Tratamiento antifibrinolítico para la prevención de la hemorragia oral en pacientes con hemofilia o enfermedad de Von Willebrand sometidos a una intervención quirúrgica oral menor o extracciones dentales
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011385.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KPM van Galen: guarantor of the review, conceiving, designing, coordinating and writing of the review, data collection, extraction and critical appraisal. Screening of search results and of retrieved papers against eligibility criteria. Data analysis and interpretation.
ET Engelen: designing search strategies and undertaking searches. Screening of search results and of retrieved papers against eligibility criteria. Data collection, extraction, writing of the review and critical appraisal.
RJJ van Es: interpretation of data, providing a clinical perspective and general advice on the review.
EP Mauser‐Bunschoten: interpretation of data, providing a methodological, clinical, consumer and policy perspective.
REG Schutgens: supervising of the review, securing funding. Verifing the data extraction of trials identified for inclusion.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Karin PM van Galen: none known.
Eveline T Engelen: none known.
Evelien Mauser‐Bunschoten: in my opinion there will be no conflict of interest. Full disclosure ‐ I have provided expert opinion to Baxter and my institution has received payments from Sanquin, NovoNordisk, Bayer, Baxter, Pfizer, Griffols for lectures I have given. My institution has also received funding in relation to a twinning program in Indonesia and education and cooperation with Indonesian haemophilia centers.
Robert JJ van Es: none known.
Roger EG Schutgens: I have no conflicts of interest to declare. Full disclosure ‐ I have provided consultancy to Bayer on new products for hemophilia and ADVANCE working group on behalf of my institution. My institution has received grants from Novo Nordisk for a project 'Project cartilage damage in hemophilia' and from Bayer for a project 'Triumph trial, immunotherapy in hemophilia'. My institution has also received payment from Biotest and Bayer for lectures I have given at hemophilia conferences.
Acknowledgements
We thank Bianca Kramer (Clinical Librarian), the Dutch Cochrane Center and the Cochrane Cystic Fibrosis and Genetic Disorders Group for their help in starting up and conducting this systematic review.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Apr 19 | Antifibrinolytic therapy for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing minor oral surgery or dental extractions | Review | Karin PM van Galen, Eveline T Engelen, Evelien P Mauser‐Bunschoten, Robert JJ van Es, Roger EG Schutgens | |
| 2015 Dec 24 | Antifibrinolytic therapy for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing minor oral surgery or dental extractions | Review | Karin PM van Galen, Eveline T Engelen, Evelien P Mauser‐Bunschoten, Robert JJ van Es, Roger EG Schutgens | |
| 2014 Dec 05 | Antifibrinolytic therapy for preventing oral bleeding in patients with a hemophilia or Von Willebrand disease undergoing oral or dental procedures | Protocol | Karin PM van Galen, Eveline T Engelen, Evelien P Mauser‐Bunschoten, Robert JJ van Es, Roger EG Schutgens | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Aminocaproic Acid [therapeutic use];
- Antifibrinolytic Agents [*therapeutic use];
- Blood Loss, Surgical [prevention & control];
- Factor IX [administration & dosage];
- Factor VIII [administration & dosage];
- Hemophilia A [complications, *drug therapy];
- Hemophilia B [complications, *drug therapy];
- Minor Surgical Procedures [adverse effects];
- Oral Hemorrhage [etiology, *prevention & control];
- Postoperative Hemorrhage [etiology, *prevention & control];
- Randomized Controlled Trials as Topic;
- Surgery, Oral;
- Tooth Extraction [*adverse effects];
- Tranexamic Acid [therapeutic use];
- von Willebrand Diseases [complications, *drug therapy];
Medical Subject Headings Check Words
Humans;
PICO
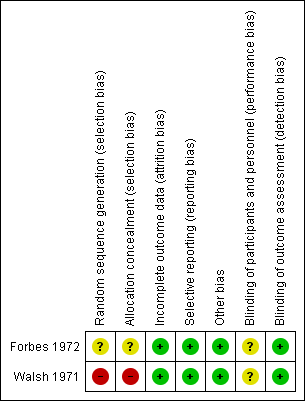
Risk of bias summary of the included studies
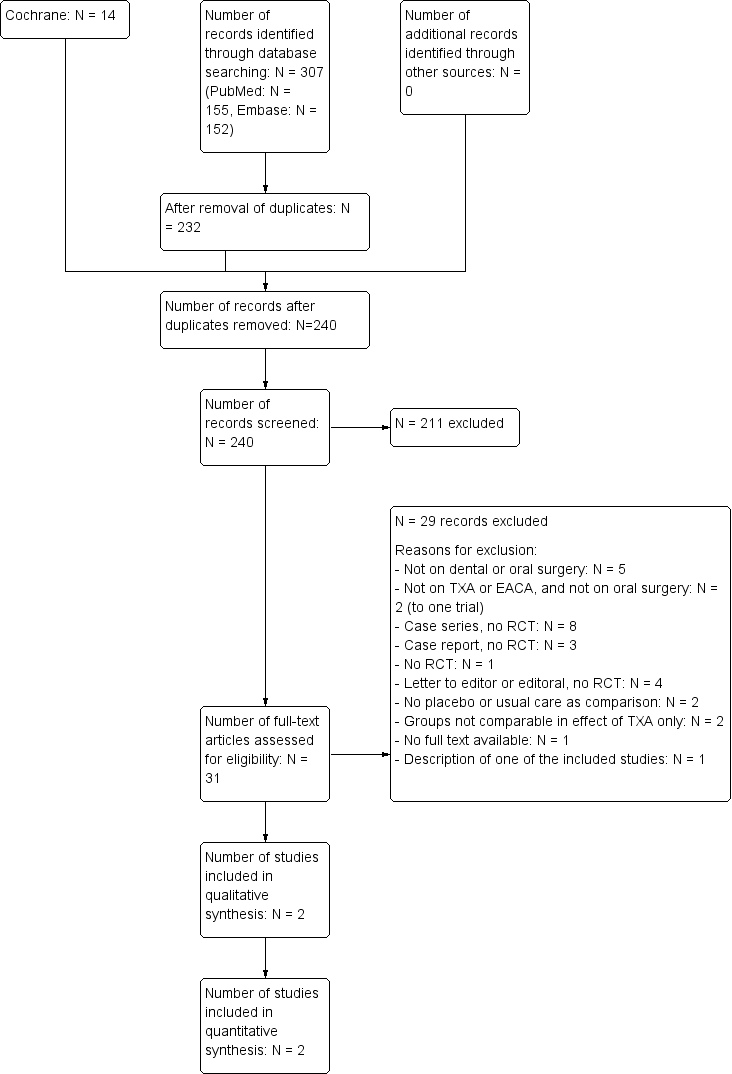
Trial flow diagram
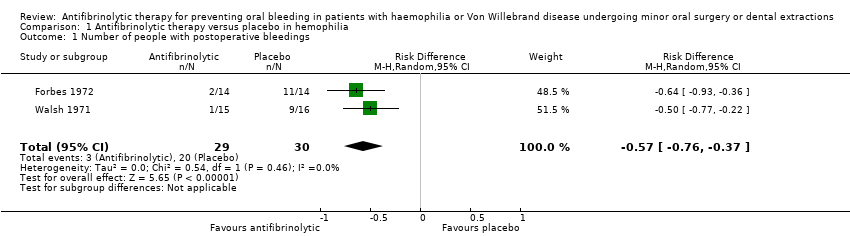
Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 1 Number of people with postoperative bleedings.

Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 2 Number of side effects requiring withdrawal.
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Outcomes | Anticipated absolute effects* | Relative effect | № of participants | Number needed to treat (NNT) | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with antifibrinolytic therapy | ||||||
| Postoperative bleedings requiring intervention | Study population | Risk difference: 57 | 59 | 1.8 | ⊕⊕⊕⊝ | ||
| 67 per 100 (20 events) | 10 per 100 (3 events) | ||||||
| Side effects or other adverse events | Study population | Risk difference: 3.4 | 59 | 0.3 | ⊕⊕⊝⊝ | ||
| 0 events | 1 event | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 The magnitude of effect was considered large in this study, therefore the quality of evidence was rated up one level. 2 Small sample sizes and lack of studies 3 Heterogeneity between the included trials regarding the proportion of severe people with haemophilia included, the concomitant standard therapy and fibrinolytic agent treatment regimens used | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Primary outcomes | 1. Postoperative bleedings requiring intervention | 2. Side effects or other adverse events | |||||
| Absolute risks % (n/ntotal) | Risk difference (RD) | Number needed to treat (NNT) | Absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | |||
| Control | Intervention | Control | Intervention | ||||
| 79% (11/14) | 14% (2/14) | 65% (37 ‐ 93) | 1.5 | 0% (0/14) | 0% (0/14) | 0% | |
| 56% (9/16) | 7% (1/15) | 49% (21 ‐ 77) | 2.0 | 0% (0/16) | 7% (1/15) | 7% (‐0.06 ‐ 20) | |
| n: number; CI: confidence interval | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | ||||||||
| 1. Number of people with postoperative bleeding | 2. Side effects or other adverse events | |||||||
| Combined absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | Number needed to treat (NNT) | Combined absolute risks % (n/ntotal) | Risk difference (RD)(% (95% CI) | Number needed to treat (NNT) | |||
| Control | Intervention | Control | Intervention | |||||
| 67% (20/30) | 10% (3/29) | 56% (36% ‐ 76%) | 1.8 | 0% (0/30) | 3.4 (1/29) | 3.4 (‐32 ‐ 38) | 0.3 | |
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||
| Patient or population: Patients with haemophilia or Von Willebrand disease Settings: Hospitals, Hemophilia centres Intervention: Tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: Placebo or no intervention or usual care with or without placebo | ||||||
| Secondary outcomes | 1. (Mean) fall in haemoglobin level from baseline | 2. Amount of postoperative blood loss: mean per patient mL (range) | 3. Need for and dose of clotting factor concentrates: Mean number of units IU (range) of replacement therapy per root extracted (A) / per patient (B) | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| 1.4 g/100mL | 0.3 g/100mL | 84.1 mL (4‐323) | 61.2 mL (1‐749) | 617 IU (0‐15800) in eleven participants | 30 IU and 65 IU in two participants | |
| Trial centre 1: 6.3% Trial centre 2: 6.3% | Trial centre 1: 3.9% Trial centre 2: 3.7% | NR | NR | Trial centre 1: 2331 IU Trial centre 2: 1881 IU | Trial centre 1: 145 IU Trial centre 2: 0 IU | |
| Abbreviations:NR: not reported | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with postoperative bleedings Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | ‐0.57 [‐0.76, ‐0.37] |
| 2 Number of side effects requiring withdrawal Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.13] |

