合成代谢类固醇治疗压力性溃疡
摘要
研究背景
压力性溃疡,也称为褥疮、压疮,是由于皮肤或下层组织或两者的局部损伤而形成的。溃疡通常发生在骨突处,并且被认为是影响长期卧床或坐轮椅人群的常见医学问题。合成代谢类固醇被用作标签外药物(未经监管批准使用的药物),并已被用作含有敷料、清创术、营养补充剂、全身性抗生素和防腐剂等被认为有助于压力性溃疡愈合的常规治疗的辅助药物。因为它们能够刺激蛋白质合成和增加肌肉量。需要综合证据来促进关于合成代谢类固醇获益和伤害的决策制定。
研究目的
本系统综述旨在评价合成代谢类固醇治疗压力性溃疡的效果。
检索策略
2017年3月,我们检索了Cochrane创伤组专业注册库(Cochrane Wound Specialised Register)、Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)、Ovid MEDLINE(包括进程中和未收录索引的引文)、Ovid Embase和EBSCO CINAHL Plus。我们还在临床试验注册库检索了正在进行和未发表的研究,并检索了相关纳入研究和系统综述的参考文献列表、meta分析和卫生技术报告以获取其他相关研究。未限制语言、出版日期及研究实施场所。
纳入排除标准
已发表或未发表的比较合成代谢类固醇与替代疗法或不同类型的合成代谢类固醇治疗压疮效果的随机对照试验(randomised controlled trials,RCTs)。
资料收集与分析
两名综述作者独立筛选研究、提取资料和评价偏倚风险。
主要结果
本系统综述仅纳入一项共涉及212名受试者的试验,所有受试者均有脊髓损伤和开放性压力性溃疡,为III期和IV期。在氧雄龙组中受试者主要是男性(98.2%, 106/108),平均年龄为58.4(标准偏差为10.4)岁,在安慰剂组中所有受试者均为男性(100%, 104/104),平均年龄为57.3(标准偏差为11.6)岁。该试验将氧雄龙(20毫克/天,口服给药)与一定剂量的安慰剂(一种由98%淀粉和2%硬脂酸镁组成的非活性物质)进行了比较,并报告了溃疡完全愈合和不良事件的资料。在24周治疗期结束时氧雄龙对溃疡完全愈合的相对效果的证据质量极低(风险比(risk ratio,RR)=0.81,95%置信区间(confidence interval,CI) [0.52,1.26])(由于95%的置信区间极宽、涵盖了获益和伤害而因不精确而降级两次;由于受试者大多是男性脊髓损伤患者而因间接性而降级一次)。因此,我们不确定氧雄龙是否会改善或减少压力性溃疡的完全愈合,我们评价证据的质量为极低。
与安慰剂相比,接受氧雄龙治疗的受试者报告的非严重性不良事件风险的证据质量为低(RR=3.85,95% CI [1.12,13.26])(一次因不精确而降级;一次因间接性而降级,因为受试者大多是男性脊髓损伤患者)。因此,氧雄龙治疗可能会增加受试者中报告非严重不良事件的风险。
与安慰剂相比,接受氧雄龙治疗的受试者报告严重不良事件风险的证据质量极低(RR=0.54,95% CI [0.25,1.17])(由于95% CI极宽、涵盖了获益和伤害,因不精确而降级两次;由于受试者大多是男性脊髓损伤患者,因间接性而降级一次)。在氧雄龙治疗组报告的5起严重不良事件中,没有一件被试验小组归类为与治疗相关。我们不确定氧雄龙是否会增加或降低严重不良事件的风险,我们评价证据的质量为极低。
纳入的试验未报告次要结局,如疼痛、住院时间、伤口大小或伤口表面积的变化、不同类型感染的发生率、治疗成本和生活质量。
总体而言,本研究中的证据质量极低(因不精确和间接性而降级)。当研究作者的无效分析(中期分析)表明氧雄龙在改善溃疡愈合方面不比安慰剂有更大获益时,该试验提前终止。
作者结论
没有高质量的证据支持在治疗压疮时使用合成代谢类固醇。
为了评价合成代谢类固醇治疗压力性溃疡的效果,未来需要设计良好的、偏倚风险低的多中心试验,但在规划未来研究时需要仔细考虑当前试验及其提前终止。
PICO
简语概要
合成代谢类固醇治疗压力性溃疡
系统综述问题
我们评价了有关合成代谢类固醇(增加肌肉量的药物)治疗压力性溃疡患者效果的证据。
系统综述背景
压力性溃疡也称为褥疮、压疮。对于长期卧床或坐轮椅的人来说,压力性溃疡是一种常见的医疗问题。身体骨骼部位(如臀部、脚后跟、下背部和肘部)的皮肤缺乏运动和持续受压会导致皮肤破裂并形成溃疡。压力性溃疡风险人群包括脊髓损伤人群、老年人和长期患病人群。压力性溃疡会影响生活质量,如果不愈合,可能会出现感染等严重并发症。除了给患者带来痛苦和麻烦之外,由于治疗涉及照护时间,压力性溃疡对医疗保健体系来说也是一笔巨大的成本。有多种常见治疗压力性溃疡的方法,包括伤口敷料和专门设计的、以减轻身体某些部位压力的床和垫子。
合成代谢类固醇是一种用于增加肌肉量的药物。它们可以作为压力性溃疡常规治疗的替代方法或辅助疗法。它们被认为可以促进骨骼肌的生长并恢复肌肉量,以此促进压力性溃疡愈合。然而,已发现氧雄龙(一种常用的合成代谢类固醇)会导致潜在的肝损伤。合成代谢类固醇也可能增加心脏病发作或中风的风险。我们想确定合成代谢类固醇是否能有效治疗压力性溃疡,以及它们是否有任何有害影响。
研究特征
我们检索了比较合成代谢类固醇与其他压疮治疗方法的随机对照试验,截至2017年3月。我们仅发现了一项试验,共涉及212名受试者。该试验比较了合成代谢类固醇(氧雄龙胶囊)和安慰剂(不含活性药物的假性治疗)对脊髓损伤患者压力性溃疡愈合的效果。氧雄龙组受试者大多为男性(98.2%),平均年龄为58.4岁,与安慰剂组受试者相当(男性:100%;平均年龄:57.3岁)。该试验进行了超过24周,并进一步随访了8周。
主要结果
因为试验作者认为中期结果表明使用氧雄龙治疗不太可能获益,该试验提前结束。由于一项试验的可用资料有限,我们仍不确定合成代谢类固醇是否对压力性溃疡的愈合有益,治疗是否会导致严重不良事件增加,以及治疗是否会增加非严重不良事件的风险。
证据质量
总的来说,这项研究的证据质量被评为极低。需要更多、设计更好的研究来提供证据,以证明合成代谢类固醇是否有益于治疗压力性溃疡。
本简语概要更新于2017年3月。
Authors' conclusions
Summary of findings
| Anabolic steroids for treating pressure ulcers | |||||
| Patient or population: people with pressure ulcers Intervention: anabolic steroids Comparison: placebo or no anabolic steroids | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Anabolic steroids | ||||
| Proportion of wounds completely healed at 24 weeks | 298 per 1000 | 241 per 1000 | RR 0.81 (0.52 to 1.26) | 212 | ⊕⊝⊝⊝ |
| Non‐serious adverse events | 29 per 1000 | 131 per 1000 | RR 3.85 (1.12 to 13.26) | 212 | ⊕⊕⊝⊝ low2,3,4 |
| Serious adverse events | 154 per 1000 | 83 per 1000 | RR 0.54 (0.25 to 1.17) | 212 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1A wide 95% CI, which spanned both benefit and harm. | |||||
Background
Description of the condition
Pressure ulcers (also known as bed sores, pressure sores or decubitus ulcers) are a common medical problem and often develop when people are confined to bed or a wheelchair for long periods of time. A pressure ulcer is commonly defined as "a localised injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure or in combination with shear" (EPUAP/NPUAP 2009). The classification of pressure ulcers agreed by the National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel (NPUAP‐EPUAP) consists of four stages (EPUAP/NPUAP 2009): stage I ‐ non‐blanchable redness of intact skin; stage II ‐ partial thickness skin loss or blister; stage III ‐ full thickness skin loss, fat visible; and stage IV ‐ full thickness tissue loss, with muscle/bone visible (see Appendix 1 for details). Pressure ulcers are a complication associated with acute illness, injuries and immobility (Kaltenthaler 2001). They develop rapidly and are dependent upon the amount of time soft tissue is compressed against underlying bone, as well as the amount of pressure exerted on the patient's skin (Versluysen 1986). Both of these factors eventually result in ischaemia (lack of blood flow to an area) and necrosis (tissue death). It has been estimated that, at any point in time, pressure ulcers affect between 4.7% and 32.1% of hospitalised patients and between 4.6% and 20.7% of people living in nursing homes (Kaltenthaler 2001).
Although it is difficult to determine the true estimates, prevalence of pressure ulcers is an established quality indicator of health care (Gunningberg, 2012), regardless of settings. It has been estimated that in the UK alone, around 412,000 people annually are likely to develop a new pressure ulcer, which is equivalent to one in every 150 people in the general population, and one in 23 people in the population aged over 65 years (Bennett 2004). A recent study estimated the point prevalence of people with pressure ulcers across a large UK city to be 0.31 people per 1000 (95% CI 0.28 to 0.36) (Cullum 2016). In a prospective cohort study in the USA, around one‐third of people with hip‐fractures (36.1 ± 2.5%, standard error (SE)) experienced one or more new pressure ulcers in the course of 32 days following hospitalisation (Baumgarten 2009). This highlights the risk of developing pressure ulcers. A recent Norwegian study amongst 1209 inpatients reported an average of 1.6 (range 1 to 1.7) pressure ulcers per person. The overall prevalence was 18.2% for pressure ulcer categories I to IV and 7.2% for categories II to IV (Bredesen 2015). This was comparable with a Swedish study in which prevalence of pressure ulcer was 16.6% in hospitals and 14.5% in nursing homes (Gunningberg, 2012).
Two cost‐analysis studies in the UK reported that treatment costs depend upon ulcer severity, the time taken to heal and the incidence of complications (Bennett 2004; Dealy 2012). According to an estimation in 2011, costs per ulcer healed ranged from GBP 1214 (category 1, comparable to stage I) to GBP 14,108 (category 4, comparable to stage IV) (Dealy 2012). Treating pressure ulcers represents a very significant resource cost to the health and social care system in the UK and elsewhere (Bennett 2004; Dealy 2012). For instance, in 1999 to 2000, the total cost of pressure ulcer care was GBP 1.4 billion to 2.1 billion, which was roughly 4% of the total National Health Service expenditure. As the majority of these costs were attributable to nursing time, any reduction in the nursing time required to manage pressure ulcers could provide a reduction in overall cost of treatment. Moreover, patients' suffering is an intangible cost, which is not possible to measure in monetary terms. Pressure ulcers from any underlying cause in any country could impair the quality of life of the person, and the burden extends to their family, society and the nation as a whole. Additionally, it is noted that people with pressure ulcers are usually at risk of developing additional pressure ulcers (EPUAP/NPUAP 2009). The impact on the cost of patient management of the transition from having no pressure ulcers to having the first pressure ulcer is greater than the impact of any increase in the number of pressure ulcers beyond the first. This is because the first pressure ulcer triggers the need for treatment interventions, as well as the prevention of subsequent pressure ulcers (Baumgarten 2009). A study in the UK has documented that the mortality of people with and without pressure ulcers in the study hospitals was 11.1% and 3.3%, respectively (Lyder 2012). In order to reduce morbidity and mortality related to pressure ulcers, it is crucial to detect an ulcer early and thus prevent progression to stage IV ulcers (Brem 2010).
Description of the intervention
Treatments that are usually used for pressure ulcers include nutrition supplementation and hydration, pressure redistribution devices (McInnes 2011), dressings (BNF 2017), debridement (EPUAP/NPUAP 2014) and systemic antibiotics and antiseptics (Norman 2016). There are a variety of regimens, which have been promoted for use as an adjuvant therapy (that supplements 'usual/conventional' treatment) in treating pressure ulcers. Anabolic steroids are used as off‐label drugs (drugs that do not have regulatory approval for use in treating pressure ulcers) and categorised into the group of adjuvants, which are considered to be supportive in healing of pressure ulcers.
Anabolic steroids are synthetic derivatives of the hormone testosterone that have anabolic (building up) and androgenic (male) properties. The USA lists many derivatives of anabolic steroids in the Designer Anabolic Steroid Control Act 2014; however, only a few are in therapeutic use, and these include nandrolone, oxandrolone and oxymetholone, which are synthetic derivatives of testosterone. A clinically important point is that there are no separate receptors for anabolic and androgenic effects and therefore, the anabolic effect cannot be entirely separated from the androgenic effect. Amongst anabolic steroids, some are more anabolic than others, for example, oxandrolone has six times the anabolic potency of methyltestosterone (Fox 1961). Anabolic androgenic steroids are widely abused in the sports world, and among high school students (FDA 2013), because of these muscle mass effects (Franke 1997).
How the intervention might work
People with severe pressure ulcers have weight loss and lean body mass resulting from a catabolic (breaking down) state. It is highly possible that wound healing will be delayed until these catabolic processes are corrected (Collins 2004b). Anabolic effects enhance the effectiveness of protein synthesis in tissues ‐ including skeletal muscle. The anabolic effect of oxandrolone, for example, is used in people with severe burns (Hart 2001).
A clinical concern associated with oxandrolone administration is an increase in liver enzymes (Jeschke 2007). This effect might limit the use of anabolic steroids in treating pressure ulcers in those with impaired liver function such as the elderly or people with chronic illness. A prospective study on 27,892 postmenopausal women showed that hormone replacement therapy (oral oestradiol in combination with testosterone‐like progestin) may increase the risk of cholecystectomy (i.e. gallbladder removal ‐ often as a result of impaired liver function) (Nordenvall 2014).
Why it is important to do this review
Pressure ulcers are a large problem in people who are in a catabolic state. Anabolic steroids, which have anabolic effects might be useful in promoting healing of pressure ulcers.
Studies report that weight loss associated with protein depletion is directly related to poor wound healing and increased surgical risk (Windsor 1988). Clinically, the anabolic effect of steroids on the growth of skeletal muscle (and bone) is potentially beneficial for the restoration of muscle mass. This is particularly useful in people with cachexia (loss of weight and muscle wastage) attributable to chronic illnesses such as HIV/AIDS (Bhasin 2000), rheumatoid arthritis (Lemmey 2013) and in the elderly with wasting (Morley 2008).
On one hand investigators have suggested that treatment of pressure ulcers with anabolic steroids might be effective for promoting lean body mass and appendicular skeletal muscle mass (combined lean body mass from all four limbs) (Sattler 2011), healing of a wound after a major operation (Jiang 1989) or chronic cutaneous wounds (Demling 2001a; Demling 2001b), and for stage IV ulcers (Collins 2004a).
On the other hand, there have been reports on the undesirable effects of the use of anabolic steroids. For instance, testosterone, as a treatment, may produce a modest increase in prostate‐specific antigen level, but does not cause changes in the signs or symptoms of prostate hyperplasia (enlargement) (Kenny 2011). A non‐Cochrane systematic review on 27 randomised controlled trials (n = 2994) reported that testosterone administration to older men increased the risk of a cardiovascular‐related event (odds ratio 1.54, 95% CI 1.09 to 2.18) (Xu 2013). An individual study on men who underwent coronary angiography reported that the use of testosterone therapy had 29% (95% CI 4% to 58%) increased risk of adverse outcomes (Vigen 2013). The generalisability of these findings to people with pressure ulcers is not known. It is, therefore, important to undertake a comprehensive assessment of all the available randomised controlled trial data on the benefits and harms of anabolic steroids in treating pressure ulcers.
Objectives
To assess the effects of anabolic steroids for treating pressure ulcers.
Methods
Criteria for considering studies for this review
Types of studies
We considered published and unpublished randomised controlled trials (RCTs) of anabolic steroids for the treatment of pressure ulcers. RCTs of anabolic steroids for the prevention of pressure ulcers were not considered.
Types of participants
We included studies with participants having a pressure ulcer (bed sore, pressure sore or decubitus ulcer) of any grade in any healthcare setting, regardless of age and underlying clinical conditions. Pressure ulcers vary in severity ranging from stage I to stage IV as classified by the National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel (NPUAP‐EPUAP) (EPUAP/NPUAP 2009). We excluded studies in which participants with a pressure ulcer were receiving systemic corticosteroids, immunosuppressive agents, anticancer agents or any radiation therapy, because these therapies can prevent or delay wound healing, which has an impact on the effect of anabolic steroids.
Types of interventions
Eligible interventions included an anabolic steroid compared with another anabolic steroid, non‐anabolic steroid therapy, placebo or alternative treatments (with or without usual pressure ulcer management). We also considered different regimens of the same anabolic steroid and different mode(s) of anabolic steroid administration.
The term 'anabolic steroid' includes testosterone and its esters, nandrolone (as the decanoate ester), oxandrolone (also known as oxandrin), mesterolone and oxymetholone, which are used clinically. Studies were only eligible for inclusion, if the use of an anabolic steroid (or an aspect of anabolic steroid use) was the only systematic difference between treatment arms. If people in different treatment arms within a trial experienced other systematic differences in co‐interventions, the study was excluded.
Types of outcome measures
Primary outcomes
The primary outcomes for this review were complete wound healing and all reported adverse events.
Complete wound healing was measured by:
-
time to complete healing/rate of healing (as defined in the trial);
-
proportion of wounds completely healed in a specified time period (as defined in the trial).
Healing is defined as "re‐epithelialisation to a cicatrix [area of new connective tissue] with a dry surface and 0 cm square of open area for a minimum of 96 hours" (Bauman 2013).
If the studies reported data on adverse events, we extracted and categorised these as either 'non‐serious adverse events' or 'serious adverse events' as stated in the trial. If the trial had explicitly reported that participants experienced no adverse events in both arms, this statement was listed.
Secondary outcomes
-
Pain (as measured by a validated pain measurement scale)
-
Length of hospital stay
-
Change in wound size or wound surface area
-
Change in pressure ulcer volume
-
Incidence of different type of infection
-
Cost of treatment
-
Quality of life (as measured by the trial investigators or measured by a validated scale).
Search methods for identification of studies
Electronic searches
We searched the following databases to identify relevant RCTs:
-
Cochrane Wounds Specialised Register (searched 3 March 2017);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library 2017, Issue 2) (searched 3 March 2017);
-
Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and Epub Ahead of Print) (1946 to 3 March 2017);
-
Ovid Embase (1974 to 3 March 2017);
-
EBSCO CINAHL Plus (1937 to 3 March 2017).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2017). There were no restrictions based on language, date of publication or study setting.
We also searched the following clinical trials registries:
-
ClinicalTrials.gov (searched 9 March 2017);
-
WHO International Clinical Trials Registry Platform (searched 9 March 2017);
-
EU Clinical Trials Register (searched 9 March 2017).
Search strategies for clinical trials registries can be found in Appendix 2.
Searching other resources
Searching reference lists of included trials and relevant reviews
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and Health Technology Assessment reports.
Handsearching
We searched the reference lists of relevant reviews and included studies to identify further relevant trials.
Contacts
We contacted an expert in this field and the author of the included study in this review (Professor William A Bauman) to request further information about his trial and whether there were any unpublished or ongoing trials.
Conference Proceedings
We searched for conference proceedings from the European Pressure Ulcer Advisory Panel and European Wound Management Association. Our searches were carried out within the Wounds UK (WUK) database from inception to 2015.
Adverse effects
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Naing 2014), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors independently screened the titles and abstracts of citations yielded from the searches in electronic databases and retrieved full‐text copies of all potentially relevant articles. The two review authors independently checked the full articles for eligibility. Any disagreement was resolved by consensus. If the eligibility of the study or the information given in the study was unclear, we contacted study authors. We recorded the reasons for excluding studies from the full‐copies retrieved. We completed a PRISMA flow chart to summarise this process (Liberati 2009).
Data extraction and management
The two reviewers extracted information from the included studies using a piloted data extraction sheet.
We extracted the following data where possible:
-
author;
-
country;
-
publication year of study;
-
setting of study (e.g. primary care, hospital);
-
participants' characteristics;
-
intervention and comparison;
-
co‐interventions ;
-
treatment regimen (drug, dosage, route of administration, frequency, duration);
-
outcomes data for primary and secondary outcomes by groups;
-
method of outcome measurements;
-
risk of bias assessment (e.g. methods of randomisation, allocation concealment, blinding, follow‐up, dropouts);
-
duration of follow‐up;
-
adverse events;
-
pain data;
-
quality‐of‐life data;
-
length of hospital stay;
-
cost of treatment.
Outcome data were collected for relevant time points as described above in the type of outcome measures.
Where studies had duplicate publications, we extracted the maximum amount of data from the available publications. We resolved disagreements by discussion. If we deemed the studies eligible but there was no clear information, we contacted the study author to obtain additional information.
Assessment of risk of bias in included studies
Two review authors independently assessed the included study using the Cochrane tool for assessing risk of bias (Higgins 2011b). The tool addresses six specific domains, namely: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias. One other bias specific to this review was comparability of ulcer surface area between treatment groups at baseline (i.e. extreme baseline imbalance). We assessed blinding (participants, trial investigators and outcome assessors) and completeness of outcome data separately for each outcome because assessment on wound healing can be subjective. We completed a 'Risk of bias' table for each included study. We discussed any disagreement amongst all review authors to achieve a consensus.
We presented the assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgements in a cross‐tabulation of the study by entry. We classified each domain as being at high, low or unclear risk of bias (See Appendix 3 for details).
Measures of treatment effect
For dichotomous outcomes, we reported the risk ratio (RR) and its 95% confidence intervals (CI). For continuous outcomes such as mean and standard deviation (SD), we planned to use the mean difference (MD) or standardised mean difference (SMD) and their corresponding 95% CIs. We would have used SMD if trials used different scales of measurement.
For studies with time‐to‐event outcomes (e.g. time to healing), we planned to report hazard ratio (HR) and its 95% CI. If the studies identified for the present review did not report HR, we planned to compute these following the formulae of Parmar 1998, implemented in a spreadsheet (Tierney 2007).
Unit of analysis issues
We recorded whether the trial measured outcomes in relation to an ulcer or a participant, or whether multiple ulcers on the same participant were randomised. We also recorded occasions where multiple ulcers on a participant had been incorrectly treated as independent without taking into account the interdependence of the ulcers. We reported this as a part of the 'Risk of bias' assessment. Unless otherwise stated, where the number of ulcers appeared to equal the number of participants, we treated the ulcer as the unit of analysis.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell, 1992). If there were missing standard deviations (SDs) for continuous outcomes, we planned to contact the corresponding author to see if data were available. If not available, we planned to calculate these using case‐analysis such as imputing SDs from SEs, CIs, t‐values or P values (as appropriate) that related to the differences between means in two groups, following the guidance described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
When there was insufficient information available to calculate the SDs, we planned to impute them. If SDs had been available from other studies included in this review for the change from baseline for the same outcome measures, we planned to use these as the missing SDs. If this approach was not applicable, assuming correlation coefficients from the two intervention groups were similar (this is reasonable for an RCT), we planned to impute an SD of the change from baseline for the experimental intervention, following a formula as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Assessment of heterogeneity
We planned to assess studies for clinical heterogeneity based on the participants, intervention, control, outcome and study design elements, and then to tabulate this in the 'Characteristics of included studies' table. We planned to assess statistical heterogeneity using the Chi2 test and the I2 statistic (Higgins 2003). The I2 statistic examines the percentage of total variation across studies due to (statistical) heterogeneity rather than to chance (Deeks 2011). Values of I2 over 50% indicates the presence of a higher level of heterogeneity. In the absence of clinical heterogeneity and in the presence of statistical heterogeneity (where I2 was greater than 50%), we planned to choose a random‐effects model.
When there was no clinical heterogeneity or low statistical heterogeneity (I2 less than 50%), we planned to use a fixed‐effect model.
Assessment of reporting biases
If there had been a sufficient number of studies (minimum 10 RCTs), we would have assessed publication bias by creating a funnel plot. In the absence of publication bias, the plot should resemble a symmetrical inverted funnel. However, an asymmetrical funnel plot may also be due to other biases such as differences in methodological quality among studies. Even when there is a symmetrical funnel plot, it does not necessarily mean there is an absence of publication bias (Sterne 2011).
Data synthesis
We planned to combine information from included studies narratively where possible. Based on assessment of clinical and methodological heterogeneity, we planned to consider meta‐analysis of outcome data in Review Manager 5 (RevMan 5) (RevMan 2014).
'Summary of findings' tables
We presented the main results of the review in a 'Summary of findings' table. This table presents key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables:
-
complete wound healing;
-
adverse events.
Sensitivity analysis
We planned to perform a sensitivity analysis by removing studies with high and unclear risk of bias from the meta‐analyses. Therefore, the analysis would have only included studies at low risk of bias in all key domains, namely, adequate generation of the randomisation sequence, adequate allocation concealment and blinding of outcome assessor for the estimates of treatment effect.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
The search generated 34 records after duplicates were removed. Of these, we excluded 11 records after screening titles and we reviewed the full texts of 23 studies. We included only one study in this review and excluded the remaining 22 studies (Figure 1). In order to ensure ongoing studies were included in this review, we contacted an expert in this field and also checked clinical trials registries. We did not identify any ongoing studies.

Study flow diagram
Included studies
This review included only one RCT (Bauman 2013), a multicenter study at 16 Veteran Affairs (VA) medical centres providing services for inpatients with spinal cord injury (SCI) in the USA. The pressure ulcers were open wounds of stage III or IV in all participants. Conventional treatments given to these participants were cleaning with normal saline or approved wound care cleaners at each dressing change, debridement, use of dressings to keep the pressure ulcer bed continuously moist and the surrounding intact skin dry, and relief of pressure on the pressure ulcer. Neither nutritional supplements nor special diets were given. Any adjuvant therapies such as ultraviolet light, laser or hyperbaric oxygen therapy were discontinued before the treatment phase was commenced.
The trial compared orally‐given oxandrolone (20 mg/day) with matching doses of placebo capsules (an inactive substance, which consisted of starch 98% and magnesium stearate 2%). The participants were mainly male (98.2%, 106/108) with the mean age of 58.4 (standard deviation 10.4) years in the oxandrolone group. The age and sex distribution of participants in the placebo group was comparable with the intervention group (male: 100%, 104/104) and the mean age was 57.3 (standard deviation 11.6) years.
Excluded studies
We excluded 22 studies from this review. The main reasons for exclusion were:
-
duplicate data (one study: Bauman 2009);
-
not a randomised controlled trial (seven studies: Crane 2013; Demling 2001a; Demling 2001b; Generali 2013; Spungen 2001; Vennits 1966; Williams 2013);
-
reviews only (three studies: Chiu 2011; Mader 2000; Morley 2002);
-
studies were on nutritional‐related issues (six studies: Bauman 2001; Collins 2004a; Collins 2004b; Himes 1999; Mikulin 2001; Phillips 2003);
-
not on pressure ulcers (two studies: Sowers 1996; Wolf 2006);
-
editorials or guideline on published studies (three studies: Phillips 2005; Salcido 1999; Salcido 2005).
Risk of bias in included studies
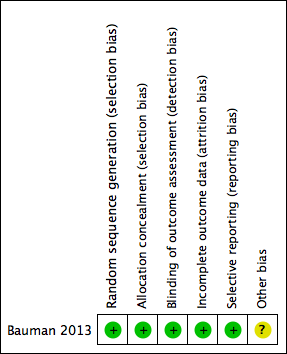
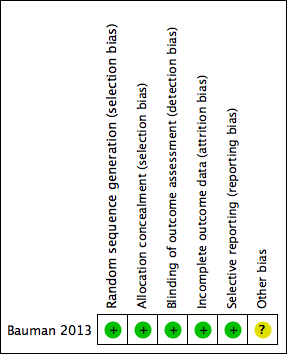
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation: Bauman 2013 had a low risk of bias, as the randomisation process used a computer‐generated randomisation sequence. We deemed the method of sequence generation adequate.
Allocation concealment: Bauman 2013 had a low risk of bias, as the participants and investigators could not foresee assignment because allocation was via an automated central telephone system. The Clinical Research Pharmacy Coordinating Center (CSP) distributed the medication or placebo through the individual pharmacy services at each participating VA medical centre.
Blinding
Blinding outcome assessment: We deemed Bauman 2013 to be at low risk of performance and detection bias because participants, clinical care providers, research study staff and statistical analyses were blinded to treatment assignment.
Incomplete outcome data
Bauman 2013 was at low risk of attrition bias as the trial report suggested that an intention‐to‐treat approach had been conducted. This was confirmed through contact with the study author (Professor William A Bauman).
Selective reporting
We deemed Bauman 2013 to be at low risk of selective reporting bias, as the outcomes reported were specified in the protocol.
Other potential sources of bias
We deemed Bauman 2013 to be at unclear risk of other potential sources of bias, as potential centre‐specific differences in rate of healing outcomes were not addressed due to limited participant numbers in each centre.
Early stopping with futility‐stopping criteria will not lead substantial bias, according to simulation evidence (Wason 2015).
The trial reported the VA institutions as its primary funding source, however, it did not mention whether the sponsor agency was involved in any processes of this study or otherwise.
Effects of interventions
See: Summary of findings for the main comparison Anabolic steroids for treating pressure ulcers
Oxandrolone compared with placebo (one study, 212 participants)
Bauman 2013 compared orally‐given oxandrolone (20 mg/day) with placebo (an inactive substance, which consisted of starch 98% and magnesium stearate 2%). In total, Bauman 2013 randomised 212 people with SCI who had pressure ulcers. We classed the study as being at low risk of selection bias, detection bias, attrition bias and reporting bias and at unclear risk of other bias. The randomised groups were similar for the baseline demographic, SCI characteristics and pressure ulcer history as well as the number of active smokers, participants with cardiovascular disease, including peripheral vascular disease and those with osteomyelitis.
Primary outcome: complete healing at week 24 of treatment
Completed ulcer healing at week 24 was measured in terms of re‐epithelialisation to a cicatrix with a dry surface and 0 cm2 of open area for a minimum of 96 hours; 26 participants in the oxandrolone group (24%; 26/108) and 31 participants in the placebo group (30%; 31/104) had a completely healed ulcer. We are uncertain whether oxandrolone increases or decreases the effect of complete healing compared with placebo (RR 0.81, 95% CI 0.52 to 1.26) (Analysis 1.1). GRADE assessment: very low‐certainty evidence, downgraded twice for imprecision (an extremely wide 95% CI, which spanned both benefit and harms) and once for indirectness (mostly male SCI participants).
Primary outcome: non‐serious adverse events and serious adverse events
Bauman 2013 reported non‐serious adverse events (Table 1) and serious adverse events (Table 2). Treatment with oxandrolone may increase the risk of non‐serious adverse events compared with placebo (11.1%; 12/108 versus 2.9%; 3/104, RR 3.85, 95% CI 1.12 to 13.26) (Analysis 1.2). The non‐serious adverse events were largely measured via biochemical or clinical assessment (e.g. measurement of liver enzyme function) and most were assessed as being possibly related to oxandrolone. GRADE assessment: low‐certainty evidence, downgraded once for imprecision (an extremely wide 95% CI in one direction) and once for indirectness (mostly male SCI participants).
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Elevated liver enzyme levels | 5 | 1 |
| Deep venous thrombosis | 3 | 0 |
| Elevated prostate specific antigen | 0 | 1 |
| Severe osteomyelitisa | 1 | 0 |
| Sepsis, secondary cellulitisa | 1 | 0 |
| Medical illnessa | 2 | 1 |
aIn the trial investigators' judgement these events were not associated with oxandrolone
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Deatha | 3 | 5 |
| Myocutaneous flap surgerya | 5 | 9 |
| Elevated bladder stone removal liver enzyme levelsa | 1 | 0 |
| Small bowel obstruction, renal failurea | 0 | 1 |
| Oral cancera | 0 | 1 |
aIn the trial investigators' judgement these events were not associated with oxandrolone
We are uncertain whether oxandrolone increases or decreases the risk of serious adverse events reported in participants (8%; 9/108 versus 15%; 16/104, RR 0.54, 95% CI 0.25 to 1.17) (Analysis 1.3). Despite serious adverse events including eight deaths (three in the oxandrolone group and five in the placebo group), the trial investigators judged that none of these serious adverse events were related to oxandrolone. GRADE assessment: very low‐certainty evidence, downgraded twice for imprecision (an extremely wide 95% CI, which spanned both benefit and harm) and indirectness (mostly male SCI participants).
Secondary outcomes
Bauman 2013 did not report any of our secondary outcomes (pain, length of hospital stay, change in wound size or wound surface area, change in pressure ulcer volume, incidence of different types of infection, cost of treatment, quality of life).
Discussion
Summary of main results
See summary of findings Table for the main comparison
We included only one trial with 212 people with SCI who had pressure ulcers in this review.
Bauman 2013 compared an anabolic steroid (oxandrolone) with placebo. The distribution of age and sex of participants was comparable in the two groups. We are uncertain whether oxandrolone is better than placebo in promoting complete healing of pressure ulcers at 24 weeks of treatment. We classed the evidence as very low certainty. The trial reported that oxandrolone may increase the risk of non‐serious adverse events by approximately four times compared with placebo. We classed this evidence as low certainty.
In summary, there is uncertain evidence on the relative effectiveness of anabolic steroids (oxandrolone) for healing pressure ulcers. We downgraded the evidence because of imprecision, with wide 95% CIs and indirectness with imbalance of male/female participants and the single trial that included those with SCI. It is very likely that further research will have an important impact on our confidence in the estimate of effect, and is likely to change the estimate. Treatment with oxandrolone may increase the risk of non‐serious adverse events: this is low‐certainty evidence, which is also very likely to be impacted by further research.
Overall completeness and applicability of evidence
The participants in the included trial were hospitalised patients with SCI. They were not likely to be representative of people seen in clinical practice with pressure ulcers as there might be elderly people with chronic illnesses other than SCI, or female SCI patients with pressure ulcers.
We identified only one trial (Bauman 2013) for inclusion in this review. Although the search strategy was comprehensive, the effect of publication bias cannot be ruled out. This trial reported uncertain treatment benefits and suggested that more non‐serious adverse events may occur in those receiving anabolic steroids. The included study was terminated after a futility analysis which was reported by the study authors to show there to be a low probability of detecting a significant difference between the groups with continued recruitment. The study authors planned to recruit 400 participants and the trial was terminated after 164 participants were recruited. The authors reported that the futility analysis indicated that the amount of healing would have had to increase in the oxandrolone group to greater than 50% (from the current figure of 25%) and decrease in the placebo group from 31% to 20% to attain a significant effect for oxandrolone administration (conditional probability of occurrence; P = 0.001) (Bauman 2013). It may be the case that the inclusion of unpublished data would add further useful information to this body of evidence. Moreover, withdrawal rates were relatively high and similar between the two groups; hence, it is likely that the people with SCI themselves were prone to develop complications that were independent of the study drugs.
Quality of the evidence
We included only one trial (Bauman 2013), which recruited 164 participants and then was terminated based on a futility analysis. Overall, we are uncertain whether oxandrolone improves complete healing and impacts on serious adverse events, as the certainty of the evidence has been assessed as very low. We used the GRADE approach to assess evidence quality. We downgraded all outcomes for indirectness and imprecision of outcomes. RCTs need to be adequately powered to detect meaningful differences, if they exist, between the experimental drug and placebo or alternatives. Although we noted in this case the futility analysis suggested that further recruitment was unlikely to change the findings.
Potential biases in the review process
We performed a thorough search of the literature and we were able to identify only one trial. Our search was not limited by language nor publication status. We also contacted the author of the trial that we included in this review (Professor William A. Bauman) for clarification of study design, and any unpublished or on‐going studies in this field. The trial author provided comprehensive information that (i) this trial was intention‐to‐treat analysis; (ii) the Data Safety Monitoring Committee performed a futility analysis and it showed the study was not going to yield a positive result to the primary objective, and thus the trial was stopped early; (iii) both participants and physicians were blinded to drug/placebo treatment and (iv) the study team investigated another study on chronic pressure ulcer in people with SCI, which was a local study and did not assess the efficacy of an anabolic agent. It is possible that there might be studies that were conducted in earlier years but were not reported before the date of the establishment of mandatory registration for clinical trials.
Agreements and disagreements with other studies or reviews
This is the first systematic review focused solely on anabolic steroid‐based products for treatment of pressure ulcers. A case study on nine people with pressure ulcers showed complete healing in 89% of cases at three to 12 months after administration of 20 mg oxandrolone (Spungen 2001). Because this was a small sample study with no rigorous methodology and no control group, it provided limited evidence. We were not able to identify any recent systematic reviews focusing on participants with pressure ulcers that evaluated anabolic steroid‐based products.
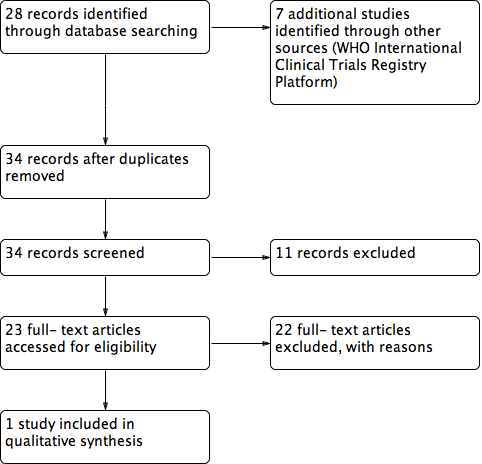
Study flow diagram
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
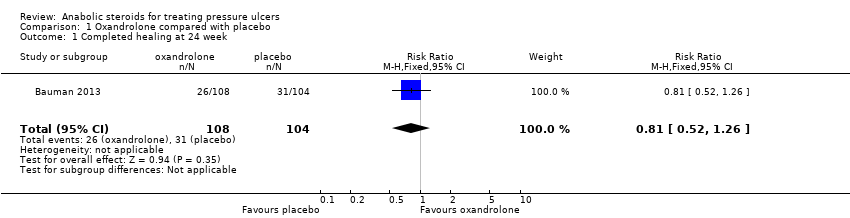
Comparison 1 Oxandrolone compared with placebo, Outcome 1 Completed healing at 24 week.

Comparison 1 Oxandrolone compared with placebo, Outcome 2 Non‐serious adverse events.
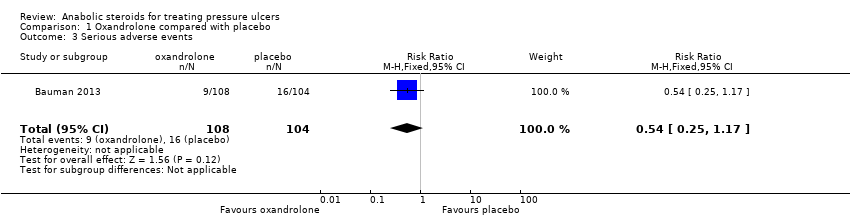
Comparison 1 Oxandrolone compared with placebo, Outcome 3 Serious adverse events.
| Anabolic steroids for treating pressure ulcers | |||||
| Patient or population: people with pressure ulcers Intervention: anabolic steroids Comparison: placebo or no anabolic steroids | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Anabolic steroids | ||||
| Proportion of wounds completely healed at 24 weeks | 298 per 1000 | 241 per 1000 | RR 0.81 (0.52 to 1.26) | 212 | ⊕⊝⊝⊝ |
| Non‐serious adverse events | 29 per 1000 | 131 per 1000 | RR 3.85 (1.12 to 13.26) | 212 | ⊕⊕⊝⊝ low2,3,4 |
| Serious adverse events | 154 per 1000 | 83 per 1000 | RR 0.54 (0.25 to 1.17) | 212 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1A wide 95% CI, which spanned both benefit and harm. | |||||
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Elevated liver enzyme levels | 5 | 1 |
| Deep venous thrombosis | 3 | 0 |
| Elevated prostate specific antigen | 0 | 1 |
| Severe osteomyelitisa | 1 | 0 |
| Sepsis, secondary cellulitisa | 1 | 0 |
| Medical illnessa | 2 | 1 |
| aIn the trial investigators' judgement these events were not associated with oxandrolone | ||
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Deatha | 3 | 5 |
| Myocutaneous flap surgerya | 5 | 9 |
| Elevated bladder stone removal liver enzyme levelsa | 1 | 0 |
| Small bowel obstruction, renal failurea | 0 | 1 |
| Oral cancera | 0 | 1 |
| aIn the trial investigators' judgement these events were not associated with oxandrolone | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completed healing at 24 week Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.26] |
| 2 Non‐serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [1.12, 13.26] |
| 3 Serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.17] |

