仙客来(Cyclamen europaeum)提取物治疗急性鼻窦炎
Abstract
研究背景
急性鼻窦炎是初诊的常见原因。它会引起显著症状,包括面部疼痛、鼻塞、头痛、鼻液粘稠、发热和咳嗽,还通常引起休工或休学。鼻窦炎治疗的重点集中在消除病因、控制炎症和感染性成分。经鼻给药的仙客来(Cyclamen europaeum)冷冻、干燥、天然液体提取物被认为是通过促进鼻腔引流来发挥缓解鼻塞的获益性疗效,并且具有抗炎作用。
研究目的
评价鼻腔局部使用仙客来提取物治疗成人和儿童急性鼻窦炎的有效性。
检索策略
我们检索了包含Cochrane急性呼吸道感染组注册库(Cochrane Acute Respiratory Infections Group's Specialised Register)在内的CENTRAL、MEDLINE、Embase和试验注册库(ClinicalTrials.gov;WHO ICTRP),检索时限至2018年1月。我们还检索了纳入文献和综述文献的参考文献以进一步获取更多相关研究,并且联系了所纳入文献的作者以获取更多关于试验的信息。
标准/纳入排除标准
经鼻给药的仙客来提取物对比安慰剂、抗生素、鼻内皮质类固醇或者无干预,治疗成人或儿童(或二者兼具)急性鼻窦炎的随机对照试验将被纳入。急性鼻窦炎由临床诊断,并通过鼻内窥镜或放射学证据确诊。
数据收集与分析
两名综述作者独立提取资料并评估试验质量。我们使用了Cochrane的标准方法程序。
主要结果
我们纳入2项随机对照试验,涉及147名借助放射学证据或鼻内窥镜被诊断为急性鼻窦炎的成人患者。这些患者被分成分别接受仙客来喷雾剂和安慰剂的2组,治疗时长为15天。由于2项研究均未报告分配隐匿及结局评价者盲,因此选择和检测偏倚的风险尚不清楚。一项研究的失访偏倚风险很高(60%),尽管它的组间脱落均衡。
2项研究均未报告我们设定的2个主要结局指标:在治疗14和30天时,症状得以解决或缓解的受试者比例。没有研究报告了关于治疗的严重不良事件和并发症;然而,仙客来治疗组发生中度不良事件(如鼻腔和喉咙刺激、轻度鼻出血和打喷嚏)的受试者人数(50%)多于安慰剂对照组(24%),风险比为2.11,95%置信区间为[1.35, 3.29],中等质量证据。
作者结论
仙客来治疗急性鼻窦炎的有效性不确定。尽管没有严重不良事件发生,但仙客来治疗组发生不良事件的受试者人数(50%)多于安慰剂对照组(24%)。
PICO
Plain language summary
仙客来(Cyclamen europaeum)提取物治疗急性鼻窦炎是否有效?
综述问题
我们评价了仙客来(Cyclamen europaeum)冷冻、干燥、天然液体提取物(一种来自仙客来块茎的草药)作为鼻腔喷雾剂,相比假治疗(安慰剂)解决或改善成人和儿童急性鼻窦炎的疗效。
研究背景
急性鼻窦炎是鼻子附近骨腔因感染而发生炎症的常见病症。症状包括鼻液粘稠、鼻塞、面部疼痛、头痛、发烧和咳嗽。症状在成人中会持续8周,在儿童中会持续12周。急性鼻窦炎通常由病毒引起;如果感染扩散,则会出现并发症。急性鼻窦炎是一种常见病症,会造成巨额花费。
一系列保守治疗可用于急性鼻窦炎的治疗。抗生素通常被使用,但临床指南并不推荐将抗生素应用于急性鼻窦炎的治疗。少数关于仙客来提取物作为鼻腔喷雾剂的研究表明,仙来客提取物或许有助于缓解鼻塞。
检索日期
证据截至2018年1月18日。
研究特征
我们纳入2项研究,涉及147名成人急性鼻窦炎。受试者被随机分配为2组,分别接受仙客来或非活性物质治疗,时长为15天。
研究经费来源
2项研究均由药企资助。
主要结局
我们想要找到在治疗14天和30天时,症状得到解决或改善的受试者比例,但是没有发现任何证据。纳入研究提供了治疗后急性鼻窦炎症状积分变化的资料。一项研究报告称,在治疗7天时,面部疼痛得以缓解。2项研究均未报告并发症、休学/休工的情况。
使用仙客来而非非活性物质治疗的患者发生了更多副作用,如鼻刺激、打喷嚏和轻度鼻出血。没有严重的不良事件发生。
我们没有发现关于仙客来是否有效的证据。
证据质量
我们对2项研究进行了质量评价,并将有关此综述唯一可评价的结局(即副作用)的证据级别评为中等质量。一项研究存在很高的脱落率(60%),但研究对其脱落情况进行了描述,且组间脱落均衡。我们发现2项研究关于不良事件的结果相一致。2项研究均未报告结局评价者是否清楚受试者所接受哪种疗法的相关信息。由于研究的小样本量和设计缺陷,我们无法得出确切结果。
Authors' conclusions
Summary of findings
| Cyclamen europaeum compared to placebo for acute sinusitis | ||||||
| Patient or population: adults with acute sinusitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Cyclamen europaeum | |||||
| Proportion of participants with resolution or improvement of symptoms up to 30 days | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Proportion of participants with any adverse event | Study population | RR 2.11 | 147 | ⊕⊕⊕⊝ Moderate | We downgraded the quality of the evidence as concealment of allocation to treatment and blinding of outcome assessors were not reported in the studies. Also, 1 study had a small sample size with a high attrition rate. | |
| 240 per 1000 | 506 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Sinusitis is a common condition that carries a large healthcare economic burden (Feldt 2013). Acute sinusitis is inflammation of one or more of the paranasal sinuses, with symptoms lasting less than eight weeks in adults and less than 12 weeks in children (Georgy 2012; Kaliner 1997). Sinusitis is one of the 10 most common reasons for visits to primary care physicians, and it is the fifth most common diagnosis for which antibiotics are prescribed. Primary care physicians tend to consider acute sinusitis to be of bacterial origin and prescribe antibiotics in 85% to 98% of cases. However, most sinusitis cases are caused by viruses. According to epidemiological estimates, only 0.2% to 2% of viral upper respiratory tract infections in adults progress to bacterial rhinosinusitis (Snow 2001). A recent systematic review and meta‐analysis reported that the prevalence of bacterial infection in acute sinusitis is likely to be greater than 2%, but this remains poorly defined (Shintani Smith 2015). Bacterial infections often resolve without antibiotic treatment. A multicentre prospective cohort study reported that antibiotics were prescribed in 71.2% of people with acute sinusitis (Dallas 2017).
Clinical signs and symptoms are not reliable indicators to effectively identify which cases of sinusitis should be treated with antibiotics. No accurate practice‐based test exists to diagnose acute bacterial sinusitis; clinicians rely mostly on clinical findings for diagnosis. Signs and symptoms of acute bacterial sinusitis and prolonged viral upper respiratory tract infection are similar, resulting in frequent misclassification of viral infections. There is no evidence to effectively distinguish bacterial from viral acute rhinosinusitis using fever and facial, dental, or both facial and dental pain (Hauer 2014). Watchful waiting is supported by current guidelines within the first seven to 10 days after symptoms of upper respiratory tract infection symptoms appear (Aring 2016). Antibiotics and intranasal steroids have been recommended for acute bacterial rhinosinusitis, although most international guidelines do not recommend the use of antibiotics to treat mild, moderate, or uncomplicated acute rhinosinusitis (Dass 2016). There is no consensus as to when to use antibiotics; furthermore, overuse of antibiotics is an alarming problem among both patients and practitioners. Antibiotics should be prescribed for people with acute rhinosinusitis whose symptoms persist for more than 10 days, in the case of onset of severe signs or symptoms of high fever (> 39 °C) and purulent nasal discharge or facial pain lasting for at least three consecutive days, or worsening symptoms following a typical viral illness lasting for five days that was initially improving (Harris 2016; Kaplan 2014).
The common cold frequently involves the upper airways, including occlusion in the sinus cavities (Gwaltney 1994). In a retrospective analysis, rhinorrhoea, purulent secretions, sinus tenderness, and a history of sinusitis were significant predictors for diagnosing sinusitis (Little 2000; Zalmanovici Trestioreanu 2013).
Diagnosis is often confirmed by sinus imaging, and computed tomography of the sinuses is helpful for people whose symptoms do not improve with treatment. Radiography is not recommended for evaluating acute uncomplicated sinusitis (Aring 2016).
Sinusitis is accompanied by inflammation of the contiguous nasal mucosa; hence, rhinosinusitis has become the preferred term (Snow 2001). Inflammation of nasal mucosa and blockage of the sinus ostium play an essential role in the development of sinusitis (Tutkun 1996). The characteristic signs and symptoms of rhinosinusitis are sinus obstruction, mucus retention, and infection. Complications can occur through intracranial extension of the infection.
Description of the intervention
The term Cyclamen europaeum has been applied to several related species of Cyclamen, but strictly applies to Cyclamen purpurascens. The tuber of Cyclamen europaeum (Cyclamen purpurascens), a member of the Primulaceae family, has been used in herbal medicine for a range of indications; it is reported to be a drastic purgative. An extract of the tuber has been used for sinusitis in the form of nasal spray known as Nasodren and Sinuforte (Micromedex 2.0).
How the intervention might work
Sinusitis treatment focuses on eliminating causative factors and controlling the inflammatory and infectious components (Becker 2003; Passali 2016). The frozen, dried, natural fluid extract of the Cyclamen europaeum plant delivered intranasally is thought to have beneficial effects in relieving congestion by facilitating nasal drainage, and has an anti‐inflammatory effect (Mashkova 2010). Beneficial effects were observed when used either as a monotherapy for mild or moderately severe disease or in combination with other agents for the treatment of acute or exacerbated chronic rhinosinusitis (Savvateeva 2010). Including Cyclamen europaeum in combined therapy for mild and moderately severe acute rhinosinusitis and exudative otitis media may make it possible to avoid drainage procedures and shorten the duration of antibacterial treatment. Simultaneous acceleration of the recovery of functional activity of the endonasal mucosa suggests a pronounced antirecurrence action of this therapy (Bogomil'skii 2010). When Cyclamen europaeum extract was used in comparison to saline for patients with chronic sinusitis and nasal polyps undergoing endoscopic sinus surgery, a significant improvement in patients' symptoms, nasal endoscopic signs, and patient satisfaction was reported. These results are thought to be connected to activities of the extract in facilitating nasal drainage and clearing the paranasal sinuses (Mullol 2009).
Why it is important to do this review
Key points of agreement in clinical guidelines regarding therapy for acute rhinosinusitis include efficacy of symptomatic treatment and the importance of reducing unnecessary antibiotic use. However, there is no consensus in guidelines regarding when antibiotics should be considered as a reasonable treatment strategy (Meltzer 2011). The management of rhinosinusitis depends on the duration and severity of symptoms. A variety of conservative and pharmacological interventions are available, although physicians can find it difficult to develop a cohesive and logical approach to sinusitis treatment (Benninger 1997; Libman 2017; Rosenfeld 2015; Sharp 2015). Antibiotics are not needed for mild to moderate sinusitis within the first week of illness. A greater likelihood of bacterial rhinosinusitis after 10 days makes antibiotic therapy a reasonable option (Van den Broek 2014). Around 80% of participants with acute sinusitis treated without antibiotics improved within two weeks (Ahovuo‐Saloranta 2014). In a Cochrane Review, a small clinical benefit was observed in participants treated with antibiotics (Ahovuo‐Saloranta 2014). A recent systematic review found that antibiotic treatment provided more symptomatic relief within the first days of treatment compared to placebo, but after 10 days both groups had similar improvement rates (Burgstaller 2016). Avoiding antibiotics for acute sinusitis could reduce antibiotic adverse effects, antibiotic resistance, and healthcare costs (Smith 2012). Another Cochrane Review did not find clear evidence for efficacy of nasal irrigations, decongestants, and antihistamines for acute sinusitis in children (Shaikh 2014). The use of adjunctive medications for acute sinusitis, such as antihistamines, decongestants, and systemic and nasal corticosteroids, also remains controversial (Shrum 2001; Venekamp 2014; Zalmanovici Trestioreanu 2013). Recent studies have tested Cyclamen europaeum extract, a novel phytotherapeutic product marketed in Europe and delivered intranasally, for its effectiveness in relieving symptoms in acute sinusitis, and found the treatment to be effective (Mullol 2009; Pfaar 2012). A recent prospective observational study reported that Cyclamen europaeum given as a monotherapy appears to be more effective than other monotherapies and combination therapies and at a lower cost per cured patient (Mullol 2013). However, a systematic review addressing the effectiveness of this therapy has not been conducted.
Objectives
To assess the effectiveness of topical intranasal Cyclamen europaeum extract on clinical response in adults and children with acute sinusitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Children and adults with acute sinusitis. Acute sinusitis was defined by a clinical diagnosis (including purulent nasal discharge and congestion, cough beyond seven days, facial pain, and fever) and radiological evidence or nasal endoscopy. We included trials in mixed populations with acute and non‐acute sinusitis if outcomes were reported separately for these subgroups.
Types of interventions
Intranasal Cyclamen europaeum extract (any preparation, dose, or duration of treatment) compared to placebo, antibiotics, intranasal corticosteroids, or no treatment. We included studies reporting combined interventions only if both treatment arms received the same co‐interventions, except for Cyclamen europaeum extract.
Types of outcome measures
Primary outcomes
-
Proportion of participants with resolution or improvement of symptoms up to 14 days.
-
Proportion of participants with resolution or improvement of symptoms up to 30 days.
Secondary outcomes
-
Proportion of participants with any adverse event.
-
Proportion of participants who developed complications.
-
Days off school or work.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1), part of the Cochrane Library (www.cochranelibrary.com) (accessed 18 January 2018), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE Ovid (1946 to 18 January 2018), and Embase.com (1974 to 18 January 2018). We searched CENTRAL and MEDLINE with the search strategy detailed in Appendix 1. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE; sensitivity‐maximising version (2008 revision): Ovid format (Lefebvre 2011). We adapted the search strategy for Embase (see Appendix 2). There were no language or publication restrictions.
Searching other resources
We searched the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en; search strategy in Appendix 3), and ClinicalTrials.gov (www.clinicaltrials.gov; search strategy in Appendix 4) on 1 February 2018. We inspected the reference lists of all identified studies and review literature for further relevant studies. We contacted trial authors for additional information (see Characteristics of excluded studies).
Data collection and analysis
Selection of studies
Two review authors (AZT, AB) independently screened titles and abstracts of all studies identified as a result of the search for potential inclusion in the review. We retrieved the full‐text study reports or publications of studies deemed potentially relevant, and two review authors (AZT, AB) independently screened the full‐text studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (BP) when necessary. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). We did not impose any language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (AZT, AB) extracted study characteristics from the included studies. We planned to extract the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (AZT, AB) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. Any disagreements were resolved by consensus or by involving a third review author (BP). One review author (AZT) transferred data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (AB) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (AZT, AB) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving a third review author (BP). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where necessary, we considered blinding separately for different key outcomes. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
We included trials if they met the following criteria:
-
a randomisation method is described that would not allow the investigator or participant to know or influence the intervention group before the eligible participant entered into the study (low risk of bias); and
-
randomisation is stated but no information on the method used is available (unclear risk of bias).
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and planned to report any deviations in the Differences between protocol and review section.
Measures of treatment effect
We analysed dichotomous data by calculating the risk ratio (RR) and risk difference (RD) for each trial with the uncertainty in each result expressed as a 95% confidence interval (CI). We analysed continuous outcomes when normally distributed by using the mean and standard deviation from each study and calculating the mean difference (MD) and the 95% CI. We expressed the results according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed all analyses on the basis of intention‐to‐treat.
Unit of analysis issues
We included randomised controlled trials with standard designs and parallel groups in the review. Participants were included only once in the analyses.
Dealing with missing data
We tried to contact study authors to obtain missing data.
Assessment of heterogeneity
We assessed heterogeneity by inspection of the graphical presentations and I² statistic for heterogeneity. Values of 25%, 50%, and 75% corresponded to low, medium, and high levels of heterogeneity.
Assessment of reporting biases
We planned a funnel plot analysis to assess possible publication bias.
Data synthesis
We used the fixed‐effect model for combining study data.
GRADE and ‘Summary of findings’ table
We created summary of findings Table for the main comparison table using the outcome proportion of participants with any adverse event. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2014).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses according to the treatment in the control group to assess the impact of this possible source of heterogeneity. We included only two studies in the review, and both used placebo in the control group.
Sensitivity analysis
We planned to use the random‐effects model to test the robustness of results when heterogeneity was present. We detected no heterogeneity between the studies. We performed a sensitivity analysis to check the robustness of the result by imputing data, considering best‐case scenario (assuming none of the dropouts in the intervention group had an adverse event and all dropouts in the control group had an adverse event) and worst‐case scenario (assuming all dropouts in the intervention group had an adverse event and none of the dropouts in the control group had an adverse event), as Ponikau 2012 had a high dropout rate.
Results
Description of studies
Results of the search
We identified 87 references from the electronic searches. After removing 21 duplicates, two review authors (AZT, AB) inspected 66 abstracts. We excluded 56 abstracts for the following reasons: not acute sinusitis, not randomised, observational study, intervention of interest not used, no relevant outcomes, review articles. We considered 10 studies for inclusion after inspecting the abstracts and obtaining full‐text reports (Figure 1). We excluded eight studies which did not meet the inclusion criteria for this review.
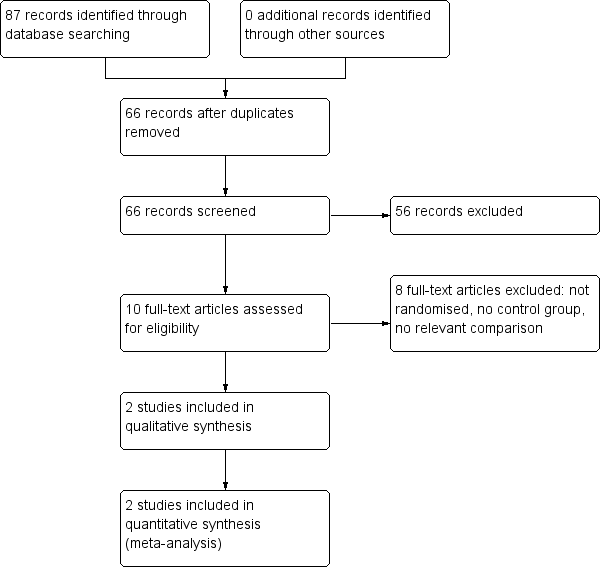
Study flow diagram.
Included studies
See Characteristics of included studies table.
Two studies (147 participants) met the inclusion criteria and were included in the review. Participants were assigned to nasal Cyclamen europaeum spray or placebo. The studies were multicentre trials: one was conducted in 13 centres in Germany (Pfaar 2012), and one study involved outpatients in 25 centres in the USA (Ponikau 2012).
Participants were adults with a documented episode of acute sinusitis confirmed by radiology or nasal endoscopy. The entry criteria and participants in the trials were similar.
Both studies used a placebo in the control group and the same dose of Cyclamen europaeum lyophilised extract, 1.3 mg once daily in each nostril, in the treatment group. Pfaar 2012 used concomitant amoxycillin in both arms or an alternative for participants who were allergic to penicillin. Treatment duration in the studies was 15 days in Pfaar 2012 and seven days in Ponikau 2012.
The outcomes in the included studies reported change from baseline in mean total symptom score, individual symptom scores, endoscopic changes, treatment failure or need for additional treatment, complications, sleep quality, and overall treatment satisfaction in Pfaar 2012; and change from baseline in sinus opacification on computed tomography (CT) scans, reduction in total symptom score, other symptom scores changes from baseline, and endoscopic inflammation in Ponikau 2012.
Information on adverse events that occurred during the trials is presented in Table 1. The studies described participants who dropped out before the end of the study, and their reasons for leaving.
| Study ID | Cyclamen europaeum | Placebo |
| 67% total 50% nasal irritation mild/moderate 27% mild epistaxis 4% sneezing 3 discontinued treatment | 29% total 4% nasal irritation 14% mild epistaxis 4% vertigo 2 discontinued treatment | |
| 15.4% total Influenza, throat irritation, migraine, sneezing No serious adverse events Did not discontinue treatment | 12.5% total Headache, ear pain, gastritis, back pain, conjunctival haemorrhage No serious adverse events Did not discontinue treatment |
Excluded studies
See Characteristics of excluded studies table.
We excluded eight studies for the following reasons: not randomised, control group not used, intervention of interest not used (Bogomil'skii 2010; Ianov 2007; Kriukov 2007; Mashkova 2010; Ovchinnikov 2009; Rybak 2008; Semenov 2011; Svistushkin 2013). We contacted one author to ask if the study was randomised; we subsequently excluded this study because quasi‐randomisation was used (Ovchinnikov 2009). All excluded studies were published in the same journal between 2007 and 2013.
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The studies were randomised, double‐blind, placebo‐controlled trials.
Allocation
Pfaar 2012 did not report randomisation methods (unclear risk of bias). Ponikau 2012 adequately reported generation of the allocation sequence (low risk of bias). Neither study reported concealment of allocation to treatment (unclear risk of bias).
Blinding
The trials were double‐blinded, and the method of blinding was described (low risk of bias). Blinding of outcome assessors was not reported in the studies (unclear risk of bias).
Incomplete outcome data
Both studies described dropouts before the end of the study and provided reasons (low risk of bias). Total losses to follow‐up were 17% in Pfaar 2012 and 60% in Ponikau 2012. Dropouts were balanced between study arms.
Selective reporting
The studies reported outcomes and results as prespecified in their protocols (low risk of bias).
Other potential sources of bias
We did not identify possible sources of bias. The studies were small and were assessed as moderate quality for the only outcome assessed (adverse events), with similar direction of results for the outcome included in our meta‐analysis. Both studies were supported by pharmaceutical companies, but funding sources did not seem to influence results.
Effects of interventions
We included two studies (147 participants) (Pfaar 2012; Ponikau 2012). See summary of findings Table for the main comparison.
Primary outcomes
1. Proportion of participants with resolution or improvement of symptoms up to 14 days
No studies reported this outcome. One study reported changes in mean total symptom score up to seven days and a trend toward greater symptomatic relief in the Cyclamen europaeum group, although this was not clear (P = 0.64) (Pfaar 2012). Uncertainty in results was observed for improvement of nasal obstruction and oedema. Improvement in facial pain up to seven days was observed, and this was obvious (mean difference ‐1.20, 95% confidence interval (CI) ‐2.32 to ‐0.08; P = 0.04) (Pfaar 2012). There was no clear difference between treatment and control groups for total or individual symptom scores in Ponikau 2012.
2. Proportion of participants with resolution or improvement of symptoms up to 30 days
No studies reported this outcome.
Secondary outcomes
1. Proportion of participants with any adverse event
Both studies reported data for this outcome (Table 1). More adverse events were reported in the Cyclamen europaeum group than in the placebo group during the treatment period, and we are confident in the result (risk ratio (RR) 2.11, 95% CI 1.35 to 3.29; risk difference 0.26, 95% CI 0.13 to 0.40; Analysis 1.1). We assessed the quality of the evidence as moderate (summary of findings Table for the main comparison).
We performed sensitivity analysis by imputing data to check the robustness of the result, considering best‐case scenario (assuming none of the dropouts in the intervention group had an adverse event and all dropouts in the control group had an adverse event) (RR 0.94, 95% CI 0.68 to 1.30; Analysis 1.2), and worst‐case scenario (assuming all dropouts in the intervention group had an adverse event and none of the dropouts in the control group had an adverse event) (RR 3.49, 95% CI 2.30 to 5.30; Analysis 1.3).
2. Proportion of participants who developed complications
Only one study reported this outcome; no complications were reported (Pfaar 2012).
3. Days off school or work
No studies reported this outcome.
We did not perform planned funnel plots and subgroup analyses due to the small number of included studies and because both used a placebo in the control group.
Discussion
Rhinosinusitis is one of the most common upper respiratory diseases treated in general practice (Marple 2006; Schappert 2006). The condition is associated with significant economic burden, which includes reduced productivity and absenteeism, as well as medical costs (Fokkens 2007; Padia 2016; Rudmik 2017). Sinusitis is a common disorder that can cause further morbidity and mortality through progression of inflammation and extension of infection. It is associated with significant patient symptomatology that adversely affects quality of life (Carr 2016). Management of uncomplicated acute rhinosinusitis includes analgesics, saline irrigations and intranasal steroids. The use of antibiotics for people with acute rhinosinusitis is widespread, and there seems to be only a slight added benefit over placebo (Sng 2015). There is a need for improved antibiotic stewardship across all settings (Sharp 2015). Alternative treatments should be considered according to severity of symptoms. We aimed to investigate whether Cyclamen europaeum extract, a novel phytotherapeutic treatment, is an effective topical treatment for acute sinusitis.
Summary of main results
We included two small studies that involved a total of 147 adult participants who received Cyclamen europaeum extract as an intranasal spray versus placebo and provided moderate‐quality evidence for the only outcome assessed (adverse events). We could not assess whether Cyclamen europaeum is effective or not for treating sinusitis. We were able to meta‐analyse results for adverse events only; data for other review outcomes were not available from the included studies. Neither study showed clear differences for total symptom scores changes between people who received Cyclamen europaeum and those who received placebo, although Pfaar 2012 reported a trend toward improvement in total symptom score and obvious difference for improvement of facial pain up to seven days. More adverse events during the treatment period were found in participants in the Cyclamen europaeum group compared to those in the placebo group, although no serious adverse events were reported in either study (Table 1).
Overall completeness and applicability of evidence
The study population included in this review was diagnosed both clinically and by radiology or endoscopy and is not identical to people diagnosed in clinical practice, among whom diagnosis is usually based on clinical symptoms and signs alone.
Cyclamen europaeum extract has been available for the treatment of acute rhinosinusitis in over 20 European countries as an intranasal product. Clinical trials in people with acute rhinosinusitis suggest that Cyclamen europaeum reduces symptoms (Semenov 2011); improves mucociliary transport time (Lopatin 2007); and increases cure rate (Mullol 2009). The saponin fraction of Cyclamen europaeum immediately stimulates nasal secretions. These actions are attributable to irritation of the trigeminal nerve endings in the nasal mucous membranes through cholinergic pathways, leading to reflex discharge of inflammatory sinus exudates through the nose and subsequent decongestion (Gedevanishvili 2007; Jurkiewicz 2016). One study found this treatment was as effective as traditional treatment, and when used as initial monotherapy, reduced drug loading (Svistushkin 2013). We could not assess in our review whether Cyclamen europaeum is effective or not.
Quality of the evidence
We included two small studies (147 participants) in this review. The studies were randomised, but neither reported concealment of allocation to treatment (Pfaar 2012; Ponikau 2012). Both studies found the same direction of effect for the outcome included in the meta‐analysis, that is more adverse events in the groups receiving Cyclamen europaeum. Ponikau 2012 had a high dropout rate (60%), but dropouts were described and balanced between study arms, and absolute numbers of dropouts were small as sample size was small in this study; we performed a sensitivity analysis considering both extreme scenarios to test the robustness of the result and this did not change the conclusion. Both studies were funded by pharmaceutical companies, among them Hartington Pharmaceutical; Cyclamen europaeum nasal spray known as Sinuforte or Nasodren is a trademark and brand of Hartington Pharmaceutical. This did not seem to influence the results, as more adverse events were reported in participants who received Cyclamen europaeum compared to the placebo group. Using GRADE principles we assessed the evidence for the only outcome meta‐analysed, that is proportion of participants with any adverse event, as of moderate quality (summary of findings Table for the main comparison), downgrading quality due to lack of reporting of concealment of allocation to treatment and blinding of outcome assessors in the studies, and a small sample size with high dropouts in Ponikau 2012.
Potential biases in the review process
We conducted searches according to Cochrane Acute Respiratory Infections Group recommendations. Two review authors (AZT, AB) independently selected studies for inclusion and extracted data. Two review authors (AZT, BP) analysed data and interpreted results to prevent possible bias in the review process. We conducted this review according to the published protocol. Participants received concomitant antibiotics in Pfaar 2012; as both groups received antibiotics, the only intervention that differed between groups was Cyclamen europaeum or placebo, and the effect could only be attributed to Cyclamen europaeum. We assessed both studies as at unclear risk of bias for blinding of outcome assessors and at low risk of bias for blinding of participants and personnel.
Agreements and disagreements with other studies or reviews
The limited existing evidence for Cyclamen europaeum treatment, which is controversial, has not been systematically reviewed, and we found the methodology of many studies to be questionable (see Characteristics of excluded studies). One study found that monotherapy with Cyclamen europaeum for participants with moderately severe acute sinusitis was associated with recovery in 73% of people (Semenov 2011). Jurkiewicz 2016 reported that Cyclamen europaeum efficiently reduced symptoms of acute sinusitis. We could not assess the effectiveness of Cyclamen europaeum for acute sinusitis in our review.
The included studies reported no serious adverse events linked to Cyclamen europaeum treatment. This was consistent with findings reported by Kriukov 2007, Ovchinnikov 2009, and Svistushkin 2013.

Study flow diagram.
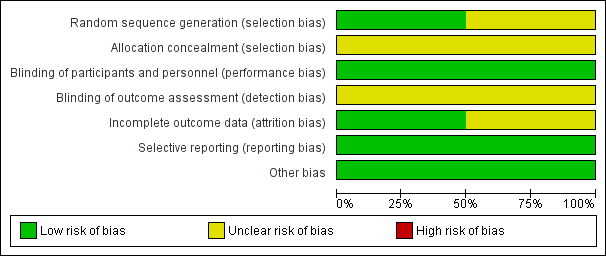
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Cyclamen europaeum versus placebo, Outcome 1 Proportion of participants with any adverse event ‐ intention‐to‐treat.

Comparison 1 Cyclamen europaeum versus placebo, Outcome 2 Proportion of participants with any adverse events ‐ best‐case sensitivity analysis.

Comparison 1 Cyclamen europaeum versus placebo, Outcome 3 Proportion of participants with any adverse event ‐ worst‐case sensitivity analysis.
| Cyclamen europaeum compared to placebo for acute sinusitis | ||||||
| Patient or population: adults with acute sinusitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Cyclamen europaeum | |||||
| Proportion of participants with resolution or improvement of symptoms up to 30 days | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Proportion of participants with any adverse event | Study population | RR 2.11 | 147 | ⊕⊕⊕⊝ Moderate | We downgraded the quality of the evidence as concealment of allocation to treatment and blinding of outcome assessors were not reported in the studies. Also, 1 study had a small sample size with a high attrition rate. | |
| 240 per 1000 | 506 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Study ID | Cyclamen europaeum | Placebo |
| 67% total 50% nasal irritation mild/moderate 27% mild epistaxis 4% sneezing 3 discontinued treatment | 29% total 4% nasal irritation 14% mild epistaxis 4% vertigo 2 discontinued treatment | |
| 15.4% total Influenza, throat irritation, migraine, sneezing No serious adverse events Did not discontinue treatment | 12.5% total Headache, ear pain, gastritis, back pain, conjunctival haemorrhage No serious adverse events Did not discontinue treatment |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with any adverse event ‐ intention‐to‐treat Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.35, 3.29] |
| 2 Proportion of participants with any adverse events ‐ best‐case sensitivity analysis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 3 Proportion of participants with any adverse event ‐ worst‐case sensitivity analysis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [2.30, 5.30] |

