Антибиотики при бессимптомной бактериурии у реципиентов почечного трансплантата
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
| Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
| Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
| Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
| Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. | Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
| Selective reporting Reporting bias due to selective outcome reporting | Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
| Other bias Bias due to problems not covered elsewhere in the table | Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
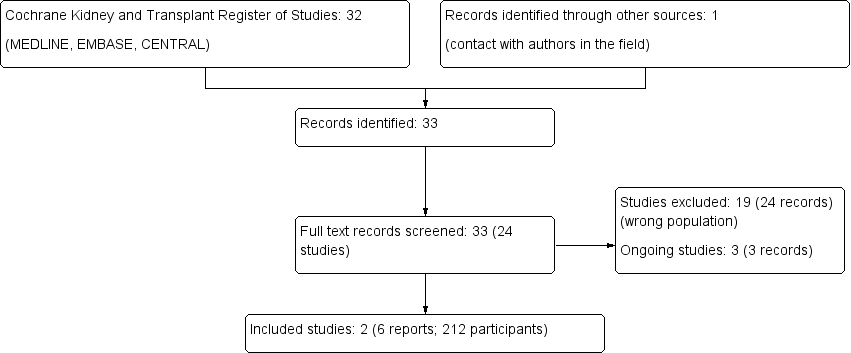
Study flow diagram.
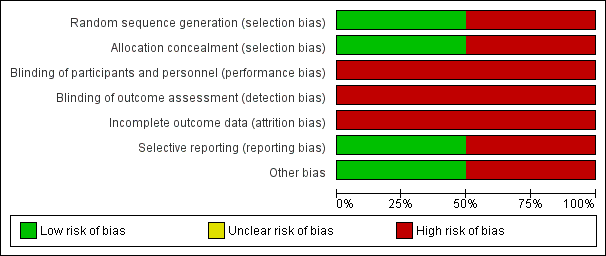
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics versus no treatment, Outcome 1 Symptomatic urinary tract infection.
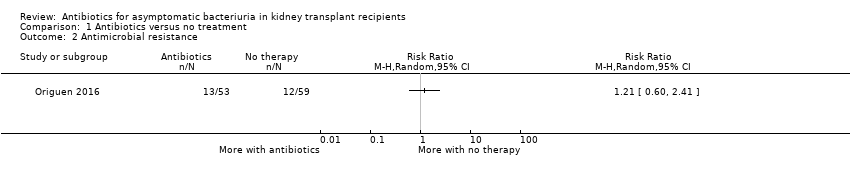
Comparison 1 Antibiotics versus no treatment, Outcome 2 Antimicrobial resistance.
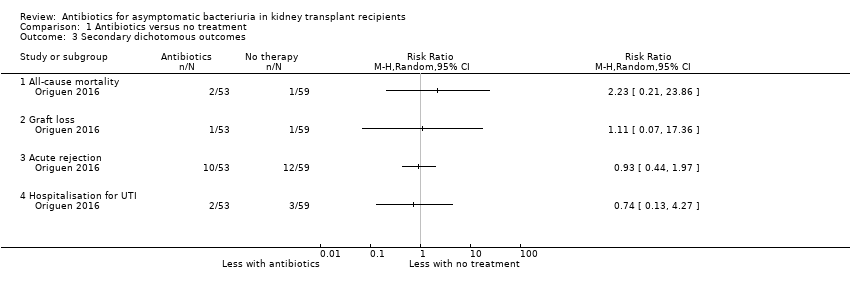
Comparison 1 Antibiotics versus no treatment, Outcome 3 Secondary dichotomous outcomes.

Comparison 1 Antibiotics versus no treatment, Outcome 4 Graft function (creatinine at end of study).
| Antibiotics versus no treatment for asymptomatic bacteriuria in kidney transplant recipients | |||||
| Patient or population: adult kidney transplant recipients | |||||
| Outcomes (follow‐up period) | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no treatment | Risk with antibiotics | ||||
| Symptomatic UTI Follow‐up: 12 to 22 months | 240 per 1,000 | 207 per 1 000 | RR 0.86 (0.51 to 1.45) | 200 2 (2 studies) | Low 3 ⊕⊕⊝⊝ |
| Antimicrobial resistance Mean follow‐up: 16.9 months | 203 per 1,000 | 245 per 1,000 | RR 1.21 (0.60 to 2.41) | 112 (1 study) | Low 4 ⊕⊕⊝⊝ |
| All‐cause mortality Mean follow‐up: 16.9 months | 17 per 1,000 | 38 per 1,000 | RR 2.23 (0.21, 23.86) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft loss Mean follow‐up: 16.9 months | 17 per 1,000 | 19 per 1,000 | RR 1.11 (0.07 to 17.36) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Acute graft rejection Mean follow‐up: 16.9 months | 203 per 1,000 | 189 per 1,000 | RR 0.93 (0.44 to 1.97) | 112 (1 study) | Low 6 ⊕⊕⊝⊝ |
| Hospitalisation for UTI Mean follow‐up: 16.9 months | 51 per 1,000 | 38 per 1,000 | RR 0.74 (0.13 to 4.27) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft function (creatinine at end of study) Follow‐up: 12 to 22 months | Mean serum creatinine in the treatment group was 0.06 mg/dL lower (0.19 mg/dL lower to 0.08 mg/dL higher) than the control group | 200 2 (2 studies) | Low 7, 8 ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; UTI: urinary tract infection 1 The two included studies compared antibiotics versus no treatment, with choice of antibiotics depending on antimicrobial susceptibility testing results. As participants could have had multiple episodes of asymptomatic bacteriuria during the follow‐up period, participants from the intervention group were retreated with antibiotics if asymptomatic bacteriuria recurred during the follow‐up period in both studies. Duration of antibiotics therapy ranged from 3 to 10 days for the first episode of asymptomatic bacteriuria. 2 212 participants included but data provided for 200 participants. 3 Neither study attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. 4 Samples could be collected both in case of symptoms of UTI or as part of routine screening. 5 The confidence interval crosses the line of no effect but does not rule out a significant effect of antibiotics on mortality and/or graft loss. 6 No systematic graft biopsy performed during the study follow‐up. Not all episodes of allograft rejection were biopsy‐proven. 7 Graft function was evaluated using creatinine at end of study, despite different values between groups at time of inclusion. We were unable to pool the data for change in graft function from baseline to end of study (data missing for one study). 8 No significant effect of antibiotics on change in graft function from baseline to end of study in both studies. | |||||
| GRADE Working Group grades of evidence | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic urinary tract infection Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.51, 1.45] |
| 2 Antimicrobial resistance Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Graft loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Acute rejection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Hospitalisation for UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Graft function (creatinine at end of study) Show forest plot | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.18] |

