Protocolos de recuperación mejorados para la cirugía pancreática, hepática y gastrointestinal superior de carácter mayor
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | Country: UK. Number randomised: 121 Post‐randomisation drop‐outs: 0 (0%) Revised sample size: 121 Average age: 64 years Females: 38 (31.4%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, mild adverse events and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified within each centre, and the randomisation sequence was generated by computer in permuted blocks of 30" |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was kept in opaque, sealed envelopes labelled with sequential study numbers in a locked box at the co‐ordinating research site" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an unblinded study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was an unblinded study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Other bias | Low risk | Quote: "The trial was funded by grant to Dr Barlow: "Leading Practice through research" from the The Health Foundation, London, UK" |
| Methods | Randomised controlled trial | |
| Participants | Country: UK. Number randomised: 104 Post‐randomisation drop‐outs: 13 (12.5%) Revised sample size: 91 Average age: 66 years Females: 37 (40.7%) Inclusion criteria All patients presenting for open liver surgery Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Preoperatively: education Postoperatively: early mobilisation and early oral feeding according to a specific schedule | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, health‐related quality of life, mild adverse events, length of hospital stay and readmissions | |
| Notes | Authors provided additional information in January 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence of group allocation by means of brown opaque envelopes was generated by an independent statistician from the University of Surrey" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization sequence of group allocation by means of brown opaque envelopes was generated by an independent statistician from the University of Surrey" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "no blinding" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "only assessor for fitness for discharge was blinded, meaning Length of stay was blinded" |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Thirteen patients were withdrawn after randomization because of changes to their original oncological staging" |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Other bias | Low risk | Quote: "Thanks also go to GUTS (Guildford Undetected Tumour Screening) and LCSA (Liver Cancer Surgery Appeal) charities who kindly provided grants helping to fund the trial" |
| Methods | Randomised controlled trial | |
| Participants | Country: Korea Number randomised: 47 Post‐randomisation drop‐outs: 3 (6.4%) Revised sample size: 44 Average age: 55 years Females: 16 (36.4%) Inclusion criteria
Exclusion criteria Factors that might impede a fast recovery:
| |
| Interventions | Participants were randomly assigned to two groups Preoperatively: education Postoperatively: pain relief, early mobilisation and early oral feeding according to a specific schedule | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, health‐related quality of life, mild adverse events, length of hospital stay, readmissions and costs | |
| Notes | Reasons for post‐randomisation drop‐outs: protocol violation (1); surgeon suspected an insecure anastomosis (2) ‐ both developed anastomotic stricture and required endoscopic stenting postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by drawing lots under stratification for gender by the co‐ordinator" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by drawing lots under stratification for gender by the co‐ordinator" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The nurses and physicians were told the result of the randomisation" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The nurses and physicians were told the result of the randomisation" |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Other bias | High risk | Quote: "The LAPD was supplied from B. Braun Korea Company just for this study" |
| Methods | Randomised controlled trial | |
| Participants | Country: New Zealand. | |
| Interventions | Participants were randomly assigned to two groups Preoperatively: education Postoperatively: pain relief, early mobilisation and early oral feeding according to a specific schedule | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, mild adverse events, mild adverse events, length of hospital stay and readmissions | |
| Notes | Reasons for post‐randomisation drop‐outs: Surgery changed to another site or cancelled or other miscellaneous reasons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by an independent researcher not involved in patient recruitment or outcome assessment using a computerized random‐number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocations were placed in sequentially numbered opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: Complications were not reported adequately |
| Other bias | Low risk | Quote: "This study required no external sources of funding" |
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 63 Post‐randomisation drop‐outs: 0 (0%) Revised sample size: 63 Average age: 61 years Females: 29 (46%) Inclusion criteria Patients undergoing gastrectomy for gastric cancer Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, mild adverse events, length of hospital stay and readmissions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed using opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "As with other fast‐track trials, it was not possible to blind this study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "As with other fast‐track trials, it was not possible to blind this study" |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: Complications were not reported adequately |
| Other bias | Unclear risk | Comment: Information on source of funding was not available |
| Methods | Randomised controlled trial | |
| Participants | Country: China. Number randomised: 297 Post‐randomisation drop‐outs: not stated Revised sample size: 297 Average age: 53 years Females: 53 (17.8%) Inclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Postoperatively: early mobilisation and early oral feeding Group 2: standard care (n = 162) | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, mild adverse events, length of hospital stay and readmissions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This trial was randomized and single‐blinded" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "This trial was randomized and single‐blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | High risk | Comment: Complications were not reported adequately |
| Other bias | Unclear risk | Comment: Information on source of funding was not available |
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Number randomised: 43 Post‐randomisation drop‐outs: 10 (23.3%) Revised sample size: 33 Average age: 60 years Females: 13 (39.4%) Inclusion criteria All patients aged 40 to 75 who underwent distal gastrectomy for gastric cancer Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups | |
| Outcomes | None of the outcomes of interest were reported | |
| Notes | Reasons for post‐randomisation drop‐outs: Onset of herpes zoster before the procedure (1), excessive intraoperative haemorrhage (> 600 mL) (2), injury to the left hepatic artery during surgery (1), changes in surgical procedure (2), metastasis to the peritoneum confirmed during surgery (1) and withdrawal of consent (3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were assigned to 1 of 2 groups using the sealed‐ envelope method and a randomized, single‐blind, parallel‐ group study was performed" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients were assigned to 1 of 2 groups using the sealed‐ envelope method and a randomized, single‐blind, parallel‐ group study was performed" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients were assigned to 1 of 2 groups using the sealed‐ envelope method and a randomized, single‐blind, parallel‐ group study was performed" |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: Complications were not reported adequately |
| Other bias | Unclear risk | Comment: Information on source of funding was not available |
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 160 Post‐randomisation drop‐outs: 0 (0%) Revised sample size: 160 Average age: 49 years Females: 35 (21.9%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Preoperatively: education Postoperatively: pain relief, early mobilisation and early oral feeding according to a specific schedule | |
| Outcomes | The outcomes reported were short‐term mortality, serious adverse events, health‐related quality of life, mild adverse events and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were assigned either to the FTS group or to the CS group by computer‐generated random numbers " |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Other bias | Low risk | Quote: "This study is supported by the StateKey Infectious Disease Project of China (2012ZX10002010, 2012ZX10002016), Science Fund for Creative Research Groups, NSFC, China 81221061, Nursing Research Fund of EHBH (12HL001)" |
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 94 Post‐randomisation drop‐outs: 2 (2.1%) Revised sample size: 92 Average age: 58 years Females: 31 (33.7%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Preoperatively: education Postoperatively: pain relief, early mobilisation and early oral feeding according to a specific schedule | |
| Outcomes | The outcomes reported were short‐term mortality, mild adverse events, length of hospital stay, readmissions and costs | |
| Notes | Reasons for post‐randomisation drop‐outs: 2 patients withdrew their consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Two patients who withdrew their consent in the FTS group were excluded from the study" |
| Selective reporting (reporting bias) | High risk | Comment: Complications were not reported adequately |
| Other bias | Unclear risk | Comment: Information on source of funding was not available |
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 80 Post‐randomisation drop‐outs: 12 (15%) Revised sample size: 68 Average age: 57 years Females: 16 (23.5%) Inclusion criteria Patients with oesophageal cancer undergoing oesophagectomy Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Preoperatively: education Postoperatively: pain relief according to a specific schedule | |
| Outcomes | The outcomes reported were serious adverse events, mild adverse events, length of hospital stay, readmissions and costs | |
| Notes | Reasons for post‐randomisation drop‐outs: Protocol violation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled patients were randomly assigned to two groups using computer‐generated random numbers (random digits from 0 to 99)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigators who designed the study prepared the envelopes and assigned participants to their groups but had no contact with the patients throughout the study. The investigator who recruited the patients, administered the interventions, and evaluated the outcomes was not involved in the randomization process" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: Complications were not reported adequately |
| Other bias | Low risk | Quote: Science Foundation of Heilongjiang health dept, the science foundation of Heilongjiang Education dept and the China postdoctoral Science foundation |
BMI: body mass index; CS: conventional surgery; FTS: fast track surgery; LAPD: local anaesthesia pump device; LSG: laparoscopic
sleeve gastrectomy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not specific to major upper gastrointestinal, liver or pancreatic surgery | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Comparison between two enhanced recovery protocols | |
| Not a randomised controlled trial | |
| Not specific to major upper gastrointestinal, liver or pancreatic surgery | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not major upper gastrointestinal, liver or pancreatic surgery | |
| Comment on an included study (Jones 2013) | |
| Not major upper gastrointestinal, liver or pancreatic surgery |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | Patients undergoing laparoscopic Roux‐en‐Y gastric bypass surgery |
| Interventions | Fast‐track surgery |
| Outcomes | Complication rate (no raw data) |
| Notes | Unclear if enhanced recovery protocol, as per definition used in the review, was used |
| Methods | Randomised controlled trial |
| Participants | Patients undergoing pancreaticoduodenectomy |
| Interventions | Fast‐track surgery |
| Outcomes | Complication rate, length of hospital stay (no raw data) |
| Notes | Unclear if enhanced recovery protocol, as per definition used in the review, was used |
| Methods | Randomised controlled trial |
| Participants | Patients undergoing laparoscopic assisted gastrectomy |
| Interventions | Enhanced recovery protocol |
| Outcomes | Length of hospital stay (no results reported) |
| Notes | Unclear if enhanced recovery protocol, as per definition used in the review, was used |
| Methods | Randomised controlled trial |
| Participants | Patients undergoing gastrectomy |
| Interventions | Fast‐track surgery |
| Outcomes | Complication rate, length of hospital stay and costs (number of people allocated to each group was not reported) |
| Notes | Unclear if enhanced recovery protocol, as per definition used in the review, was used |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | JPRN‐UMIN000011572 |
| Methods | Randomised controlled trial |
| Participants | Patients requiring pancreaticoduodenectomy |
| Interventions | Enhanced recovery after protocol |
| Outcomes | Length of postoperative hospital stay, postoperative morbidity and mortality, medical cost and safety |
| Starting date | May 2014 |
| Contact information | Ryuichi Yoshida (ryuichi‐[email protected]‐u.ac.jp) |
| Notes |
| Trial name or title | JPRN‐UMIN000014068 |
| Methods | Randomised controlled trial |
| Participants | Patients requiring gastrectomy |
| Interventions | Enhanced recovery after protocol |
| Outcomes | Length of postoperative hospital stay, postoperative morbidity and mortality |
| Starting date | September 2013 |
| Contact information | Kazuhisa Uchiyama ([email protected]‐med.ac.jp) |
| Notes |
| Trial name or title | NCT01766765 |
| Methods | Randomised controlled trial |
| Participants | Patients requiring laparoscopic gastrectomy |
| Interventions | Enhanced recovery after protocol |
| Outcomes | Length of postoperative hospital stay and mortality |
| Starting date | April 2013 |
| Contact information | Qi Mao ([email protected]) |
| Notes |
| Trial name or title | NCT01938313 |
| Methods | Randomised controlled trial |
| Participants | Patients requiring laparoscopic gastrectomy |
| Interventions | Enhanced recovery after protocol |
| Outcomes | Length of postoperative hospital stay, postoperative complications and quality of life |
| Starting date | August 2012 |
| Contact information | Hyung‐Ho Kim ([email protected]) |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 7 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.44, 17.73] |
| Analysis 1.1  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 1 Short‐term mortality. | ||||
| 2 Serious adverse events (proportion) Show forest plot | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [0.68, 45.89] |
| Analysis 1.2  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 2 Serious adverse events (proportion). | ||||
| 3 Serious adverse events (number) Show forest plot | 7 | 859 | Rate Ratio (Fixed, 95% CI) | 0.72 [0.45, 1.13] |
| Analysis 1.3  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 3 Serious adverse events (number). | ||||
| 4 Health‐related quality of life Show forest plot | 4 | 373 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| Analysis 1.4  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 4 Health‐related quality of life. | ||||
| 5 Mild adverse events (proportion) Show forest plot | 4 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.39, 0.85] |
| Analysis 1.5  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 5 Mild adverse events (proportion). | ||||
| 6 Mild adverse events (number) Show forest plot | 9 | 1014 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.39, 0.70] |
| Analysis 1.6  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 6 Mild adverse events (number). | ||||
| 7 Length of hospital stay Show forest plot | 9 | 1014 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐2.53, ‐1.85] |
| Analysis 1.7  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 7 Length of hospital stay. | ||||
| 8 Readmissions Show forest plot | 7 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.69, 2.87] |
| Analysis 1.8  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 8 Readmissions. | ||||
| 9 Costs Show forest plot | 4 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.84, ‐0.42] |
| Analysis 1.9  Comparison 1 Enhanced recovery protocol versus standard care, Outcome 9 Costs. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 6 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.17] |
| Analysis 2.1  Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 1 Short‐term mortality. | ||||
| 1.1 Oesophagectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Gastrectomy | 3 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.17] |
| 1.3 Liver surgery | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (proportion) Show forest plot | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [0.68, 45.89] |
| Analysis 2.2  Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 2 Serious adverse events (proportion). | ||||
| 2.1 Oesophagectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gastrectomy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.87] |
| 2.3 Liver surgery | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.39 [0.44, 161.01] |
| 3 Serious adverse events (number) Show forest plot | 6 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.53, 1.43] | |
| Analysis 2.3  Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 3 Serious adverse events (number). | ||||
| 3.1 Oesophagectomy | 1 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.01, 8.12] | |
| 3.2 Gastrectomy | 2 | Rate Ratio (Fixed, 95% CI) | 1.10 [0.35, 3.49] | |
| 3.3 Liver surgery | 3 | Rate Ratio (Fixed, 95% CI) | 0.84 [0.48, 1.48] | |
| 4 Health‐related quality of life Show forest plot | 4 | 373 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| Analysis 2.4  Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 4 Health‐related quality of life. | ||||
| 4.1 Oesophagectomy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gastrectomy | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.32] |
| 4.3 Liver surgery | 2 | 251 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.27, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life Show forest plot | 3 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.19, 0.53] |
| Analysis 3.1  Comparison 3 Enhanced recovery protocol versus standard care (sensitivity analysis), Outcome 1 Health‐related quality of life. | ||||
| 2 Length of hospital stay Show forest plot | 3 | 267 | Mean Difference (IV, Fixed, 95% CI) | ‐2.31 [‐2.93, ‐1.69] |
| Analysis 3.2  Comparison 3 Enhanced recovery protocol versus standard care (sensitivity analysis), Outcome 2 Length of hospital stay. | ||||
| 3 Costs Show forest plot | 3 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.81, ‐0.39] |
| Analysis 3.3  Comparison 3 Enhanced recovery protocol versus standard care (sensitivity analysis), Outcome 3 Costs. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
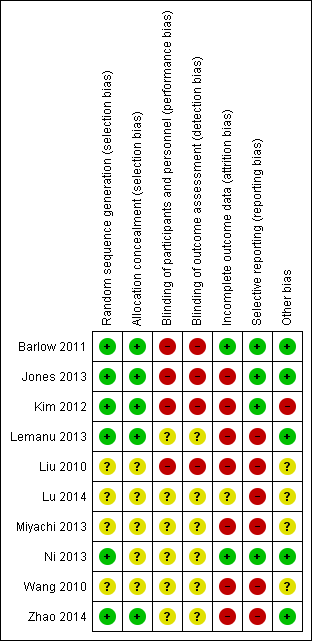
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 1 Short‐term mortality.

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 2 Serious adverse events (proportion).

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 3 Serious adverse events (number).
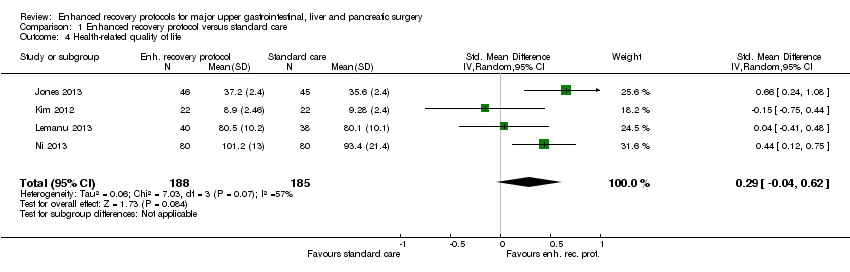
Comparison 1 Enhanced recovery protocol versus standard care, Outcome 4 Health‐related quality of life.

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 5 Mild adverse events (proportion).
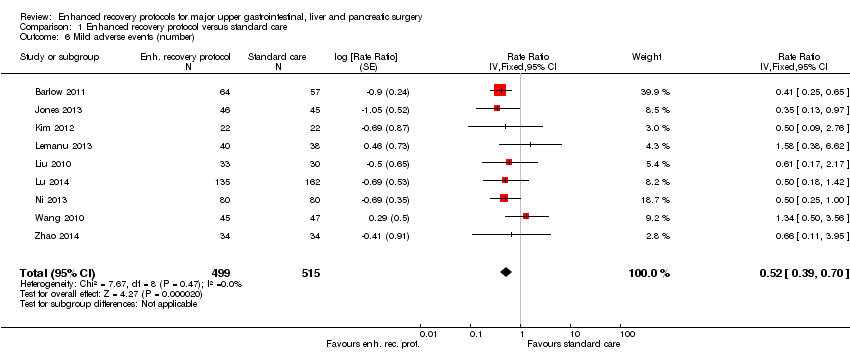
Comparison 1 Enhanced recovery protocol versus standard care, Outcome 6 Mild adverse events (number).

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 7 Length of hospital stay.

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 8 Readmissions.

Comparison 1 Enhanced recovery protocol versus standard care, Outcome 9 Costs.

Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 1 Short‐term mortality.
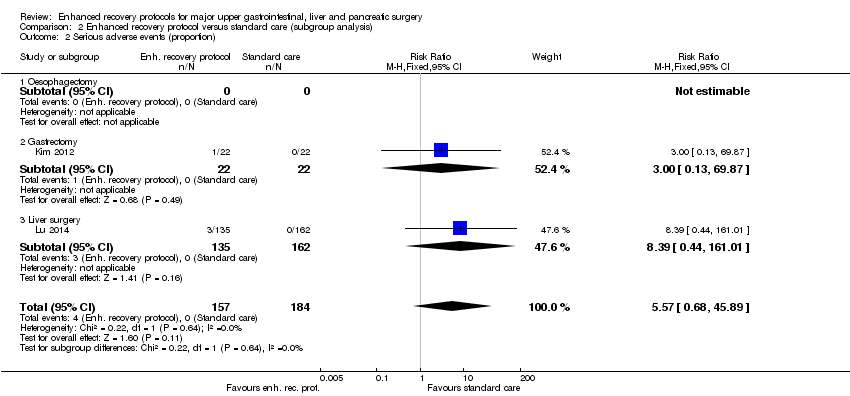
Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 2 Serious adverse events (proportion).

Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 3 Serious adverse events (number).

Comparison 2 Enhanced recovery protocol versus standard care (subgroup analysis), Outcome 4 Health‐related quality of life.

Comparison 3 Enhanced recovery protocol versus standard care (sensitivity analysis), Outcome 1 Health‐related quality of life.
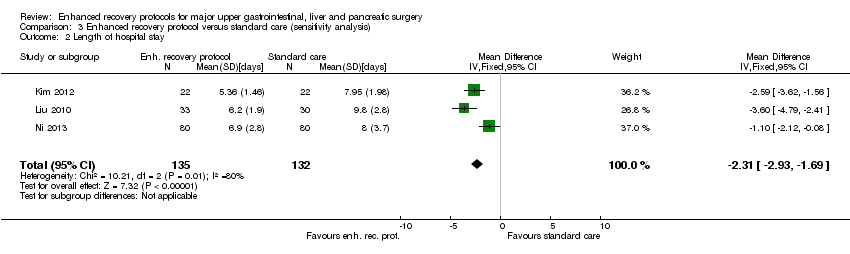
Comparison 3 Enhanced recovery protocol versus standard care (sensitivity analysis), Outcome 2 Length of hospital stay.

Comparison 3 Enhanced recovery protocol versus standard care (sensitivity analysis), Outcome 3 Costs.
| Enhanced recovery protocols versus standard care for major upper gastrointestinal, liver and pancreatic surgery | ||||||
| Patient or population: people with major upper gastrointestinal, liver and pancreatic surgery Control: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Enhanced recovery protocol versus standard care | |||||
| None of the trials reported long‐term mortality, medium‐term health‐related quality of life (3 months to 1 year), time to return to normal activity, or time to return to work. | ||||||
| Short‐term mortality | 2 per 1000 | 6 per 1000 | RR 2.79 | 868 | ⊕⊝⊝⊝ | |
| Serious adverse events (proportion) | 1 per 1000 | 6 per 1000 | RR 5.57 (0.68 to 45.89) | 341 | ⊕⊝⊝⊝ | Since there were no serious adverse events in the control group (in the two trials that reported the proportion of participants with serious adverse events), the control group risk was stated as 0.1% for this outcome alone |
| Serious adverse events (number) | 105 per 1000 | 76 per 1000 | Rate ratio 0.72 | 859 | ⊕⊝⊝⊝ | |
| Health‐related quality of life (until 3 months) | The mean health‐related quality of life in the intervention groups was | 373 | very low1,4 | SMD 0.29 (‐0.04 to 0.62) | ||
| Mild adverse events (proportion) | 188 per 1000 | 109 per 1000 | RR 0.58 | 525 | ⊕⊕⊝⊝ | |
| Mild adverse events (number) | 249 per 1000 | 129 per 1000 | Rate ratio 0.52 | 1014 | ⊕⊕⊝⊝ | |
| Length of hospital stay | The mean length of hospital stay in the control groups was | The mean length of hospital stay in the intervention groups was | 1014 | ⊕⊕⊝⊝ | The length of hospital stay reported in the trials included only the length of hospital stay during the admission for surgery and does not include the readmissions | |
| Readmissions | 24 per 1000 | 33 per 1000 | RR 1.4 | 733 | ⊕⊝⊝⊝ | |
| Costs | The mean costs in the control groups were | The mean costs in the intervention groups were | 282 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk is the mean control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The risk of bias was high in all the trials. | ||||||
| Study name | Surgery | Preoperative education | Pain relief protocol | Early mobilisation protocol | Nutritional protocol | Growth factors | Outcomes reported |
| Major upper gastrointestinal surgery (oesophagectomy, gastrectomy and pancreatectomy) | No | No | No | Yes | No |
| |
| Open liver surgery | Yes | No | Yes | Yes | No |
| |
| Laparoscopic distal gastrectomy | Yes | Yes | Yes | Yes | No |
| |
| Laparoscopic sleeve gastrectomy | Yes | Yes | Yes | Yes | No |
| |
| Gastrectomy | Yes | Yes | Yes | Yes | No |
| |
| Liver surgery | No | No | Yes | Yes | No |
| |
| Gastrectomy | No | No | No | No | Yes | None of the outcomes of interest were reported | |
| Partial liver resection | Yes | Yes | Yes | Yes | No |
| |
| Gastrectomy | Yes | Yes | Ys | Yes | No |
| |
| Oesophagectomy | Yes | Yes | N | No | No |
| |
| The table shows the surgeries that the participants underwent, the elements of enhanced recovery protocol that were different between the intervention and control, and the outcomes reported in the trials. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 7 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.44, 17.73] |
| 2 Serious adverse events (proportion) Show forest plot | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [0.68, 45.89] |
| 3 Serious adverse events (number) Show forest plot | 7 | 859 | Rate Ratio (Fixed, 95% CI) | 0.72 [0.45, 1.13] |
| 4 Health‐related quality of life Show forest plot | 4 | 373 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| 5 Mild adverse events (proportion) Show forest plot | 4 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.39, 0.85] |
| 6 Mild adverse events (number) Show forest plot | 9 | 1014 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.39, 0.70] |
| 7 Length of hospital stay Show forest plot | 9 | 1014 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐2.53, ‐1.85] |
| 8 Readmissions Show forest plot | 7 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.69, 2.87] |
| 9 Costs Show forest plot | 4 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.84, ‐0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 6 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.17] |
| 1.1 Oesophagectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Gastrectomy | 3 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.17] |
| 1.3 Liver surgery | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (proportion) Show forest plot | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [0.68, 45.89] |
| 2.1 Oesophagectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gastrectomy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.87] |
| 2.3 Liver surgery | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.39 [0.44, 161.01] |
| 3 Serious adverse events (number) Show forest plot | 6 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.53, 1.43] | |
| 3.1 Oesophagectomy | 1 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.01, 8.12] | |
| 3.2 Gastrectomy | 2 | Rate Ratio (Fixed, 95% CI) | 1.10 [0.35, 3.49] | |
| 3.3 Liver surgery | 3 | Rate Ratio (Fixed, 95% CI) | 0.84 [0.48, 1.48] | |
| 4 Health‐related quality of life Show forest plot | 4 | 373 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| 4.1 Oesophagectomy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gastrectomy | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.32] |
| 4.3 Liver surgery | 2 | 251 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.27, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life Show forest plot | 3 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.19, 0.53] |
| 2 Length of hospital stay Show forest plot | 3 | 267 | Mean Difference (IV, Fixed, 95% CI) | ‐2.31 [‐2.93, ‐1.69] |
| 3 Costs Show forest plot | 3 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.81, ‐0.39] |

