合成代谢类固醇治疗压力性溃疡
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT Parallel‐group (1:1), placebo‐controlled A separate screening and treatment phase without a crossover Undertaken in 16 Veteran Affairs (VA) medical centres providing services for inpatients with spinal cord injury (SCI) in the USA | |
| Participants | 212 participants completed at least 4 weeks of treatment. Oxandrolone: 108; placebo: 104 Inpatients with SCI and stage III or IV pressure ulcers Inclusion criteria: inpatients who were ≥ 18 years old with SCI or equivalent spinal cord damage and at least 1 stage III or IV pressure ulcer of the ischial, trochanteric, perineal, and sacral regions of the pelvis and provided written informed consent for the treatment and follow‐up phases Exclusion criteria (screening phase) Patients for
Patients with:
Exclusion criteria (treatment phase) Patients having:
Patients who were:
| |
| Interventions | Group A (treatment group): orally‐given oxandrolone (10 mg, twice daily with morning and evening meals Participants remained in the treatment phase until full healing of the ulcer Targeted pressure ulcer healing at 24 weeks. Group B (control group): placebo capsules 1500 mg (294 mg starch: 6 mg magnesium stearate, 98% and 2%) In screening phase: participants were enrolled in a 28 ± 2 day observation period to identify hard‐to‐heal pressure ulcers (i.e. wound healed ≤ 30% during the 1‐month screening phase). Non‐drug adjunctive therapies were allowed In treatment phase: the targeted pressure ulcers were examined and measured weekly. Participants remained until full healing of the targeted pressure ulcer or 24 weeks. Any adjunctive therapies were not allowed. If targeted pressure ulcer healed in treatment phase, participants were followed up at 4 and 8 weeks after termination of the treatment phase to determine whether the targeted pressure ulcer remained closed. | |
| Outcomes | Primary outcome The percentage of participants who had complete healing of the targeted pressure ulcer by 24 weeks (defined as re‐epithelialisation to a cicatrix with a dry surface and 0 cm2 of open area for a minimum of 96 hours) Secondary outcome VA Nutrition Status Classification (NSC) score at 12 and 24 weeks | |
| Notes | Study period was between 1 August 2005 and 30 November 2008 Trial was reviewed when complete data for 164 participants were obtained. Based on the findings of a futility analysis, this study was terminated. The original sample size was 400. Trial stopped 2 months later and 7 participants (3 Oxandrone and 4 placebo) completed only 4 weeks instead of 24 weeks Source of funding: The Veterans Affairs Clinical Science Research and Development Service, Cooperative Study #535; Rehabilitation Research and Development, National Center of Excellence for the Medical Consequences of Spinal Cord Injury (B2648C, B4162C, and B9212C); and Department of Veterans Affairs, Spinal Cord Injury Services. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation process used a computer‐generated randomisation sequence. Comments: method of sequence generation deemed adequate |
| Allocation concealment (selection bias) | Low risk | Comments: allocation concealment deemed appropriate as the participants and investigators could not foresee assignment because an automated central telephone system was used. The Clinical Research Pharmacy Co‐ordinating Center (CSP) distributed the medication or placebo through the individual pharmacy services at each participating VA medical centre. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients, clinical care providers, research study staff and statistical analyst were blinded to treatment assigns" Comments: blinding of outcome assessment deemed adequate |
| Incomplete outcome data (attrition bias) | Low risk | 40% (43/108) of the participants in group A and 36% (37/104) in group B did not complete the treatment. Details were listed. All 212 were included in the current data analysis according to the intention‐to‐treat principle. Comments: an intention‐to‐treat approach was followed; this was confirmed the trial investigator |
| Selective reporting (reporting bias) | Low risk | Many outcomes listed in methods were reported |
| Other bias | Unclear risk | Potential centre‐specific differences in targeted pressure ulcer healing by 24 weeks or in targeted pressure ulcer healing ≥ 30% at 4 weeks were not explored due to limited participant numbers at each centre. Comment: site‐specific healing not addressed |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| A study on anabolic hormones on body composition changes in spinal cord injury | |
| Duplicate data | |
| A review | |
| Nutrition assessment on people with pressure ulcers | |
| Nutrition assessment on people with pressure ulcers | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Review on the role of protein in anabolic action in wound healing | |
| A treatment guideline | |
| Assessed nutrition on wound healing | |
| A review | |
| Not an RCT; study on nutritional assessment to determine the need of using anabolic agents in wound healing | |
| Not RCT; guideline information of oxandrolone and nutritional assessment | |
| An editorial on anabolic steroids of published studies | |
| An editorial on anabolic steroids of published studies | |
| Not an RCT and assessed people with osteoarthritis | |
| A single arm trial of oxandrolone with no comparator drug | |
| Not an RCT, although double‐blind assessment was done | |
| Not an RCT; an awareness initiative programme for the risks and preventive strategies of pressure ulcers | |
| RCT of oxandrolone on people with severe burns |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completed healing at 24 week Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.26] |
| Analysis 1.1 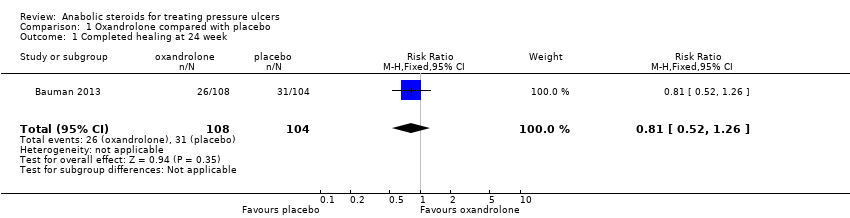 Comparison 1 Oxandrolone compared with placebo, Outcome 1 Completed healing at 24 week. | ||||
| 2 Non‐serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [1.12, 13.26] |
| Analysis 1.2  Comparison 1 Oxandrolone compared with placebo, Outcome 2 Non‐serious adverse events. | ||||
| 3 Serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.17] |
| Analysis 1.3 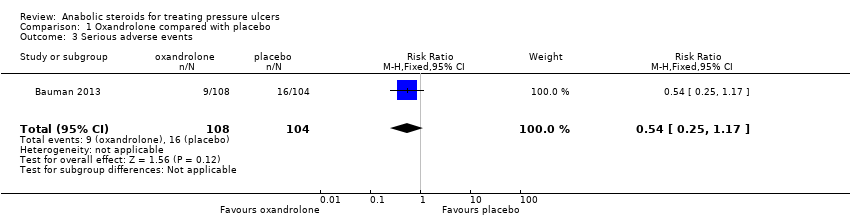 Comparison 1 Oxandrolone compared with placebo, Outcome 3 Serious adverse events. | ||||
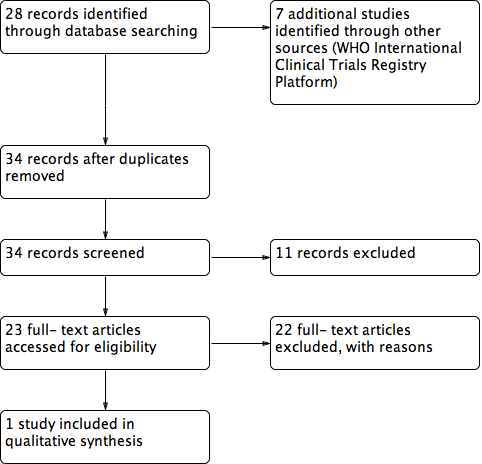
Study flow diagram
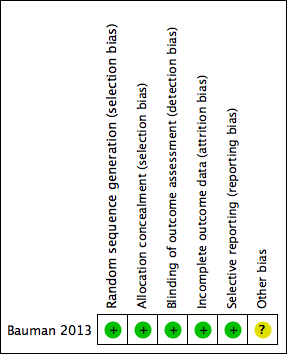
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
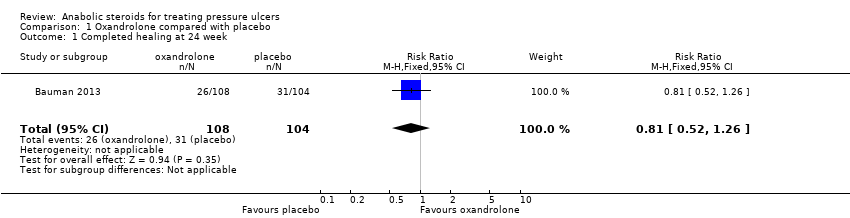
Comparison 1 Oxandrolone compared with placebo, Outcome 1 Completed healing at 24 week.

Comparison 1 Oxandrolone compared with placebo, Outcome 2 Non‐serious adverse events.
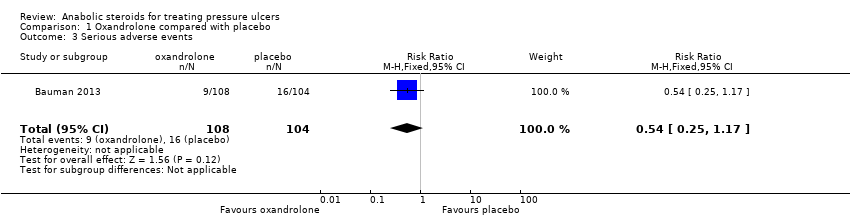
Comparison 1 Oxandrolone compared with placebo, Outcome 3 Serious adverse events.
| Anabolic steroids for treating pressure ulcers | |||||
| Patient or population: people with pressure ulcers Intervention: anabolic steroids Comparison: placebo or no anabolic steroids | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Anabolic steroids | ||||
| Proportion of wounds completely healed at 24 weeks | 298 per 1000 | 241 per 1000 | RR 0.81 (0.52 to 1.26) | 212 | ⊕⊝⊝⊝ |
| Non‐serious adverse events | 29 per 1000 | 131 per 1000 | RR 3.85 (1.12 to 13.26) | 212 | ⊕⊕⊝⊝ low2,3,4 |
| Serious adverse events | 154 per 1000 | 83 per 1000 | RR 0.54 (0.25 to 1.17) | 212 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1A wide 95% CI, which spanned both benefit and harm. | |||||
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Elevated liver enzyme levels | 5 | 1 |
| Deep venous thrombosis | 3 | 0 |
| Elevated prostate specific antigen | 0 | 1 |
| Severe osteomyelitisa | 1 | 0 |
| Sepsis, secondary cellulitisa | 1 | 0 |
| Medical illnessa | 2 | 1 |
| aIn the trial investigators' judgement these events were not associated with oxandrolone | ||
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Deatha | 3 | 5 |
| Myocutaneous flap surgerya | 5 | 9 |
| Elevated bladder stone removal liver enzyme levelsa | 1 | 0 |
| Small bowel obstruction, renal failurea | 0 | 1 |
| Oral cancera | 0 | 1 |
| aIn the trial investigators' judgement these events were not associated with oxandrolone | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completed healing at 24 week Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.26] |
| 2 Non‐serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [1.12, 13.26] |
| 3 Serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.17] |

