health workers and participants were not blinded to treatment or outcome.
All the carotid endarterectomies were performed by the same vascular surgeon, using a standard surgical technique. Participants were followed up by the trial coordinator seven days after treatment and then again at two, six, and 12 months following randomization. No reference was made as to whether the outcome assessor was blinded to the timing of surgery.
Incomplete outcome data
Of the 21 participants randomized to 'delayed' surgery, nine participants did not undergo carotid endarterectomy at two months. Data were analyzed on an intention‐to‐treat basis. The included study reported losses, and therefore, was classified as having a high risk of attrition bias
Selective reporting
Outcome measures were recorded using the Barthel Activities of Daily Living score for disability and the Modified Rankin Scale for independence. All of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported
Other potential sources of bias
We assessed possible bias by small size of the included study, as small studies have been shown to overestimate treatment effects, allowing critical criteria to be compromised. We considered studies to be at low risk of bias if they had 200 participants or more per arm, at unclear risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants (Dechartres 2013). Therefore, we considered the included study was at high risk.
Effects of interventions
In the one included study, both study groups achieved an adequate distribution of patient characteristics such as age, sex, and the severity of carotid disease or stroke (McCollum 2004).
Nineteen participants were allocated to early surgery (usually within two to six hours) and 21 to late surgery (six to eight weeks). Outcome was determined 12 months after the stroke in this study. Nine of the 21 participants randomized to the delayed group did not undergo surgery due to occlusion of internal carotid following randomization (three participants), participants refusal of surgery (three participants), considered to be medically unfit for surgery after stroke progression (two participants), and regression of carotid stenosis to less than 70% (one participant).
We used a random‐effects model because, after inclusion of future studies, it is possible that the interventions themselves will differ from one study to the next. The timing of the intervention, or the 'best medical therapy' can be very different across time, as seen in other studies (Ballota 2002; McCollum 2004).
Relative risks between early and late surgery did not attain statistical significance for any of the outcomes assessed (see Data and analyses). When we considered the cumulative unweighted risk of stroke or death, or both, within 30 days of surgery, the risks for late and early surgery were 5% (1/21) and 16% (3/19), respectively. Three post‐operative strokes were recorded in the early group and none in the delayed group. Late surgery was favored, although confidence intervals were wide (RR 3.32; 95% CI 0.38 to 29.23; Analysis 1.1). However, when we considered the combined outcome of perioperative death and strokes, the risks for the early and delayed groups were 16% (3/19) and 33% (7/21), respectively. Perioperative death rate was 5% in the delayed surgical group and 0% in the early group. In addition, six neurologic events were recorded before surgery in the late group and none in the early group. The relative risk between early and late surgery showed a trend for better outcome in participants undergoing early surgery. The benefits or hazards of early surgery in perioperative death and stroke risks from these data remains unclear (RR 0.47; CI 0.14 to 1.58; Analysis 1.2).
The average length of hospital stay was 11 days for the early group and 28 days for the delayed group. However, we were unable to estimate 95% CIs of the difference due to the lack of standard deviations.
Discussion
Summary of main results
Most studies that examine the influence of timing of cerebral revascularization on outcomes after a neurological event are not randomized. We identified only one completed, randomized study that compared timing of surgery after a stroke, and there were not enough data to draw any reliable conclusions about the preferred timing of surgery (McCollum 2004). Hence there is a need for further randomized trials.
There is one ongoing RCT that compares the timing of surgery after an acute neurological event (SPREAD‐STACI). The data should be available within the next two years.
Although the study compared very early surgery with surgery performed after 14 days of the onset of symptoms, there is already evidence that carotid endarterectomy performed within 14 days of the symptoms produces better long‐term results than if it is delayed (Rothwell 2003). Therefore, it is unclear whether the risk of stroke, death, or both in the delayed group was related to the carotid surgery or to the conditions of the participants.
Overall completeness and applicability of evidence
The primary outcome of this review, the combined outcome of stroke, death, or both occurring within 30 days of intervention, was poorly reported by the study, and the relative risks between early and late surgery did not attain statistical significance. Therefore, it was difficult to analyze and draw reliable conclusions.
Quality of the evidence
Overall, the included study was at a very high risk of bias. The reasons for downgrading the quality of the evidence related to the uncertainty in outcome measurement, the incompleteness of outcome data available in the study, and a very small sample size. The quality of all outcomes included in summary of findings Table for the main comparison was downgraded three levels, resulting in a very low‐quality of the evidence for all outcomes (GRADE 2015). Another issue is that medical management has changed over the study period, as more advanced pharmacological agents and more stringent management of various risk factors of atherosclerosis have led to an overall decline in the incidence of stroke.
Potential biases in the review process
We strived to prevent bias in the review process by involving two independent review authors at each step of the review, and by performing a comprehensive search with no language restrictions.
Agreements and disagreements with other studies or reviews
We identified other systematic reviews that examined the timing of interventions for recently symptomatic patients, which included non‐randomized clinical trials. They found no substantially elevated risk with early surgery in stable patients (Rerkasem 2009), but found an elevated risk in people with unstable neurological symptoms (Naylor 2009; Rerkasem 2009).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
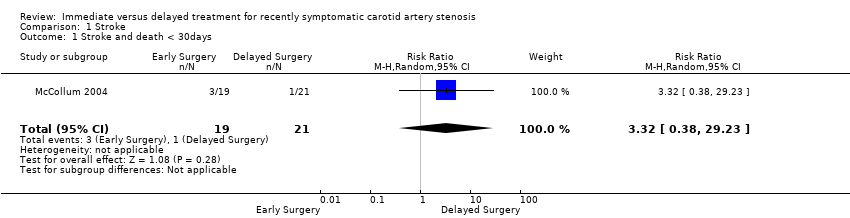
Comparison 1 Stroke, Outcome 1 Stroke and death < 30days.

Comparison 1 Stroke, Outcome 2 Perioperative death and all strokes.
| Very early cerebral revascularization compared with delayed treatment for recently symptomatic carotid artery stenosis | ||||||
| Patient or population: people with recently symptomatic carotid artery stenosis Settings: hospital Intervention: very early cerebral revascularization (within two days) Comparison: delayed treatment (after two days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Very early cerebral revascularization | Delayed Treatment | |||||
| Stroke and Death < 30 days | This outcome was poorly reported | RR 3.3 (0.4 to 29.2) | 40 (1 study) | ⊝⊝⊝ | ||
| Perioperative death and strokes | This outcome was poorly reported | RR 0.5 (0.1 to 1.6) | 40 (1 study) | ⊝⊝⊝ | ||
| Lenght of hospital stay | This outcome was poorly reported: One study reported no difference between groups. No standard deviation was reported. | |||||
| Myocardial infarction < 30 days | This outcome was not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level: one study with small sample size 2Downgraded one level due to risk of bias: incomplete outcome data 3Downgraded one level due to uncertainty in outcome measurement | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stroke and death < 30days Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 3.32 [0.38, 29.23] |
| 2 Perioperative death and all strokes Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.14, 1.58] |

