Radioterapia de haz externo para el carcinoma hepatocelular no resecable
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial with three arms: transarterial chemoembolisation (TACE) (arm A), or radiotherapy (arm B), or radiotherapy +TACE (arm C). Parrallel group design | |
| Participants | 107 people with primary liver cancer Median age: 46 years Male/female: 80/27 TACE = 39; RT = 32; TACE + RT = 36 Recruitment: September 1990 to June 1995 Inclusion criteria:
| |
| Interventions | TACE: hepatic artery infusion. Two drugs from (cisplatin 60 mg to 120 mg, doxorubicin , THP 50 mg to 100 mg, mitomycin 16 mg to 20 mg, 5‐fluorouracil 1.0 g to 2.0 g, and cyclophosphamide 1.2 g) were chosen. 40% iodinated oil 10 mL to 30 mL, gel foam particles 1 mm to 2 mm every 4 to 8 weeks; average 3.2 times. | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not mention the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other biases |
| Methods | Randomised clinical trial with two arms: transarterial chemoembolisation (TACE) versus three dimensional conformal radiotherapy (DCRT) and TACE). Parrallel group design. | |
| Participants | 48 people with unresectable liver cancer TACE = 24; 3‐DCRT and TACE = 24 Recruitment: November 2005 to November 2007 | |
| Interventions | TACE: femoral artery by Seldinger technique. 5‐fluorouracil 1000 mg to 1250 mg, DDP 70 mg to 90 mg, Epi‐ADM 50 mg to 60 mg, iodinated oil 5 mL to 20 mL. | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: Quzhou Science and Technology Bureau (NO. 20051120) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article mentioned that the study was "randomised", but they did not provide the randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was unclearly reported. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were assessed by apparatus. |
| Incomplete outcome data (attrition bias) | Low risk | No missing participants |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Low risk | The study was funded by Quzhou Science and Technology Bureau (grant NO. 20051120). |
| Other bias | Unclear risk | Unclear risk of other biases |
| Methods | Randomised clinical trial with 2 arms: hepatic arterial embolisation versus hyperfractionated radiotherapy and hepatic arterial embolisation. Parallel‐group design | |
| Participants | 91 people with unresectable liver cancer Male/female: 82/9 Average age: 52 years Hepatic arterial embolisation = 48; hyperfractionated radiotherapy and hepatic arterial embolisation = 43 Recruitment: September 1988 to December 1997 | |
| Interventions | The total dose is 4000 to 5000 cGy, 34 to 42 fractions, over 3 to 4 weeks. | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other biases |
| Methods | Randomised clinical trial with 2 arms: three dimensional conformal radiotherapy (DCRT) + transarterial chemoembolisation (TACE) versus TACE. Parrallel group design | |
| Participants | 76 people with primary liver cancer 3‐DCRT + TACE = 40; TACE = 36 Median age: 52 years Male/female: 48/28 Recruitment: May 2003 to March 2007 Inclusion criteria: Chinese Medical Association guideline:
3‐DCRT + TACE:
TACE:
| |
| Interventions | TACE: femoral artery by Seldinger technique. | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to make contact with the corresponding author to provide missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article was described as a "prospective randomised clinical study", but randomisation method was not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were assessed by apparatus. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
| Methods | Randomised clinical trial with 3 arms: radiotherapy versus transarterial chemoembolisation (TACE) versus radiotherapy + TACE. Parallel‐group design | |
| Participants | 60 people with histologically proven inoperable/unresectable advanced primary hepatocellular carcinoma. Radiotherapy = 20 participants; TACE = 20 participants; radiotherapy + TACE = 20 participants. Median age: 52 years Male/female: 45/16 Recruitment: January 1990 to January 1998 Inclusion criteria: Histologically or cytologically proven hepatocellular carcinoma and measurable bipolar disease. Patients with solitary lesion were also included if they were not candidates for surgery. Exclusion criteria: Patients were excluded if they were older than 75 years, had bilirubin level more than 3, TLC less than 3000, platelet less than 60,000, creatinine more than 2. | |
| Interventions | Radiotherapy: given as whole‐liver irradiation with the moving‐strip technique 150 to 180 cGy until tumour dose at the centre reached 20 to 25 Gy, then the residual foci as localised by ultrasound were treated with a boost until 50 Gy with cobalt‐60 machine. TACE: Seldinger technique was used. Chemotherapy consisted of cisplatin, adriamycin, and mitomycin or floxuridine. The embolisation agent was 40% iodised oil. Treatment was repeated at 4, 6, and 8 weeks. Participants received TACE 4 to 5 times. Radiotherapy + TACE: radiotherapy started 2 weeks after TACE until 20 to 25 Gy, then 1 week rest followed by the 2nd TACE. Another 2‐week gap, then radiotherapy boost until total dose up to 50 Gy. A further 2‐week gap, then 3rd TACE was given. 4th TACE was given after 6 to 8 weeks. TACE was administered in this group until 5 times. | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope method |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Detection bias was unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
| Methods | Randomised clinical trial with 2 arms: three dimensional conformal radiotherapy (3‐DCRT) plus transarterial chemoembolisation (TACE) versus TACE. Parallel‐group design | |
| Participants | 60 people with unresectable primary hepatic carcinoma. TACE + 3‐DCRT = 30; TACE = 30 Median age: not reported Male/female: 45/15 Recruitment: January 2002 to June 2006 Inclusion criteria: Chinese Medical Association guideline:
| |
| Interventions | TACE: femoral artery by Seldinger technique 3‐DCRT: total average dose = 55 Gy | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to make a contact with the corresponding author to request missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was generated by random table number. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome was assessed by apparatus. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source. |
| Other bias | Unclear risk | Unclear risk of other bias. |
| Methods | Randomised clinical trial including 4 arms: transarterial chemoembolisation (TACE) with immediate administration of doxorubicin versus TACE with delayed administration of doxorubicin versus TACE plus external beam radiotherapy (EBRT) versus EBRT alone. Parallel‐group design | |
| Participants | 82 people with hepatocellular carcinoma Average age: 48.3 years Male/female: 69/13 Recruitment: March 1991 to April 1993 | |
| Interventions | TACE with immediate administration of doxorubicin (20 participants) versus TACE with delayed administration of doxorubicin (20 participants) versus EBRT + TACE (21 participants) versus EBRT alone (21 participants) | |
| Outcomes |
| |
| Notes | Child‐Pugh A: 36 Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as a randomised clinical trial, but the method of randomisation was not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The trial did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
| Methods | Randomised clinical trial with 2 arms: transarterial chemoembolisation (TACE) + Gamma Knife versus TACE. Parallel‐group design | |
| Participants | 259 people with primary hepatocellular carcinoma. Gamma Knife + TACE = 135; TACE = 124 Median age: 52 years Male/female: 240/55 Recruitment: not mentioned Inclusion criteria Chinese Medical Association guideline:
TACE + Gamma Knife: elevated alpha‐fetoprotein: 101; single lesion: 92 | |
| Interventions | TACE: femoral artery by Seldinger technique. 5‐fluorouracil 500 mg to 1000 mg, DDP 200 mg to 300 mg, Epi‐ADM 30 mg to 50 mg, iodinated oil 5 mL to 20 mL. | |
| Outcomes |
| |
| Notes | Country of the study: China We were unable to make contact with the corresponding author to request missing data. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as a randomised clinical trial, but the method of randomisation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were assessed by apparatus. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
| Methods | Randomised clinical trial with 2 arms: three dimensional conformal radiotherapy (3‐DCRT) plus transarterial chemoembolisation (TACE) versus TACE | |
| Participants | 96 people with inoperable primary liver cancer. TACE + 3‐DCRT = 49; TACE = 47 Median age: 52 years Male/female: 59/37 Recruitment: January 1998 to April 2000 Diagnostic criteria:
79 participants had pathologic diagnosis; others were diagnosed by clinical symptoms, imaging, and alpha‐fetoprotein. | |
| Interventions | Planning scan by spiral computed tomography, design CTV, and PTV. | |
| Outcomes |
| |
| Notes | Country of the study: China We contacted the corresponding author on 19 March 2016 to provide missing data, but have not yet received any feedback. Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as a randomised clinical trial, but the method of randomisation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
Epi‐ADM: doxorubicin and epirubicin; cGy: centigray; CTV: clinical target volume; DDP: cisplatin; Gy: gray; KPS: Karnofsky performance score; PTV: planning target volume; THP: tetrahydropalmatine; TLC: total leukocytic count; TNM: tumour, node and metastasis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| All included participants were eligible for transplantation (participants were required to have disease within the Milan or San Francisco criteria, without vascular invasion). | |
| Radiotherapy was part of the therapeutic strategy in the 3 randomised arms, thus it was not possible to formally assess the added benefit or harm of radiotherapy in these trial participants. | |
| Not randomised | |
| Not randomised | |
| Not randomised |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer |
| Methods | Phase III trial |
| Participants | People with hepatocellular carcinoma unsuitable for resection, radiofrequency ablation, or transarterial chemoembolisation (TACE) |
| Interventions | Experimental: Arm 1 (sorafenib tosylate): Sorafenib tosylate given orally twice a day on days 1 to 28. Treatment repeats every 28 days for up to 5 years in the absence of disease progression or unacceptable toxicity. Experimental: Arm 2 (stereotactic body radiotherapy and sorafenib tosylate): stereotactic body radiotherapy administered every 24 to 72 hours for a total of 5 fractions over 5 to 15 days. Within 1 to 5 days post‐stereotactic body radiotherapy, treatment with sorafenib tosylate commences, given orally twice a day on days 1 to 28. Treatment repeats every 28 days for up to 5 years in the absence of disease progression or unacceptable toxicity. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Health‐related quality of life assessments measured by FACT‐Hep |
| Starting date | April 2013 |
| Contact information | Christopher M Iannuzzi; [email protected] |
| Notes | Inclusion criteria:
Exclusion criteria:
|
| Trial name or title | Transarterial chemoembolisation plus radiotherapy or sorafenib in hepatocellular carcinoma with major vascular invasion (START) |
| Methods | Randomised phase II trial |
| Participants | People with hepatocellular carcinoma invading major intrahepatic vessels Country: Korea |
| Interventions | transarterial chemoembolisation (TACE) + external beam radiotherapy versus sorafenib |
| Outcomes | Primary endpoint
Secondary endpoints
|
| Starting date | July 2013 |
| Contact information | Young‐Suk Lim, Associate Professor, Asan Medical Center |
| Notes | Estimated enrolment: 90 Inclusion criteria:
|
| Trial name or title | Comparison between radiofrequency ablation and hypofractionated proton beam radiation for recurrent/residual hepatocellular carcinoma |
| Methods | Phase III trial |
| Participants | People with recurrent/residual hepatocellular carcinoma |
| Interventions | Experimental: Arm A (radiofrequency ablation) Experimental: Arm B (proton beam radiotherapy) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | October 2013 |
| Contact information | Joong Won Park, PhD; [email protected] |
| Notes | Estimated enrolment: 144 Inclusion criteria:
Exclusion criteria:
|
| Trial name or title | A trial on stereotactic body radiotherapy after incomplete transarterial chemoembolisation (TACE) versus exclusive TACE for treatment of inoperable hepatocellular carcinoma |
| Methods | Phase III trial |
| Participants | People with inoperable hepatocellular carcinoma |
| Interventions | Experimental: Stereotactic body radiation therapy Active comparator: transarterial chemoembolisation (TACE) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2014 |
| Contact information | Marta Scorsetti, MD, PhD; [email protected] |
| Notes | Estimated enrolment: 80 Inclusion criteria:
Exclusion criteria:
|
| Trial name or title | Proton radiotherapy versus radiofrequency ablation for patients with medium or large hepatocellular carcinoma |
| Methods | Phase III trial |
| Participants | People with medium (> 3, ≤ 5 cm) or large (> 5, ≤ 7 cm) treatment‐naive hepatocellular carcinoma |
| Interventions | Proton beam radiotherapy versus radiofrequency ablation |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | January 2016 |
| Contact information | Bing‐Shen Huang, MD; [email protected] |
| Notes | Inclusion criteria:
Exclusion criteria:
*Baseline laboratories results must be within the protocol range prior to signing informed consent. Repeat lab tests are permitted to evaluate eligibility during the screening period. |
| Trial name or title | Transarterial chemoembolisation compared with stereotactic body radiation therapy or stereotactic ablative radiation therapy in treating patients with residual or recurrent liver cancer undergone initial transarterial chemoembolisation |
| Methods | Phase III trial |
| Participants | People with residual or recurrent liver cancer who have undergone initial transarterial chemoembolisation |
| Interventions | Active comparator: Arm I ‐ transarterial chemoembolisation (TACE) Experimental: Arm II (stereotactic body radiotherapy) |
| Outcomes | Primary objectives:
Secondary objectives:
|
| Starting date | May 2016 |
| Contact information | Rachel Freiberg; [email protected] |
| Notes | Inclusion criteria:
Exclusion criteria:
|
| Trial name or title | Transarterial chemoembolisation (TACE) versus TACE + stereotactic body radiotherapy for unresectable hepatocellular cancer (TACE ‐ stereotactic body radiotherapy) |
| Methods | Phase III trial |
| Participants | People with unresectable hepatocellular carcinoma |
| Interventions | Active comparator: drug‐eluting beads ‐ TACE arm Experimental: drug‐eluting beads ‐ TACE + stereotactic body radiotherapy arm |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2014 |
| Contact information | Supriya Chopra; [email protected] |
| Notes | Inclusion criteria:
Exclusion criteria:
|
CTCAE: Common Terminology Criteria for Adverse Events; EASL: European Association for the Study of the Liver; ECOG: Eastern Cooperative Oncology Group; FACT‐Hep: Functional Assessment of Cancer Therapy ‐ Hepatobiliary; MRI: magnetic resonance imaging; RECIST: Response Evaluation Criteria in Solid Tumours.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (at 1 year) Show forest plot | 9 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.41, 0.62] |
| Analysis 1.1 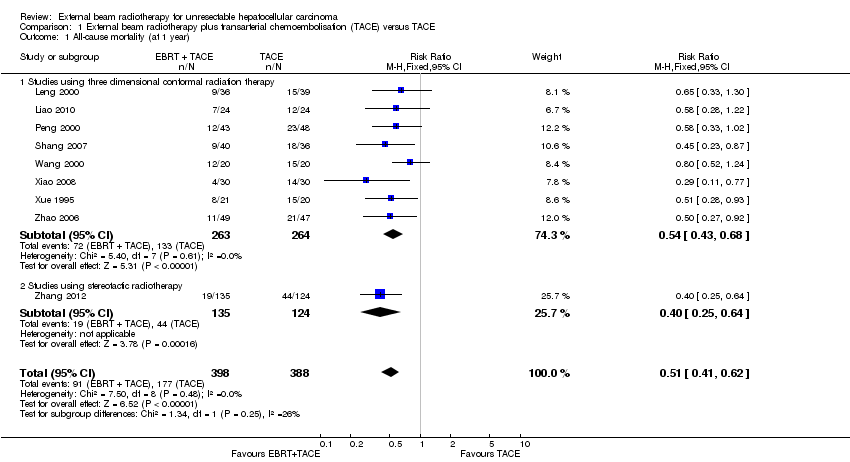 Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 1 All‐cause mortality (at 1 year). | ||||
| 1.1 Studies using three dimensional conformal radiation therapy | 8 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.68] |
| 1.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.25, 0.64] |
| 2 Complete response Show forest plot | 7 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.47, 3.13] |
| Analysis 1.2  Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 2 Complete response. | ||||
| 2.1 Studies using three dimensional conformal radiation therapy | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.34, 3.37] |
| 2.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.12, 4.21] |
| 3 Complete response + partial response Show forest plot | 7 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.40, 1.78] |
| Analysis 1.3 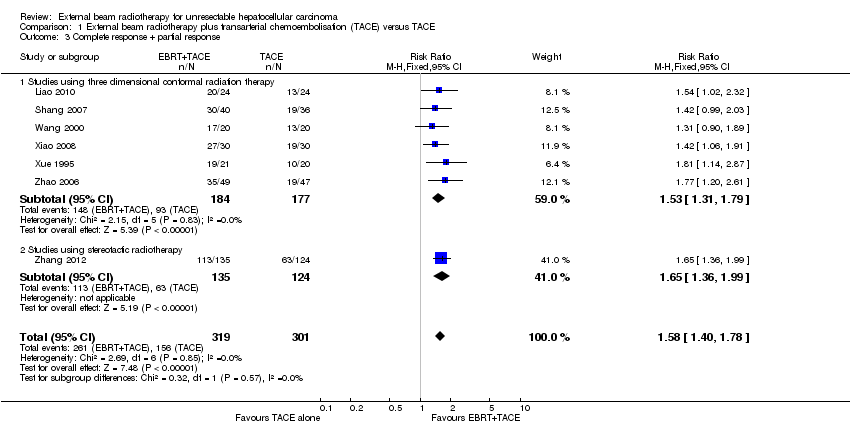 Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 3 Complete response + partial response. | ||||
| 3.1 Studies using three dimensional conformal radiation therapy | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.31, 1.79] |
| 3.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.36, 1.99] |
| 4 Elevated alanine aminotransferase Show forest plot | 3 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.08, 1.84] |
| Analysis 1.4  Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 4 Elevated alanine aminotransferase. | ||||
| 5 Elevated total bilirubin Show forest plot | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.34, 5.40] |
| Analysis 1.5 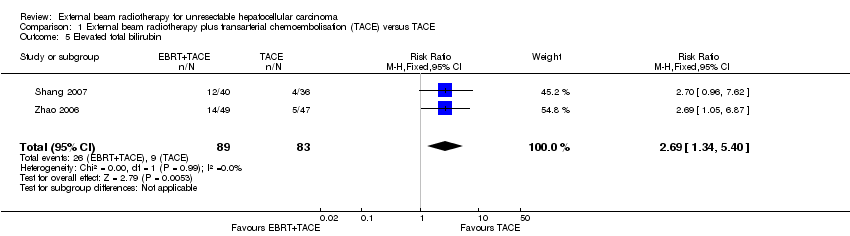 Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 5 Elevated total bilirubin. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.97, 1.50] |
| Analysis 2.1  Comparison 2 External beam radiotherapy versus transarterial chemoembolisation, Outcome 1 All‐cause mortality. | ||||
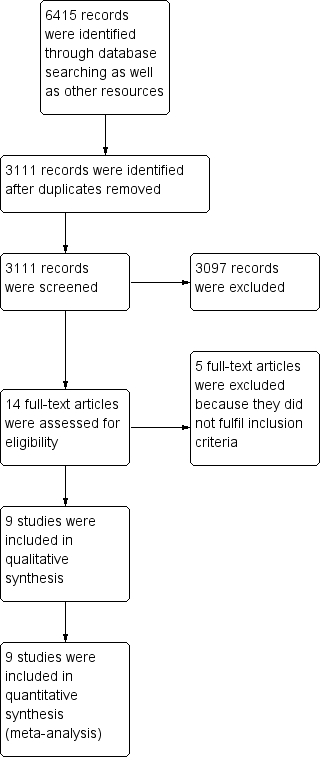
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis comparing external beam radiotherapy (EBRT) plus transarterial chemoembolisation (TACE) versus TACE alone on the outcome 'all‐cause mortality at one year'. A subgroup of studies used three‐dimensional conformal radiotherapy. The diversity‐adjusted required information size (DARIS) of n = 1029 patients was calculated based upon a proportion of mortality of 50.3% of patients in the TACE group, a relative risk reduction of 20% in the EBRT + TACE group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. The blue curve presents the cumulative meta‐analysis Z‐score, and the inward‐sloping dotted red curves present the adjusted threshold for statistical significance according to the two‐sided trial sequential monitoring boundaries.

Trial Sequential Analysis comparing external beam radiotherapy (EBRT) versus transarterial chemoembolisation (TACE) on the outcome 'all‐cause mortality at one year'. The diversity‐adjusted required information size (DARIS) of n = 808 patients was calculated based upon a proportion of mortality of 57% of patients in the TACE group, a relative risk reduction of 20% in the EBRT group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. The blue curve presents the cumulative meta‐analysis Z‐score, and the inward‐sloping dotted red curves present the adjusted threshold for statistical significance according to the two‐sided trial sequential monitoring boundaries.

Trial Sequential Analysis comparing external beam radiotherapy (EBRT) plus transarterial chemoembolisation (TACE) versus TACE alone on the outcome 'complete response plus partial response ‐ subgroup of studies using three‐dimensional conformal radiotherapy'. The diversity‐adjusted required information size (DARIS) of n = 951 patients was calculated based upon a proportion of response of 52.5% of patients in the TACE group, a relative risk reduction of 20% in the TACE + EBRT group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. The blue curve presents the cumulative meta‐analysis Z‐score, and the inward‐sloping red curves present the adjusted threshold for statistical significance according to the two‐sided trial sequential monitoring boundaries.
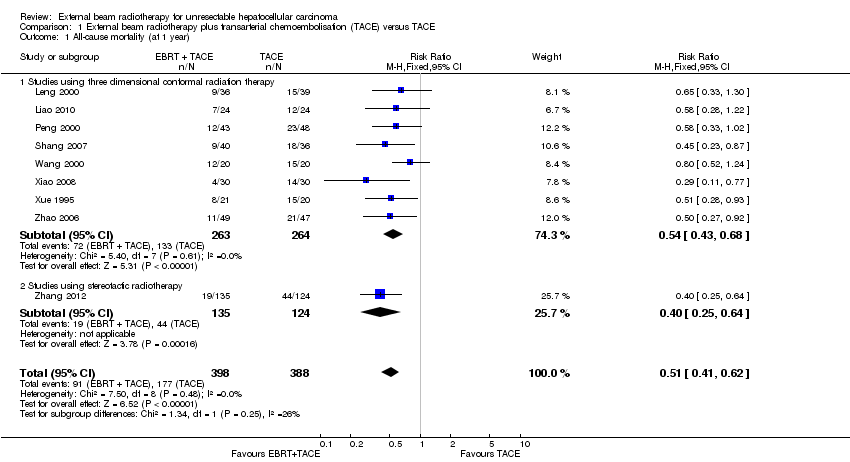
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 1 All‐cause mortality (at 1 year).

Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 2 Complete response.

Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 3 Complete response + partial response.

Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 4 Elevated alanine aminotransferase.
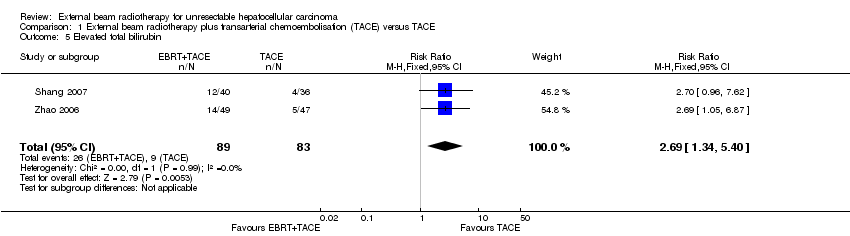
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 5 Elevated total bilirubin.
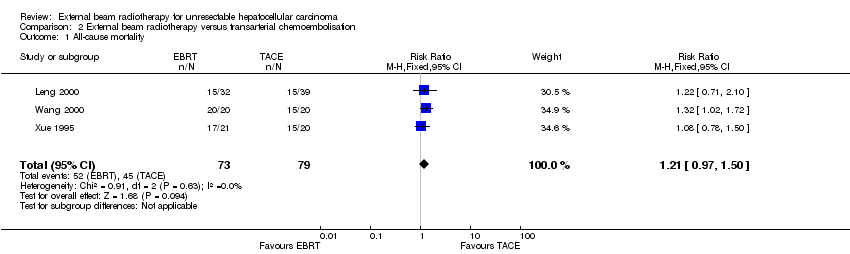
Comparison 2 External beam radiotherapy versus transarterial chemoembolisation, Outcome 1 All‐cause mortality.
| External beam radiotherapy (EBRT) plus transarterial chemoembolisation(TACE) versus TACE alone for unresectable hepatocellular carcinoma | ||||||
| Patient or population: people with unresectable hepatocellular carcinoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TACE | Risk with EBRT + TACE | |||||
| All‐cause mortality (at maximum 1‐year follow‐up) | Study population | RR 0.51 | 786 | ⊕⊕⊝⊝ | ||
| 456 per 1000 | 233 per 1000 | |||||
| Health‐related quality of life | No data were available for this outcome. | |||||
| Serious adverse events | No data were available for this outcome. | |||||
| Complete response plus partial response Length of follow‐up: 1 year | Study population | RR 1.58 | 620 | ⊕⊕⊝⊝ | ||
| 518 per 1000 | 819 per 1000 | |||||
| Elevated alanine aminotransferase Length of follow‐up: 1 year | Study population | RR 1.41 | 232 | ⊕⊝⊝⊝ | ||
| 319 per 1000 | 449 per 1000 | |||||
| Elevated total bilirubin Length of follow‐up: 1 year | Study population | RR 2.69 | 172 | ⊕⊝⊝⊝ | ||
| 108 per 1000 | 292 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels (‐2) due to: i) within‐study risk of bias: high risk of bias in all included trials; ii) publication bias: cannot be assessed. | ||||||
| External beam radiotherapy (EBRT) versus transarterial chemoembolisation(TACE) for unresectable hepatocellular carcinoma | ||||||
| Patient or population: people with unresectable hepatocellular carcinoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TACE | Risk with EBRT | |||||
| All‐cause mortality (at 1 year) | Study population | RR 1.21 | 152 | ⊕⊝⊝⊝ | ||
| 570 per 1000 | 689 per 1000 | |||||
| Serious adverse events | No data were available for this outcome. | |||||
| Complete response plus partial response Length of follow‐up: 1 year | 10 out of 20 trial participants attained partial response in the TACE arm. | 3 out of 21 trial participants attained a response in the EBRT arm. | ‐ | 41 (1 RCT) | ⊕⊝⊝⊝ | |
| Elevated alanine aminotransferase | No data were available for this outcome. | |||||
| Elevated total bilirubin | No data were available for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded three levels (‐3) due to i) within‐study risk of bias: high risk of bias in all included trials; ii) publication bias: cannot be assessed; iii) imprecision: small number of trials. | ||||||
| Examples from table | Explanation |
| Outcomes | The tables provide the findings for the most important outcomes for someone making a decision. These include potential benefits and harms, whether the included studies provide data for these outcomes or not. Additional findings may be reported elsewhere in the review. |
| Assumed control group risk | Assumed control group risks can be based either on the control group risks reported in the included studies or on epidemiological data from elsewhere. When only one control group risk is provided, it is normally the median control group risk across the studies that provided data for that outcome. Risk is the probability of an outcome occurring. The control group risk is the risk of an outcome occurring in the comparison group (without the intervention). |
| Corresponding intervention group risk | Risk is the probability of an outcome occurring. The intervention group risk is the risk of an outcome occurring in the group receiving the intervention. |
| Relative effect | Relative effect or RR (risk ratio) Relative effects are ratios. Here the relative effect is expressed as a risk ratio. Risk is the probability of an outcome occurring. A RR is the ratio between the risk in the intervention group and the risk in the control group. If the risk in the control group is 10% (100 per 1000) and the risk in the intervention group is 1% (10 per 1000), the RR is 10/100 or 0.10. If the RR is exactly 1.0, this means that there is no difference between the occurrence of the outcome in the intervention and the control group. It is unusual for the RR to be exactly 1.0, and understanding what it means if it is above or below this value depends on whether the outcome being counted is judged to be good or bad. If the RR is greater than 1.0, the intervention increases the risk of the outcome. If it is a good outcome (e.g. the birth of a healthy baby), a RR > 1.0 indicates a desirable effect for the intervention, whereas if the outcome is bad (e.g. death), a RR > 1.0 indicates an undesirable effect. If the RR is less than 1.0, the intervention decreases the risk of the outcome. This indicates a desirable effect if it is a bad outcome (e.g. death) and an undesirable effect if it is a good outcome (e.g. birth of a healthy baby). |
| What is the difference between absolute and relative effects? The effect of an intervention can be described by comparing the risk of the intervention group with the risk of the control group. Such a comparison can be made in different ways. One way to compare two risks is to calculate the difference between the risks. This is the absolute effect. Consider the risk for blindness in a person with diabetes over a five‐year period. If the risk for blindness is found to be 20 in 1000 (2%) in a group of people treated conventionally and 10 in 1000 (1%) in people treated with a new drug, the absolute effect is derived by subtracting the intervention group risk from the control group risk: 2% ‐ 1% = 1%. Expressed in this way, it can be said that the new drug reduces the five‐year risk for blindness by 1% (absolute effect is 10 fewer per 1000). Another way to compare risks is to calculate the ratio of the two risks. Given the data above, the relative effect is derived by dividing the two risks, with the intervention risk being divided by the control risk: 1% ÷ 2% = ½ (0.50). Expressed in this way, as the 'relative effect', the five‐year risk for blindness with the new drug is 1/2 the risk with the conventional drug. Here the table presents risks as x per 1000 (or 100, etc.) instead of %, as this tends to be easier to understand. Whenever possible, the table presents the relative effect as the RR. The absolute effect is usually different for groups that are at high and low risk, whereas the relative effect is often the same, therefore, when it is relevant, we have reported indicative risks for groups at different levels of risk. Two or three indicative control group risks and the corresponding intervention group risks are presented when there are important differences across different populations. | |
| Mean difference | The mean difference (MD) is the average difference between the intervention group and the control group across studies. Here a weighted MD is used, which means the results of some of the studies make a greater contribution to the average than the results of others. Studies with more precise estimates for their results (narrower confidence intervals) are given more weight. This way of measuring effect is used when combining or comparing data for continuous outcomes such as weight, blood pressure, or pain measured on a scale. When different scales are used to measure the same outcome, e.g. different pain scales, a standardised mean difference (SMD) may be provided. This is a weighted mean difference standardised across studies giving the average difference in standard deviations for the measures of that outcome. |
| Confidence interval | A confidence interval (CI) is a range around an estimate that conveys how precise the estimate is; in this example the result is the estimate of the intervention group risk. The CI is a guide to how sure we can be about the quantity we are interested in (here the true absolute effect). The narrower the range between the two numbers, the more confident we can be about what the true value is; the wider the range, the less sure we can be. The width of the CI reflects the extent to which chance may be responsible for the observed estimate (with a wider interval reflecting more chance). |
| 95% confidence interval | As explained above, the CI indicates the extent to which chance may be responsible for the observed numbers. In the simplest terms, a 95% CI means that we can be 95% confident that the true size of effect is between the lower and upper confidence limit (e.g. 0 and 3 in the blindness drugs example mentioned above). Conversely, there is a 5% chance that the true effect is outside of this range. |
| Not statistically significant | Statistically significant means that a result is unlikely to have occurred by chance. The usual threshold for this judgement is that the results, or more extreme results, would occur by chance with a probability of less than 0.05 if the null hypothesis (no effect) was true. When results are not statistically significant, as in this example, this is stated to alert users to the possibility that the results may have occurred by chance. |
| No. of participants (studies) | The table provides the total number of participants across studies and the number of studies that provided data for that outcome. This indicates how much evidence there is for the outcome. |
| Quality of the evidence | The quality of the evidence is a judgement about the extent to which we can be confident that the estimates of effect are correct. These judgements are made using the GRADE system, and are provided for each outcome. The judgements are based on the type of study design (randomised trials versus observational studies), the risk of bias, the consistency of the results across studies, and the precision of the overall estimate across studies. For each outcome, the quality of the evidence is rated as high, moderate, low, or very low using the following definitions:
|
| ‐ | A "‐" indicates that the information is not relevant. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (at 1 year) Show forest plot | 9 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.41, 0.62] |
| 1.1 Studies using three dimensional conformal radiation therapy | 8 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.68] |
| 1.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.25, 0.64] |
| 2 Complete response Show forest plot | 7 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.47, 3.13] |
| 2.1 Studies using three dimensional conformal radiation therapy | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.34, 3.37] |
| 2.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.12, 4.21] |
| 3 Complete response + partial response Show forest plot | 7 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.40, 1.78] |
| 3.1 Studies using three dimensional conformal radiation therapy | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.31, 1.79] |
| 3.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.36, 1.99] |
| 4 Elevated alanine aminotransferase Show forest plot | 3 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.08, 1.84] |
| 5 Elevated total bilirubin Show forest plot | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.34, 5.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.97, 1.50] |

