Análogos de insulina de acción rápida subcutáneos para la cetoacidosis diabética
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011281.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 enero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Trastornos metabólicos y endocrinos
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Carlos A Andrade‐Castellanos (CAC): protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation.
Luis E Colunga‐Lozano (LCL): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates.
Netzahualpilli Delgado‐Figueroa (NDF): protocol drafting, acquiring trial reports, data analysis, data interpretation, review drafting, and future review updates.
Daniel A Gonzalez‐Padilla (DGP): acquiring trial reports, data analysis, review drafting, and future review updates.
Sources of support
Internal sources
-
Department of Emergency Medicine, Hospital Civil de Guadalajara "Dr. Juan I. Menchaca", Mexico.
Moral support
External sources
-
No sources of support supplied
Declarations of interest
CAC: none known.
LCL: none known.
NDF: none known.
DGP: none known.
Acknowledgements
We thank Karla Bergerhoff and Maria‐Inti Metzendorf, Trials Search Co‐ordinators of the Cochrane Metabolic and Endocrine Disorders Group, for developing the electronic search strategies.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jan 21 | Subcutaneous rapid‐acting insulin analogues for diabetic ketoacidosis | Review | Carlos A Andrade‐Castellanos, Luis Enrique Colunga‐Lozano, Netzahualpilli Delgado‐Figueroa, Daniel A Gonzalez‐Padilla | |
| 2014 Sep 01 | Subcutaneous rapid‐acting insulin analogues for diabetic ketoacidosis | Protocol | Carlos A Andrade‐Castellanos, Luis E Colunga‐Lozano, Netzahualpilli Delgado‐Figueroa, Daniel A Gonzalez‐Padilla | |
Notes
We have based parts of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review on a standard template established by the CMED Group.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Diabetic Ketoacidosis [*drug therapy];
- Hypoglycemic Agents [adverse effects, *therapeutic use];
- Injections, Subcutaneous;
- Insulin [therapeutic use];
- Insulin Aspart [therapeutic use];
- Insulin Lispro [therapeutic use];
- Insulin, Short‐Acting [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Child; Humans; Young Adult;
PICO
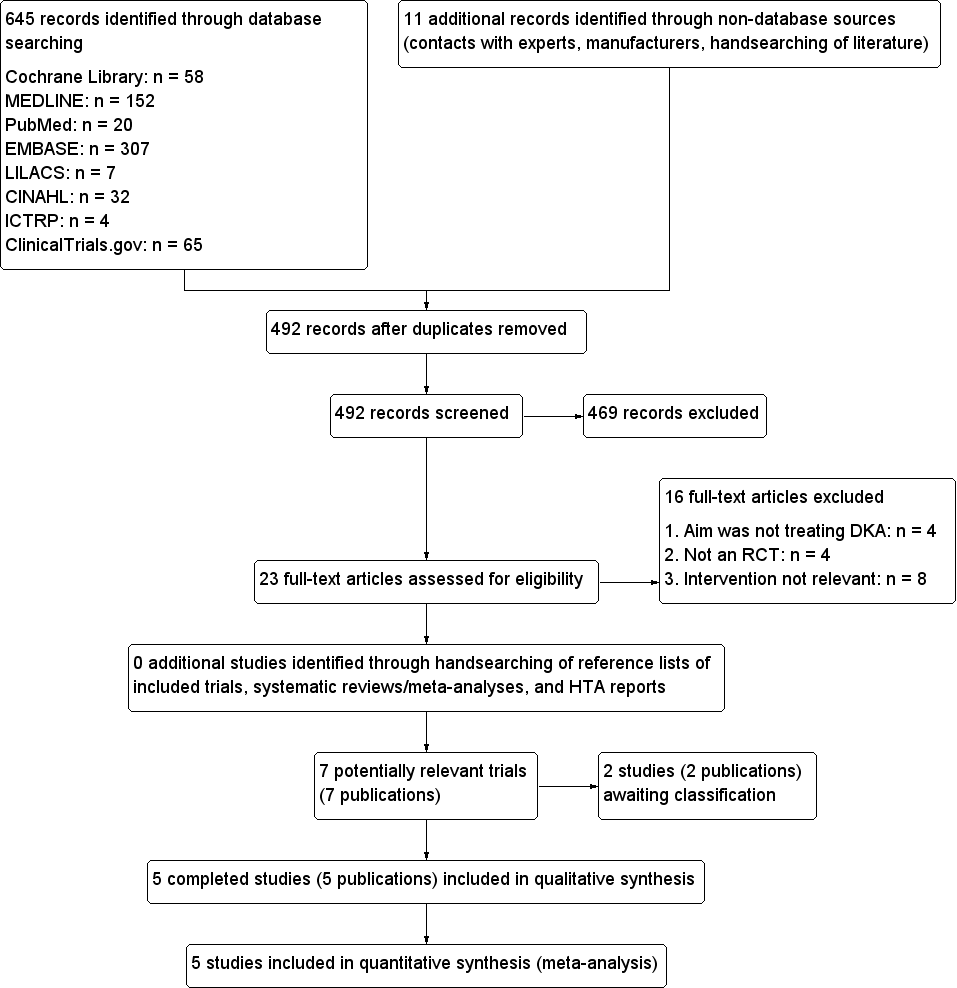
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some trials).

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome).
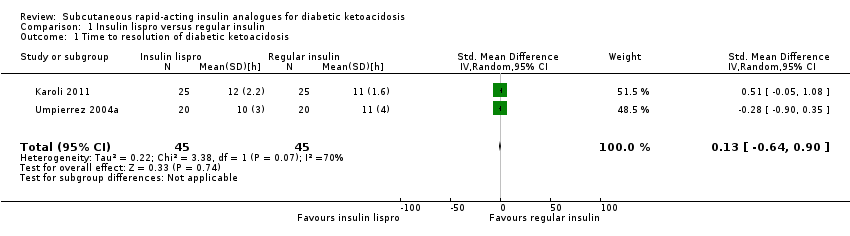
Comparison 1 Insulin lispro versus regular insulin, Outcome 1 Time to resolution of diabetic ketoacidosis.

Comparison 1 Insulin lispro versus regular insulin, Outcome 2 All‐cause mortality.

Comparison 1 Insulin lispro versus regular insulin, Outcome 3 Hypoglycaemic episodes.
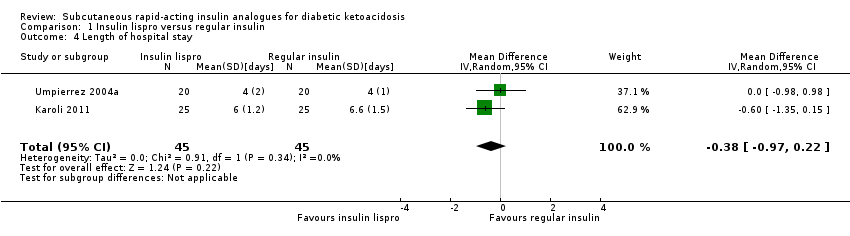
Comparison 1 Insulin lispro versus regular insulin, Outcome 4 Length of hospital stay.

Comparison 2 Insulin aspart versus regular insulin, Outcome 1 Time to resolution of diabetic ketoacidosis.
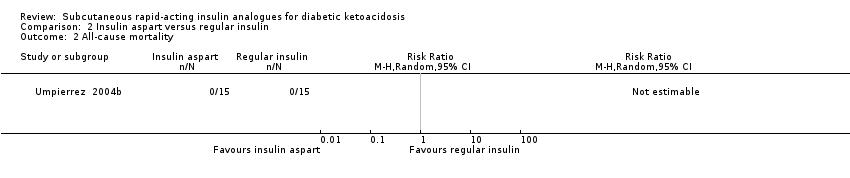
Comparison 2 Insulin aspart versus regular insulin, Outcome 2 All‐cause mortality.

Comparison 2 Insulin aspart versus regular insulin, Outcome 3 Hypoglycaemic episodes.

Comparison 2 Insulin aspart versus regular insulin, Outcome 4 Length of hospital stay.
| Subcutaneous insulin lispro versus intravenous regular insulin for diabetic ketoacidosis | ||||||
| Patient: participants with diabetic ketoacidosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous regular insulin | Subcutaneous insulin lispro | |||||
| All‐cause mortality (N) Mean hospital stay: 2‐7 days | See comment | See comment | Not estimable | 156 (4) | ⊕⊕⊕⊝ | No deaths reported |
| Hypoglycaemic episodes (N) Mean hospital stay: 2‐7 days | 118 per 1000 | 70 per 1000 | RR 0.59 | 156 (4) | ⊕⊕⊝⊝ | Comparable risk ratios for adults (4 trials) and children (1 trial) |
| Morbidity (N) Mean hospital stay: 2‐7 days | See comment | See comment | Not estimable | 96 (2) | See comment | No cases of cerebral oedema, venous thrombosis, adult respiratory distress syndrome, hyperchloraemic acidosis |
| Adverse events other than hypoglycaemic episodes | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Time to resolution of diabetic ketoacidosis (h) Mean hospital stay: 2‐4 days | The mean time to resolution of diabetic ketoacidosis across the intravenous regular insulin groups was 11 h | The mean time to resolution of diabetic ketoacidosis in the subcutaneous insulin lispro groups was 0.2 h higher (1.7 h lower to 2.1 h higher) | ‐ | 90 (2) | ⊕⊝⊝⊝ | Metabolic acidosis and ketosis took longer to resolve in the subcutaneous insulin lispro group in 1 trial (60 children); no exact data published |
| Patient satisfaction | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects: length of hospital stay (days) Mean hospital stay: 4‐7 days | The mean length of hospital stay in the intravenous regular insulin groups ranged between 4 and 6.6 days | The mean length of hospital stay in the subcutaneous insulin lispro groups was 0.4 days shorter (1 day shorter to 0.2 days longer) | ‐ | 90 (2) | ⊕⊕⊝⊝ | US setting: treatment of diabetic ketoacidosis in a non–intensive care setting (step‐down unit or general medicine ward) was associated with a 39% lower hospitalisation charge than was treatment with intravenous regular insulin in the intensive care unit (USD 8801 (SD USD 5549) vs USD 14,429 (SD USD 5243); the average hospitalisation charges per day were USD 3981 (SD USD 1067) for participants treated in an intensive care unit compared with USD 2682 (SD USD 636) for those treated in a non–intensive care setting |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Assumed risk was derived from the event rates in the comparator groups. | ||||||
| Subcutaneous insulin aspart versus intravenous regular insulin for diabetic ketoacidosis | ||||||
| Patient: participants with diabetic ketoacidosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous regular insulin | Subcutaneous insulin aspart | |||||
| All‐cause mortality (N) Mean hospital stay: 3‐5 days | See comment | See comment | Not estimable | 45 (1) | ⊕⊕⊝⊝ | No deaths reported |
| Hypoglycaemic episodes (N) Mean hospital stay: 3‐5 days | 67 per 1000 | 67 per 1000 | RR 1.00 | 30 (1) | ⊕⊕⊝⊝ | ‐ |
| Morbidity | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Adverse events other than hypoglycaemic episodes | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Time to resolution of diabetic ketoacidosis (h) Mean hospital stay: 3‐5 days | The mean time to resolution of diabetic ketoacidosis across the intravenous regular insulin groups was 11 h | The mean time to resolution of diabetic ketoacidosis in the subcutaneous insulin aspart group was 1 h lower (3.2 h lower to 1.2 h higher) | ‐ | 30 (1) | ⊕⊝⊝⊝ | ‐ |
| Patient satisfaction | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects: length of hospital stay (days) Mean hospital stay: 3‐5 days | The mean length of hospital stay in the intravenous regular insulin group was 4.5 days | The mean length of hospital stay in the subcutaneous insulin aspart group was 1.1 days shorter (3.3 days shorter to 1.1 days longer) | ‐ | 30 (1) | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Assumed risk was derived from the event rates in the comparator groups | ||||||
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible | Randomised | Analysed | Finishing trial | Randomised finishing trial | Follow‐up timeb | |
| Umpierrez 2004a | I: s.c. insulin lispro | Arbitrary estimation of a difference between groups of ≥ 5 hours to determine ketoacidosis as being clinically important; a sample size of 20 participants was needed in each group to provide a power of 0.93, given an alpha level of 0.05, a SD of 4, and a 1:1 inclusion ratio | ‐ | 20 | 20 | 20 | 100 | Mean hospital stay: 4 days |
| C: i.v. regular insulin | 20 | 20 | 20 | 100 | ||||
| total: | 40 | 40 | 40 | 100 | ||||
| Umpierrez 2004b | I1: s.c. insulin aspart, every hour | Arbitrary estimation of a difference between groups of ≥ 4 hours to determine ketoacidosis as being clinically significant. A sample size of 15 participants was needed in each group to provide a power of 0.81, given an alpha error of 0.05 and a SD of 3 | ‐ | 15 | 15 | 15 | 100 | Mean hospital stay: 3.4 days |
| I2: s.c. insulin aspart, every 2 h | 15 | 15 | 15 | 100 | Mean hospital stay: 3.9 days | |||
| C: i.v. regular insulin | 15 | 15 | 15 | 100 | Mean hospital stay: 4.5 days | |||
| total: | 45 | 45 | 45 | 100 | ||||
| Della Manna 2005 | I: s.c. insulin lispro | ‐ | ‐ | 25 | 25 | 25 | 100 | Mean hospital stay: 2‐3 days |
| C: i.v. regular insulin | 21 | 21 | 21 | 100 | ||||
| total: | 46 | 46 | 46 | 100 | ||||
| Ersöz 2006 | I: s.c. insulin lispro | ‐ | ‐ | 10 | 10 | 10 | 100 | ‐ |
| C: i.v. regular insulin | 10 | 10 | 10 | 100 | ||||
| total: | 20 | 20 | 20 | 100 | ||||
| Karoli 2011 | I: s.c. insulin lispro | ‐ | ‐ | 25 | 25 | 25 | 100 | Mean hospital stay: 6 days |
| C: i.v. regular insulin | 25 | 25 | 25 | 100 | Mean hospital stay: 6.6 days | |||
| total: | 50 | 50 | 50 | 100 | ||||
| Grand total | All interventions | 110 | 110 | |||||
| All comparators | 91 | 91 | ||||||
| All interventions and comparators | 201 | 201 | ||||||
| aAccording to power calculation in study publication or report ‐ denotes not reported C: comparator; I: intervention; i.v.: intravenous; s.c.: subcutaneous; SD: standard deviation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of diabetic ketoacidosis Show forest plot | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.64, 0.90] |
| 2 All‐cause mortality Show forest plot | 4 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Hypoglycaemic episodes Show forest plot | 4 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.52] |
| 3.1 Adults | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 3.94] |
| 3.2 Children | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.72] |
| 4 Length of hospital stay Show forest plot | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.97, 0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of diabetic ketoacidosis Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Hypoglycaemic episodes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

