Антибиотики при бессимптомной бактериурии у реципиентов почечного трансплантата
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "According to the order of patients' transplant code, they were divided into two groups of case and control, in every other one manner" Comment: high‐risk of selection bias is associated with quasi‐RCTs |
| Allocation concealment (selection bias) | High risk | Quote: "According to the order of patients' transplant code, they were divided into two groups of case and control, in every other one manner" Comment: high‐risk of selection bias is associated with quasi‐RCTs |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "In case group, a 10‐day oral antibiotic therapy was administered (...). The patients in control group were left untreated" Comment: as symptoms of UTIs are in part subjective, the absence of blinding may impact the number of symptomatic infections observed |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "In case group, a 10‐day oral antibiotic therapy was administered (...). The patients in control group were left untreated" Comment: as symptoms of urinary tract infections are in part subjective, the absence of blinding may impact the number of symptomatic infections observed |
| Incomplete outcome data (attrition bias) | High risk | Quote: "The patients with lost follow‐up visits, acute rejection, and pyelonephritis leading to hospitalization during the study were excluded. (...) Twelve patients were excluded of the study, 11 because of lost follow‐up visits and 1 because of acute pyelonephritis, and eventually, data from 88 patients were analyzed" Comment: Twelve enrolled patients (12%) were excluded from the analysis (11 subjects lost to follow‐up and 1 patient due to occurrence of acute graft pyelonephritis). A high‐risk of attrition bias was suspected. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol. Authors did not divided outcomes into primary and secondary outcomes. Some expected outcomes such as incidences of antimicrobial resistance, pyelonephritis, graft rejection and graft loss, all‐cause mortality, incidence of hospitalisation for UTI and incidence of adverse reactions to antimicrobial agents were not reported |
| Other bias | High risk | Comment: Moradi 2005 did not provide a specific definition of the term "symptomatic UTI" and there were no details on the episodes of symptomatic UTI. Attempts to contact the corresponding author were unsuccessful. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomised (1:1 ratio) using a predetermined computer‐generated sequence and consecutively numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were randomised (1:1 ratio) using a predetermined computer‐generated sequence and consecutively numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label, parallel‐group, randomised trial" Comment: as symptoms of UTIs are in part subjective, the absence of blinding may impact the number of symptomatic infections observed |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "open‐label, parallel‐group, randomised trial" Comment: as symptoms of UTIs are in part subjective, the absence of blinding may impact the number of symptomatic infections observed |
| Incomplete outcome data (attrition bias) | High risk | Quote: "the 12‐ and 24‐month follow‐up periods were completed in 98 (86.6%) and 61 patients (54.4%), respectively" Comment: all the 112 participants were included into the intention‐to‐treat analysis. However, little more than half reached the end of the two year study period. Participants were withdrawn after they developed acute pyelonephritis, which could have biased results for the other outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: all the expected outcomes were reported. |
| Other bias | Low risk | Quote: 'this work was partially supported by the Fundación Mutua Madrileña de Investigación Médica (FMM Grant 2010/0015), by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias [FIS] 12/02269 and Proyecto Integrado de Excelencia [PIE] 13/00045), and by the European Development Regional Fund (EDRF) "A way to achieve Europe". J.O. holds a research‐training contract "Rio Hortega" (CM13/00180) from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III). M.F.R. holds a clinical research contract “Juan Rodés” (JR14/00036) from the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III" Comment: a low‐risk of sponsorship bias is expected due to the nature of the study. |
IDSA ‐ Infectious Diseases Society of America; MDRD ‐ Modification of Diet in Renal Disease; M/F ‐ male/female; RCT ‐ randomised controlled trial; SD ‐ standard deviation; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong population: evaluated the role of perioperative antibiotic prophylaxis | |
| Wrong population: evaluated the role of perioperative antibiotic prophylaxis | |
| Wrong population: evaluated the role of perioperative antibiotic prophylaxis | |
| Wrong population: evaluated the effect of long‐term prophylaxis with cotrimoxazole following kidney transplantation | |
| Wrong population: evaluated the risk of leucopenia associated with the use of cotrimoxazole in kidney transplant recipients having UTI | |
| Wrong population: compared two different regimen of long‐term antibiotic prophylaxis following kidney transplantation | |
| Wrong population: compared various doses of prophylaxis with cotrimoxazole following kidney transplantation | |
| Wrong population: evaluated the effect of antibiotics prophylaxis following kidney transplantation | |
| Wrong population: evaluated the effect of perioperative antibiotic prophylaxis | |
| Wrong population: evaluated the effect of a 10‐days antimicrobial prophylaxis following kidney transplantation | |
| Wrong population: evaluated the effect of long‐term prophylaxis with ciprofloxacin following kidney transplantation | |
| Wrong population: compares two regimen of long‐term prophylaxis following kidney transplantation | |
| Wrong population: evaluated the role of perioperative antibiotic prophylaxis | |
| Wrong population: evaluated the effect of intravesical administration of antibiotics at the time of transplantation | |
| Wrong population: evaluated the effect of intravesical administration of antibiotics just before transplantation | |
| Wrong population: evaluated different regimen of postoperative short‐term antibiotics prophylaxis | |
| Wrong population: evaluated the effect of long‐term prophylaxis with cotrimoxazole following kidney transplantation | |
| Wrong population: evaluated the role of perioperative antibiotic prophylaxis | |
| Wrong population: evaluated the effect of antibiotic prophylaxis in kidney transplant recipients |
UTI ‐ urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Bacteriuria in Renal Transplantation (BiRT) study: a prospective, randomised, parallel‐group, multicenter, open‐label, superiority trial comparing antibiotics versus no treatment in the prevention of symptomatic urinary tract infection in kidney transplant recipients with asymptomatic bacteriuria |
| Methods |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control arm
|
| Outcomes | Primary outcome
Secondary outcomes (to be evaluated during the 12 months of follow‐up)
Specific strategies to obtain good quality urine samples
|
| Starting date | April 2014 |
| Contact information | Julien Coussement, MD (co‐ordinating investigator) Dept. of Nephrology, Dialysis and Kidney Transplantation, Hôpital Erasme – Université Libre de Bruxelles Route de Lennik, 808, 1070 Brussels, Belgium. Phone: +32.2.555.30.49 / Fax: + 32.2.555.64.99 / E‐mail: [email protected] |
| Notes | Protocol published by The Lancet (reference: 14PRT/5447): http://www.thelancet.com/protocol‐reviews/14PRT‐5447 |
| Trial name or title | Antibiotic treatment versus no therapy in kidney transplant recipients with asymptomatic bacteriuria. A prospective randomised study (BAC01) |
| Methods |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment arm
Control arm
|
| Outcomes | Primary outcome
Secondary outcomes
Specific strategies to obtain good quality urine samples: not specified |
| Starting date | January 2013 |
| Contact information | Núria Sabé Fernàndez Hospital Universitari de Bellvitge L'Hospitalet de Llobregat, Barcelona, Spain, 08907 Phone number: +34932607625 E‐mail: [email protected] |
| Notes | Estimated study completion date: December 2015 |
| Trial name or title | The impact of antimicrobial treatment for asymptomatic bacteriuria in renal transplant patients |
| Methods |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcome
Secondary outcomes
Specific strategies to obtain good quality urine samples: not specified |
| Starting date | April 2014 |
| Contact information | Ruth Rahamimov Head of Transplant investigator service Rabin Medical centre, Israel |
| Notes |
CFU ‐ colony forming units; ESKD ‐ end‐stage kidney disease; eGFR ‐ estimated glomerular filtration rate; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; UTI ‐ urinary tract infection; WBC ‐ white blood cells
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic urinary tract infection Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.51, 1.45] |
| Analysis 1.1 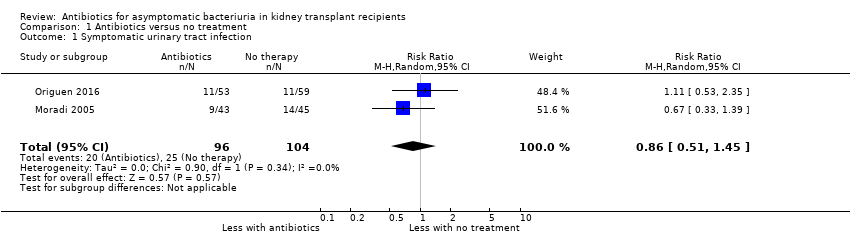 Comparison 1 Antibiotics versus no treatment, Outcome 1 Symptomatic urinary tract infection. | ||||
| 2 Antimicrobial resistance Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2 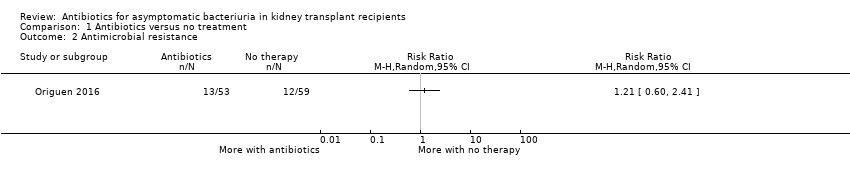 Comparison 1 Antibiotics versus no treatment, Outcome 2 Antimicrobial resistance. | ||||
| 3 Secondary dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Antibiotics versus no treatment, Outcome 3 Secondary dichotomous outcomes. | ||||
| 3.1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Graft loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Acute rejection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Hospitalisation for UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Graft function (creatinine at end of study) Show forest plot | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.18] |
| Analysis 1.4 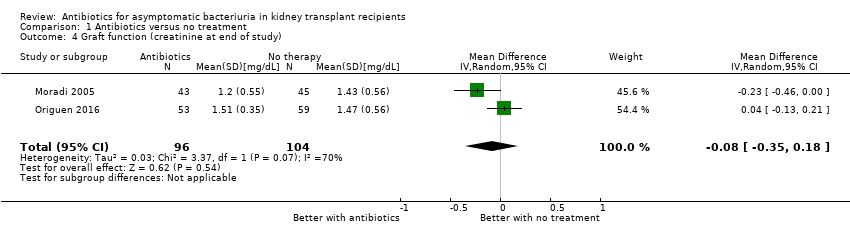 Comparison 1 Antibiotics versus no treatment, Outcome 4 Graft function (creatinine at end of study). | ||||
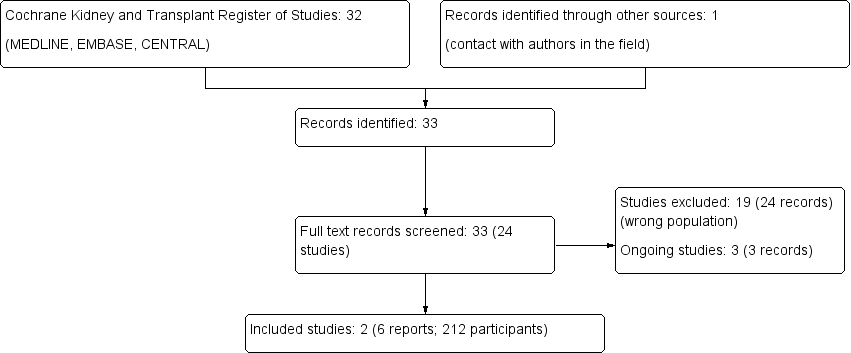
Study flow diagram.
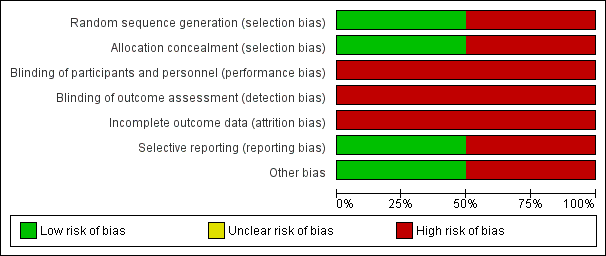
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics versus no treatment, Outcome 1 Symptomatic urinary tract infection.
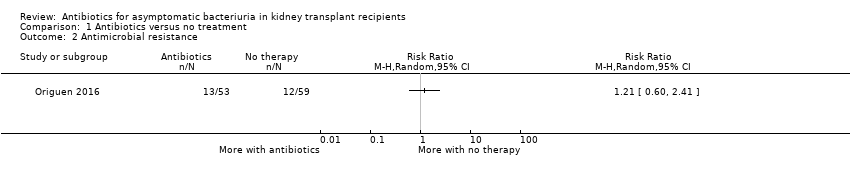
Comparison 1 Antibiotics versus no treatment, Outcome 2 Antimicrobial resistance.
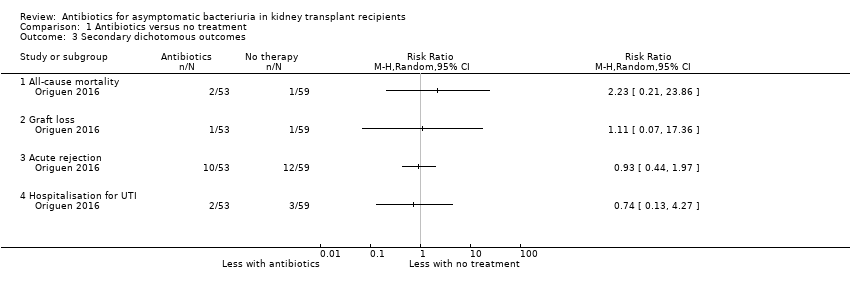
Comparison 1 Antibiotics versus no treatment, Outcome 3 Secondary dichotomous outcomes.

Comparison 1 Antibiotics versus no treatment, Outcome 4 Graft function (creatinine at end of study).
| Antibiotics versus no treatment for asymptomatic bacteriuria in kidney transplant recipients | |||||
| Patient or population: adult kidney transplant recipients | |||||
| Outcomes (follow‐up period) | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no treatment | Risk with antibiotics | ||||
| Symptomatic UTI Follow‐up: 12 to 22 months | 240 per 1,000 | 207 per 1 000 | RR 0.86 (0.51 to 1.45) | 200 2 (2 studies) | Low 3 ⊕⊕⊝⊝ |
| Antimicrobial resistance Mean follow‐up: 16.9 months | 203 per 1,000 | 245 per 1,000 | RR 1.21 (0.60 to 2.41) | 112 (1 study) | Low 4 ⊕⊕⊝⊝ |
| All‐cause mortality Mean follow‐up: 16.9 months | 17 per 1,000 | 38 per 1,000 | RR 2.23 (0.21, 23.86) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft loss Mean follow‐up: 16.9 months | 17 per 1,000 | 19 per 1,000 | RR 1.11 (0.07 to 17.36) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Acute graft rejection Mean follow‐up: 16.9 months | 203 per 1,000 | 189 per 1,000 | RR 0.93 (0.44 to 1.97) | 112 (1 study) | Low 6 ⊕⊕⊝⊝ |
| Hospitalisation for UTI Mean follow‐up: 16.9 months | 51 per 1,000 | 38 per 1,000 | RR 0.74 (0.13 to 4.27) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft function (creatinine at end of study) Follow‐up: 12 to 22 months | Mean serum creatinine in the treatment group was 0.06 mg/dL lower (0.19 mg/dL lower to 0.08 mg/dL higher) than the control group | 200 2 (2 studies) | Low 7, 8 ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; UTI: urinary tract infection 1 The two included studies compared antibiotics versus no treatment, with choice of antibiotics depending on antimicrobial susceptibility testing results. As participants could have had multiple episodes of asymptomatic bacteriuria during the follow‐up period, participants from the intervention group were retreated with antibiotics if asymptomatic bacteriuria recurred during the follow‐up period in both studies. Duration of antibiotics therapy ranged from 3 to 10 days for the first episode of asymptomatic bacteriuria. 2 212 participants included but data provided for 200 participants. 3 Neither study attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. 4 Samples could be collected both in case of symptoms of UTI or as part of routine screening. 5 The confidence interval crosses the line of no effect but does not rule out a significant effect of antibiotics on mortality and/or graft loss. 6 No systematic graft biopsy performed during the study follow‐up. Not all episodes of allograft rejection were biopsy‐proven. 7 Graft function was evaluated using creatinine at end of study, despite different values between groups at time of inclusion. We were unable to pool the data for change in graft function from baseline to end of study (data missing for one study). 8 No significant effect of antibiotics on change in graft function from baseline to end of study in both studies. | |||||
| GRADE Working Group grades of evidence | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic urinary tract infection Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.51, 1.45] |
| 2 Antimicrobial resistance Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Graft loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Acute rejection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Hospitalisation for UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Graft function (creatinine at end of study) Show forest plot | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.18] |

