Interrupción de los agonistas beta2 de acción prolongada (ABAP) para niños con asma bien controlada con ABAP y corticosteroides inhalados
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011316.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 mayo 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Shaleen Ahmad searched clinicaltrials.gov and drug company trial registries. Shaleen Ahmad and Kayleigh Kew sifted the main electronic search and the results from additional searching, and compiled the study shortlist. Sean Beggs and Kayleigh Kew made the final decisions on study exclusion. Kayleigh Kew wrote up the draft manuscript with clinical and editorial appraisal from Sean Beggs.
Sources of support
Internal sources
-
Kayleigh Kew, UK.
St George's University, London
-
Shaleen Ahmad, UK.
St George's University, London
-
Sean Beggs, Other.
No such funding was received for this review
External sources
-
Kayleigh Kew, UK.
National Institute of Health Research: Evidence to guide care in adults and children with asthma, 13/89/14
Declarations of interest
Kayleigh Kew: none known
Sean Beggs: none known
Shaleen Ahmad: none known
Acknowledgements
We would like to thank Rebecca Normansell, who contributed to the related protocol and review addressing this question in adults (Ahmad 2014), which informed the design of this review. We also thank Elizabeth Stovold for designing the search strategy and running the main electronic searches.
Jimmy Chong was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 May 21 | Stopping long‐acting beta2‐agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids | Review | Kayleigh M Kew, Sean Beggs, Shaleen Ahmad | |
| 2014 Sep 22 | Stopping long‐acting beta2‐agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids | Protocol | Kayleigh M Kew, Sean Beggs | |
Differences between protocol and review
No trials met the inclusion criteria for this review, we were unable to conduct meta‐analyses, implement any of the data analysis plan, or conduct any of the planned subgroup or sensitivity analyses.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Humans;
PICO
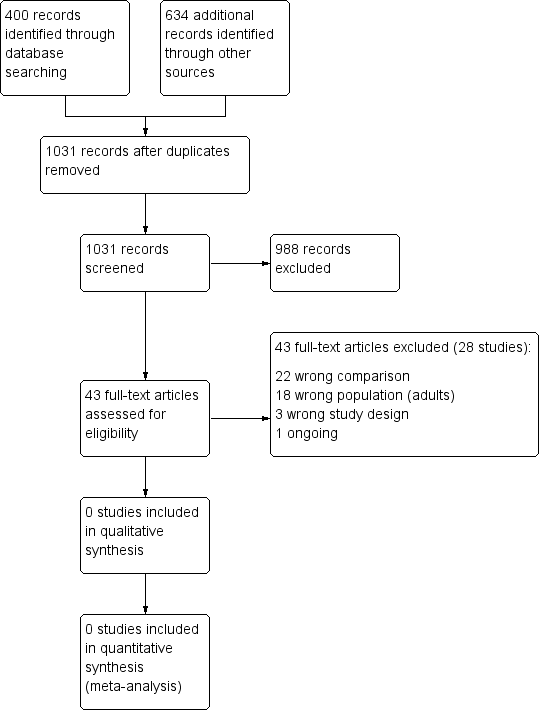
Results of the search and study selection process
| Stopping long‐acting beta2‐agonists (LABA) compared with continuing inhaled corticosteroids (ICS) + LABA for children with well‐controlled asthma | ||
| Patient or population: children (aged 18 years or younger) whose asthma is well controlled on combination ICS + LABA Settings: outpatient Intervention: stopping LABA Comparison: continuing ICS + LABA | ||
| Outcomes | No of Participants | Comments |
| Exacerbations requiring systemic corticosteroids | 0 (0) | No studies met the inclusion criteria for the review |
| Asthma control (validated scales) | 0 (0) | |
| Serious adverse events (all cause) | 0 (0) | |
| Quality of life (validated scales) | 0 (0) | |
| Exacerbations requiring hospitalisation or emergency department visit | 0 (0) | |
| Adverse events (all cause) | 0 (0) | |
| Withdrawals | 0 (0) | |

