Injerto de piel y reemplazo tisular para el tratamiento de las úlceras del pie en personas con diabetes
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre RCT (one foot and ankle clinic in the USA) with 16 weeks' follow‐up | |
| Participants | Twenty‐eight diabetic patients with Wagner grade 2, chronic non‐healing lower extremity wounds and present for at least 6 weeks Mean age: (SD) 61.43 (7.18) in intervention group, 66.21 (4.37) in control group Mean ulcer size: not stated Mean ulcer duration: not stated | |
| Interventions | Group 1 (n = 14): a human acellular regenerative tissue matrix onlay (Graft jacket) combined with sharp debridement Group 2 (n = 14): gauze dressings and sharp debridement | |
| Outcomes | 1. Incidence of complete wound closure after 16 weeks: Group 1: 12/14 (85.7%) Group 2: 4/14 (28.6%) 2. Mean time to complete wound closure: Group 1: 11.92 (2.87) weeks Group 2: 13.50 (3.42) weeks 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''full epithelization of the wound with the absence of drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''Patients were then randomized to one of two treatment groups'' Comment: method of generating the random schedule was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “All patients completed the 16‐week study” Comment: there were no dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "Stephen A. Brigido, DPM is a consultant for Wright Medical Technology" Comment: not clear if variables (e.g. ulcer size, ulcer duration) were balanced at baseline At least one of the authors is connected to a commercial organisation |
| Methods | Multicentred RCT (6 centres in Italy) with 11 weeks' follow‐up | |
| Participants | Seventy‐nine patients with non‐infected diabetic plantar and dorsal foot ulcers > 2 cm², Wagner grade 1 or 2 and without signs of healing for at least one month Mean age: not stated Mean ulcer size (SD): 5.3 cm² (6.76) in intervention group, 6.2 cm² (7.58) in control group Mean ulcer duration (SD): 4.0 months (10.0) in intervention group, 4.0 months (6.0) in control group | |
| Interventions | Group 1 (n = 43): autologous tissue‐engineered grafts (fibroblasts on Hyalograft 3D® and keratinocytes grown on Laserskin) Group 2 (n = 36): non‐adherent paraffin gauze with traditional absorbent secondary dressing | |
| Outcomes | 1. Incidence of complete wound closure after 11 weeks: Group 1: 60.4% (total 26/43; plantar 12/22 and dorsal 14/21) Group 2: 41.7% (total 15/36; plantar 10/20 and dorsal 15/36) 2. Median time to complete wound closure: Group 1: 57 days Group 2: 77 days 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''complete re‐epithelialization without residual exudate or crusting'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Randomization was done by telephone, and the randomization list was generated and held by the sponsor'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''Randomization was done by telephone, and the randomization list was generated and held by the sponsor'' Comment: allocation concealed using an independent central randomisation service |
| Blinding of participants and personnel (performance bias) | High risk | Quote: ''This was an open, stratified, randomized and controlled trial'' Comment: open‐label RCT, so no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: ''The primary efficacy parameters […] were evaluated by the investigators at every weekly visit'' Comment: blinding of investigators not likely in this open‐label RCT |
| Incomplete outcome data (attrition bias) | Low risk | Quote:''Details of discontinued patients are presented in Table 1'' Comment: the numbers and reasons for dropouts and withdrawals were balanced and described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was supported by a research grant from Fidia Advanced Biopolymers" Comment: funded by commercial organisation |
| Methods | Single‐centre RCT (podiatric practice in the USA) with 20 weeks' follow‐up | |
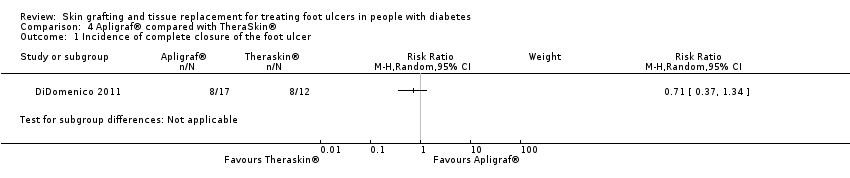
| Participants | Twenty‐nine wounds from 28 diabetic patients with Wagner 1 or Texas 1 diabetic foot ulcers with a size between 0.5 and 4 cm² and present for at least four weeks Mean age: not stated Mean ulcer size: 1.89 cm² in Apligraf®/BSS group, 1.82 cm² in TheraSkin®/SSA group Mean ulcer duration: not stated | |
| Interventions | Group 1 (n = 17): a bioengineered skin substitute (BSS; Apligraf®) Group 2 (n = 12): a split‐skin allograft (SSA; TheraSkin®) | |
| Outcomes | 1. Incidence of complete wound closure after 20 weeks: Group 1: 47.1% (exact numbers not stated, most likely 8/17) Group 2: 66.7% (exact numbers not stated, most likely 8/12) 2. Mean time to complete wound closure: Group 1: 6.86 (4.12) weeks Group 2: 5.00 (3.43) weeks 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: ''Due to an unintentional error in the randomization scheme, more patients were enrolled in the BSS group than in the SSA group.'' Comment: randomised, but with errors |
| Allocation concealment (selection bias) | Unclear risk | Comment: apparent block randomisation, but not specifically stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: authors gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was funded by Soluble Systems, LLC" Comment: funded by commercial organisation |
| Methods | International multicentre RCT (24 hospitals in the European Union and Australia) with 12 weeks' follow‐up for efficacy | |
| Participants | Eighty‐two patients with neuropathic diabetic foot ulcers with a size between 1 and 16 cm² and present for at least two weeks Mean age (SD) 56.4 (11.6) in intervention group, 60.6 (9.8) in control group Median ulcer size (range): 2.5 cm² ( 0.8 ‐ 9.3) in intervention group, 2.25 cm² (0.5 ‐ 10.0) in control group Median ulcer duration (range): 1.1 years (0.1 ‐ 8.0) in intervention group, 1.2 (2 weeks ‐ 7.0 years) in control group | |
| Interventions | Group 1 (n = 40; 33 in per‐protocol analysis): Apligraf®, living keratinocytes and fibroblasts Group 2 (n = 42; 39 in per‐protocol analysis): Standard therapy; polyamide and saline‐moistened gauze dressings | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 17/40 (42.5%) Group 2: 10/42 (23.8%) 2. Median time to complete wound closure: Group 1: 84 days Group 2: median could not be estimated 3. Total incidence of lower limb amputations: Not reported as separate outcome, but at least 1 transmetatarsal amputation occurred in group 2 | |
| Notes | Definition of complete closure: ''full epithelialization with no drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Eligible patients were randomized in a 1:1 ratio to either the Apligraf® group or the control group by means of sealed allocation cards'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''Eligible patients were randomized in a 1:1 ratio to either the Apligraf® group or the control group by means of sealed allocation cards'' Comment: allocation concealed using sealed allocation cards |
| Blinding of participants and personnel (performance bias) | High risk | Quote: ''This was a prospective, multicenter, randomized, controlled, open‐label study'' Comment: No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not stated, but Apligraf® was applied in addition to standard treatment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: ''One of the standard therapy subjects had a fractured femur following the baseline treatment visit, did not have a follow‐up efficacy visit, subsequently dropped out of the study'' Comment: all withdrawals and protocol violations are described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | Quote: ''Because of difficulties encountered during the registration process in the European Union, the study sponsor elected to prematurely halt enrolment in the study. This was done for external reasons, and was not due to any safety concerns'' Comment: study was stopped prematurely and at least one of the authors is connected to a commercial organisation |
| Methods | Multicentred RCT (5 centres in the USA) with a follow‐up period of 12 weeks for wound healing and a mean follow‐up period of 14 months for ulcer recurrence assessment | |
| Participants | Fifty patients with full‐thickness, diabetic foot ulcers of the plantar surface or heel > 1 cm² Mean age: group 1 62.7, group 2 66.2 and 62.7 years in group 3. In the control group the mean age was 53.8 years Median ulcer size was respectively 2.2, 2.3 and 3.3 cm² in the intervention groups and 1.9 cm² in the control group Mean ulcer duration in weeks was respectively 50.4, 40.7 and 43.2 weeks in the intervention groups and 87.0 weeks in the control group | |
| Interventions | Group 1 (n = 12): one piece of Dermagraft® applied weekly for a total of eight pieces and eight applications Group 2 (n = 14): two pieces of Dermagraft® applied every 2 weeks for a total of eight pieces and four applications Group 3 (n = 11): one piece of Dermagraft® applied every 2 weeks for a total of four pieces and four applications Group 4 (n = 13): conventional therapy | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 6/12 (50.0%) Group 2: 3/14 (21.4%) Group 3: 2/11 (18.2%) Group 4: 1/13 (7.7%) 2. Median time to complete wound closure: Group 1: 12 weeks; Group 2, 3 and 4: > 12 weeks 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''full epithelialization with no drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''When patients and their wounds met study criteria, they were given a study number, and sealed randomization envelopes were used to assign them to one of four study treatments'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''When patients and their wounds met study criteria, they were given a study number, and sealed randomization envelopes were used to assign them to one of four study treatments" Comment: allocation concealed using sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “This was a controlled prospective multicenter randomized single‐blinded pilot study'' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “This was a controlled prospective multicenter randomized single‐blinded pilot study'' Comment: single‐blinded but not specifically explained the blinding process |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Three Dermagraft patients died after 2, 6, and 11 months of follow‐up, respectively, at which time their ulcers had not recurred. […] the one healed control‐treated ulcer had not recurred after 2 months, after which the patient was lost to follow up" Comment: all patients completed the 12 week follow‐up, patients died or lost‐to‐follow‐up were described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "Advances Tissue Sciences, Inc., La Jolla, California, provided financial support for this study" Comment: ulcer duration and age were not balanced at baseline and the study was funded by a commercial organisation. Furthermore, there is no information about how these patients were randomised over the five institutions, which is relevant in this small patient sample |
| Methods | Multicentred RCT (4 foot clinics in the USA) with a follow‐up duration of 20 weeks | |
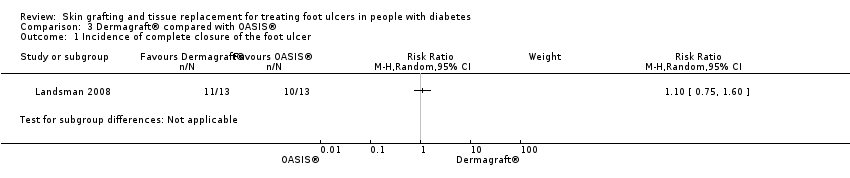
| Participants | Twenty‐six patients with neuropathic, full‐thickness diabetic foot ulcers with a size between 1 and 16 cm² and present for at least four weeks Mean age (SD) 63.4 (9.84) in Dermagraft group, 62.17 (12.17) in OASIS group. Mean ulcer size (SD): 1.88 cm² (1.39) in Dermagraft group, 1.85 cm² (1.83) in OASIS group. Mean ulcer duration: not stated | |
| Interventions | Group 1 (n = 13): Dermagraft, a living skin equivalent Group 2 (n = 13): OASIS, an acellular, porcine‐derived, bioactive collagen matrix material | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 11/13 (84.6%) Group 2: 10/13 (76.9%) 2. Mean time to complete wound closure: Group 1: 40.90 (32.32) days Group 2: 35.67 (41.47) days 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''full epithelialization without any evidence of drainage or bleeding'' A cost‐effectiveness analysis of this clinical trial is published by Gilligan 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was accomplished when investigative site identified a qualified candidate and contacted an independent site that randomly assessed patients to one of the two study arms” Comment: the method of generating the random sequence was not reported |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was accomplished when investigative site identified a qualified candidate and contacted an independent site that randomly assessed patients to one of the two study arms” Comment: allocation concealed using an central independent unit |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “In a randomized, non‐blinded study” Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “In a randomized, non‐blinded study” Comment: no blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: authors gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Unclear risk | Quote: “At subsequent weekly evaluations, the wound was cleaned, evaluated, photographed, and measured at each time point" Comment: change in ulcer area was not stated, but measurements are described in the method section |
| Other bias | Unclear risk | Comment: there is a different maximum number of treatments allowed in each group. This influences costs and, possibly, outcome; furthermore, at least one of the authors is connected to a commercial organisation |
| Methods | Multicentred RCT (8 centres in the USA) with 12 weeks’ follow‐up | |
| Participants | Forty patients with neuropathic diabetic foot ulcers, Texas grade 1A, with a size between 1 and 12 cm² and present for at least 30 days Mean age (SD) 59.0 (12.7) in intervention group, 57.4 (10.6) in control group Mean ulcer size (SD): 6.0 cm² (7.6) in intervention group, 5.5 cm² (4.3) in control group Mean ulcer duration (SD): 11.9 months (11.8) in intervention group, 12.2 months (10.8) in control group | |
| Interventions | Group 1 (n = 20): BCM Group 2 (n = 20): Standard care | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks (all wounds): Group 1: 7/20 (35.0%) Group 2: 4/20 (20.0%) 1a. Incidence of complete wound closure after 12 weeks for ulcers with baseline size ≤ 6 cm² Group 1: 7/15 (46.6%) Group 2: 3/13 (23.1%) 1b. Incidence of complete wound closure after 12 weeks for ulcers with baseline size ≥ 6cm² Group 1: 0/5 (0.0%) Group 2: 1/7 (14.3%) 2. Average time to complete wound closure: not reported 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''100% epithelialization with no drainage or need for absorbent dressing'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Those patients who continued to meet eligibility criteria were individually assigned to the BCM treatment group or to the control group according to a computer‐generated randomization code” |
| Allocation concealment (selection bias) | Low risk | Quote: “Those patients who continued to meet eligibility criteria were individually assigned to the BCM treatment group or to the control group according to a computer‐generated randomization code” Comment: allocation concealed using a computer‐generated randomization code |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “This study was an open label, multicenter, controlled, randomized, parallel‐group pilot study'' Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Film was processed at a central facility and read in randomized order by two separate wound care experts who were blinded to specific protocol, patient, and visit date.” Comment: the primary outcomes were assessed blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the numbers and reasons for dropouts and withdrawals were balanced and described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This work was supported by a grant from Ortec International" Comment: funded by commercial organisation |
| Methods | Multicentred RCT (35 centres in the USA) with 12‐week follow‐up | |
| Participants | 314 patients with chronic diabetic foot ulcers sized ≥ 1 cm² and present for at least two weeks Mean age (range): 55.8 (27‐83) in intervention group, 55.5 (31‐79) in control group Mean ulcer size (range): 2.31 cm² (0.75 ‐ 16.7) in intervention group, 2.53 cm² (0.5 ‐ 18.0) in control group Mean ulcer duration: 41 weeks in intervention group, 67 weeks in control group | |
| Interventions | Group 1 (n = 163; 130 per‐protocol): Dermagraft®, a cryopreserved human fibroblast derived dermal substitute Group 2 (n = 151: 115 per‐protocol): Saline‐moistened gauze, dry gauze and fixation sheets (Hypafix) | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 39/163 (23.9%) Group 2: 21/151 (13.9%) 2. Average time to complete wound closure: exact numbers not stated Quote: ''The Dermagraft treated group had a significantly faster time to complete wound closure than the control group (P = 0.04)'' 3. Total incidence of lower limb amputations: Group 1: 9/163 amputations or bone resections (5.5%) Group 2: 19/151 amputations or bone resections (12.6%) | |
| Notes | Definition of complete closure: ''full epithelialization of the wound with the absence of drainage" Results of this study are also published by Frykberg 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized into either the Dermagraft or the control group. Patients were not informed as to which treatment they received" Comment: multicentre study design suggests central randomisation procedure, but this was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: concealment method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The study was a prospective, single‐blind, randomized, controlled investigation'' Comment: patient was blinded, blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “The study was a prospective, single‐blind study'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “The reasons for discontinuation were comparable between the two treatment groups. The majority of the patients who discontinued had an adverse event requiring' treatment that warranted withdrawal from the study'' Comment: numbers and reasons of discontinuation are stated |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was supported by a research grant from Advanced Tissue Sciences, Inc., and Smith & Nephew, Inc" Comment: funded by commercial organisation |
| Methods | Multicentred RCT (20 centres in the USA) with 32 weeks of follow‐up | |
| Participants | 281 patients with neuropathic, full‐thickness diabetic foot ulcers with a size ≥ 1 cm² and present for at least two weeks Baseline comparability for age, ulcer size and ulcer duration was not reported | |
| Interventions | Group 1 (n = 139 randomised, 109 per‐protocol): Dermagraft®, a three‐dimensionally cultivated human diploid fibroblast cells on a polymer scaffold Group 2 (n = 142 randomised; 126 per‐protocol): standard therapy only | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 42/109 (38.5%; 30.2% of all 139 patients randomised) Group 2: 40/126 (31.7%; 28.2% of all 142 patients randomised) 2. Median time to complete wound closure: Group 1: 13 weeks Group 2: 28 weeks 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''full epithelialization of the wound with absence of drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''A prospective, randomised controlled single‐blind design was used'' Comment: insufficient information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: concealment method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Single‐blind study'' Comment: patient was blinded, blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “The study was a prospective, single‐blind study'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: ''A total of 281 patients were enrolled in this study (139 Dermagraft®, 142 control); of these, 235 patients (83.6%) could be evaluated for the primary effectiveness endpoint (109 Dermagraft®, 126 control)'' Comments: reasons for drop‐outs were not adequately described |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary outcome changed to the number of 'active implants' resulting in less patients receiving the best treatment |
| Other bias | Unclear risk | Quote: ''The study enrolled diabetic patients with neuropathic full‐thickness plantar surface foot ulcers of the forefoot or heel, 31.0 cm2 in size, and eliminated ulcers that showed initial rapid healing in response to standard care during a screening period'' Comment: patients were included in the study with ulcers that initially did not respond to standard treatment. This might result in ulcers being in the control group that already had not responded to standard treatment. Furthermore, basic demographic information is not shown and the study was funded by a commercial organisation as authors are supported by Advanced Tissue Sciences, Inc |
| Methods | Single‐centre RCT (one foot centre in Thailand) with 6 months of follow‐up | |
| Participants | Eighty diabetic patients with infected lower extremity wounds Mean age (SD): 56.84 (8.96) in meshed skin graft group, 55.02 (10.12) in split‐skin graft group Mean ulcer size (SD): 104.24 cm² (152.0) in meshed skin graft group, 82.0 cm² (73.07) in split‐skin graft group Mean ulcer duration: not stated | |
| Interventions | Group 1 (n = 36): Meshed skin graft Group 2 (n = 44): Split‐skin graft | |
| Outcomes | 1. Incidence of complete wound closure after 6 months: not specifically stated, but this seems to be 100% in both groups 2. Mean time to complete wound closure: Group 1: 19.84 (7.37) days Group 2: 20.36 (7.21) days 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: Excellent (95% < 14 days with smooth scar); good (< 21 days), fair (> 21 days), or poor (> 28 days with poor scar or recurrence) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This prospective randomized control study'' Comment: insufficient information about the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “This prospective randomized control study'' Comment: insufficient information about the randomisation procedure |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient information about dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: complete wound healing was not assessed as primary outcome parameter |
| Other bias | Unclear risk | Comment: statistical analysis is dubious (t‐test) |
| Methods | Multicentred RCT (11 centres in the USA) with 12 weeks’ follow‐up | |
| Participants | Eighty‐six patients with diabetic foot ulcers, University of Texas grade 1 or 2, with a size between 1 and 25 cm² Mean age (SD): 55.4 (9.6) in intervention group, 58.9 (11.6) in control group Mean ulcer size (SD): 3.6 cm² (4.3) in intervention group, 5.1 cm² (4.8) in control group Mean ulcer duration (SD): 23.3 weeks (22.4) in intervention group, 22.9 weeks (29.8) in control group | |
| Interventions | Group 1 (n = 47; 46 per‐protocol): Graftjacket®, a human acellular dermal tissue matrix Group 2 (n = 39): Standard care | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 32/47 (68.1%) Group 2: 18/39 (46.2%) 2. Mean time to complete wound closure: Group 1: 5.7 weeks (SD 3.5) Group 2: 6.8 weeks (SD 3.3) 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''100% re‐epithelialisation without drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:''A prospective, randomised, multicenter study'' Comment: insufficient information about the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote:''A prospective, randomised, multicenter study'' Comment: insufficient information about the method of randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Patients were evaluated by the investigators at least once every 7 days to obtain ulcer measurements and to perform dressing changes" Comment: investigators actively participated in treatment and assessments |
| Incomplete outcome data (attrition bias) | Low risk | Comment: numbers and reasons of discontinuation are shown in figure 1 |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This clinical trial was supported by Wright Medical Technology, Inc." Comment: funded by commercial organisation |
| Methods | Multicentred RCT (2 hospital‐based wound care centres in the USA) with 20 weeks of follow‐up | |
| Participants | Twenty‐three patients with full‐thickness diabetic foot ulcers with a size between 1 and 10 cm² and present for at least 30 days Mean age (SD): 56.58 (14.96) in HFDS group, 60.0 (15.74) in HSA group Mean ulcer size (SD): 4.78 cm² (3.95) in HFDS group, 5.45 cm² (5.58) in HSA group Mean ulcer duration (SD): 11.71 weeks (8.02) HFDS group, 43.58 weeks (78.08) in HSA | |
| Interventions | Group 1 (n = 12): HFDS, an invitro‐engineered, human fibroblast‐derived dermal skin substitute (Dermagraft®) Group 2 (n = 11): HSA, a biologically active cryopreserved human skin allograft (TheraSkin®) | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 4/12 (33.3%) Group 2: 7/11 (63.6%) 2. Mean time to complete wound closure: Group 1: 12.5 weeks (range 7‐20 weeks) Group 2: 8.9 (range 5‐20) 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''only fully epithelialized wounds were considered healed'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a series of sealed envelopes that designated the biologically active treatment to be applied" Comment: allocation concealed using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Quote: "Envelopes were randomized in blocks of six; however, the investigators were unaware of the block size or randomization scheme" Comment: allocation concealed using sealed envelopes and the investigators were unaware of the randomisation scheme |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Because the grafts have a different physical appearance, it was not possible to disguise the type of graft used at the time of evaluation" Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Because the grafts have a different physical appearance, it was not possible to disguise the type of graft used at the time of evaluation" Comment: blinding of outcome assessment was not possible |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: authors gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "Dr. Sanders and Dr. A. Landsman are paid consultants" Comment: at least one of the authors is connected to a commercial organisation |
| Methods | Multicentred RCT (7 specialised diabetic foot centres in Italy) with 20 weeks of follow‐up for efficacy and 18 months for safety | |
| Participants | One hundred and eighty patients with diabetic foot ulcers, Wagner grades 1 or 2, with a size ≥ 2 cm² and present for at least 1 month Mean age (SD): 61 (10) in intervention group, 62 (11) control group Mean ulcer size (SD): 8.8 cm² (9.4) in intervention group, 6.7 cm² (7.7) in control group Mean ulcer duration (SD): 6.82 months (5.09) in intervention group, 5.43 months (4.83) in control group | |
| Interventions | Group 1 (n = 90, 80 in analyses): Hyalograft 3D® and Laserskin® autograft Group 2 (n = 90, 80 in analyses): Non‐adherent paraffin gauze, control group | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 19/90 (21.1%) Group 2: 17/90 (18.9%) 2. Mean time to complete wound closure: Group 1: 50 days Group 2: 58 days 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''complete reepithelialization without exudates ad eschar'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''At the first visit, eligible patients were randomized in a 1:1 ratio, using a computer‐generated method, in a block |
| Allocation concealment (selection bias) | Low risk | Quote: ''For randomization, each site used sealed envelopes opened in numerical order'' |
| Blinding of participants and personnel (performance bias) | High risk | Quote: ''This was an open, randomized, controlled study'' |
| Blinding of outcome assessment (detection bias) | High risk | Comment: investigators actively participated in treatment and assessments |
| Incomplete outcome data (attrition bias) | High risk | Quote: ''A total of 180 patients were screened and randomized (n = 90 per group). Of these, 7 patients had an ulcer area < 1 cm2 after the run‐in period and were excluded, and 13 patients did not return to the investigational site after the baseline visit. Thus, 160 patients were included in the intention‐to treat analysis (n = 80 per group)'' Comment: all randomised patients should have been included in the intention‐to treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote 1: ''It was ended prematurely because of the low enrolment with fewer number of randomized patients than initially planned, with larger ulcers at baseline in the treated group, which may have underpowered the trial and included hard‐to‐heal ulcers in the treated group'' Quote 2: "This study was supported by a research grant from Anika Therapeutics srl" Comment 1: the study was prematurely ended and funded by commercial organisation Comment 2: basic demographic information is not shown Comment 3: only patients not healed after 2 weeks of control treatment were enrolled |
| Methods | Multicentred (24 centres in the USA) RCT with 12 weeks of follow‐up | |
| Participants | 208 patients with non‐infected, non‐ischaemic neuropathic diabetic foot ulcers with a size between 1 and 16 cm² and present for at least two weeks Mean age (SD): 58 (10) in intervention group, 56 (10) control group Mean ulcer size (SD): 2.97 cm² (3.10) in intervention group, 2.83 cm² (2.45) in control group Mean ulcer duration (SD): 11.5 months (13.3) in intervention group, 11.1 months (12.5) in control group | |
| Interventions | Group 1 (n = 112): Graftskin® Group 2 (n = 96): Saline‐moistened gauze, control group | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 63/112 (56.3%) Group 2: 36/96 (37.5%) 2. Median time to complete wound closure: Group 1: 65 days Group 2: 90 days 3. Total incidence of lower limb amputations (on study limb) Group 1: 7/112 (6.3%) Group 2: 15/96 (15.6%) | |
| Notes | Definition of complete closure: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Patients were randomized at the end of the screening visit according to a computer generated randomization schedule provided by the sponsor'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''For randomization, each site used sealed envelopes opened in numerical order'' |
| Blinding of participants and personnel (performance bias) | High risk | Quote:''Patients were informed about the results of randomization during their next visit'' Comment: participants and clinicians were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: ''Complete dressing changes were performed by the investigator at visits scheduled for study weeks 1, 2, 3, and 4'' Comment: investigators actively participated in treatment and assessments |
| Incomplete outcome data (attrition bias) | Low risk | Comment: numbers and reasons of discontinuation are described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Comment: patients were pretreated with moist saline gauze and the non‐responders were then randomised; control patients again received moist saline, who had already shown not to respond Furthermore, the study was funded by a commercial organisation |
| Methods | Multicentred RCT (three university hospitals in Korea) with a follow‐up duration of 12 weeks for efficacy and 6 months for safety and recurrence | |
| Participants | Fifty‐nine patients with diabetic foot ulcers, Texas grade 1 or 2, with a size of ≥ 1 cm² and without signs of healing for at least six weeks Mean age (SD) in per‐protocol set: 63.5 (9.0) in intervention group, 62.4 (9.4) control group Mean ulcer size (SD) in per‐protocol set: 4.0 cm² (3.5) in intervention group, 5.2 cm² (6.4) in control group Mean ulcer duration (SD) in per‐protocol set: 0.33 years (0.24) in intervention group, 0.40 years (0.68) in control group | |
| Interventions | Group 1 (n = 27, 20 in per‐protocol set): Allogenic keratinocyte treatment Group 2 (n = 32, 26 in per‐protocol set): Vaseline gauze, control group | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 20/27(100% in per‐protocol group; 74.1% in intention‐to treat analysis, authors report 85%) Group 2: 18/32 (69.2% in per‐protocol group; 56.3% in intention‐to treat analysis, authors report 59%) 2. Mean time to complete wound closure Group 1 (SD): 41.6 (26.1) days in intention‐to treat analysis Group 2 (SD): 43.6 (19.4) days in intention‐to treat analysis 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Randomization schedules were stratified according to clinical center with the use of a permuted‐block method with a block size of four to six using the Statistical Analysis System and treatment allocation ratio of 1:1 and stratification at the three sites'' |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information concerning method of concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: ''Wound evaluation was performed in a single‐blinded fashion. The patients did not know whether or not their wounds had been treated with the keratinocytes, but the wound evaluators were aware of the method of treatment'' Comment: patient was blinded, blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) | High risk | Quote: ''Wound evaluation was performed in a single‐blinded fashion. The patients did not know whether or not their wounds had been treated with the keratinocytes, but the wound evaluators were aware of the method of treatment'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Comment: numbers and reasons of discontinuation are shown in figure 1. However, not all exact numbers in intention‐to treat analysis were reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: not all exact numbers in intention‐to treat analysis were reported |
| Other bias | Unclear risk | Quote: "This study was supported by grants from Tego Science" Comment: funded by commercial organisation |
| Methods | Multicentre RCT (two university hospitals in Korea) with 12 weeks of follow‐up | |
| Participants | Sixty‐five patients with diabetic foot ulcers, Wagner grade 1 or 2, with a size ≥ 1 cm² and without signs of healing for at least six weeks Mean age (SD): 61.2 (11.4) in intervention group, 63.8 (10.7) in control group Mean ulcer size (SD): 3.5 cm² (3.7) in intervention group, 2.9 cm² (2.7) in control group Mean ulcer duration (SD): 6.1 months (16.4) in intervention group, 6.2 months (19.7) in control group | |
| Interventions | Group 1 (n = 33; 31 per‐protocol): autologous fibroblast‐hyaluronic acid complex Group 2 (n = 32): polyurethane foam dressing | |
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 26/33 (78.79%) Group 2: 11/32 (34.38%) 2. Mean time to complete wound closure for patients that healed: Group 1: 36.4 days (SD 17.6) Group 2: 48.4 days (SD 13.1) 3. Total incidence of lower limb amputations: not reported | |
| Notes | Definition of complete closure: ''a completely epithelialised state in the absence of any discharge and which allowed the patient to shower'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomiation schedules were stratified with the use of a permuted block method with a block size of four to six using the statistical analysis system and a treatment allocation ratio of 1:1" |
| Allocation concealment (selection bias) | Unclear risk | Quote: Randomiation schedules were stratified with the use of a permuted block method with a block size of four to six using the statistical analysis system and a treatment allocation ratio of 1:1" Comment: insufficient information about the concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not stated, but blinding not likely |
| Incomplete outcome data (attrition bias) | High risk | Quote: ''Two patients in the treatment group were excluded before application of the autologous fibroblast‐hyaluronic acid complex owing to contamination during cell culture Comment: numbers and reasons of discontinuation are shown in figure 2A. However, all randomised patients should have been included in the intention‐to treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was supported by grants from ChaBio & Diostec" Comment: funded by commercial organisation |
| Methods | Single‐centre RCT (one hospital in the USA) with 6 weeks of follow‐up | |
| Participants | Twenty‐five patients with diabetic foot ulcers between 1 and 25 cm² and present for at least four weeks Mean age (SD): 56.4 (14.7) in intervention group, 61.7 (10.3) in control group Mean ulcer size (SD): 2.6 cm² (1.9) in intervention group, 3.4 cm² (2.9) in control group Mean ulcer duration (SD): 14.1 weeks (13.0) in intervention group, 16.4 weeks (15.5) in control group | |
| Interventions | Group 1 (n = 13): Dehydrated human amniotic membrane (EpiFix®) Group 2 (n = 12): Moist wound therapy, standard care | |
| Outcomes | 1. Incidence of complete wound closure after 6 weeks: Group 1: 12/13 (92.3%) Group 2: 1/12 (8.3%) 2. Mean time to complete wound closure for patients that healed: Group 1: 2.5 weeks (SD 1.9, n = 12) Group 2: 5 weeks (n = 1) 3. Total incidence of lower limb amputations: no amputations reported | |
| Notes | Definition of complete closure: ''complete epithelialisation of the open area of the wound'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial [...] The randomisation schedule was balanced and permuted in blocks of 10'' |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information concerning the concealment of the allocation schedule |
| Blinding of participants and personnel (performance bias) | High risk | Quote: ''A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial'' Comment: participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: ''A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: ''Patients were exited from the study and allowed to seek alternative treatment if the index ulcer did not achieve 50% area reduction at 6 weeks'' 'Comment: no dropouts were reported at the 6‐week time point, one dropout was reported at the final endpoint after 12 weeks. After 65 weeks there were still 12 patients in the EpiFix® and 13 in the standard care group |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Comment: surgical debridement of all necrotic tissue was performed only in EpiFix® group. Furthermore, at least one of the authors is connected to a commercial organisation |
BCM: bilayered cellular matrix
BSS: bioengineered skin substitute
HFDS: human fibroblast‐derived dermal skin
HSA: human skin allograft
RCT: randomised controlled trial
SD: standard deviation
SSA: split‐thickness skin substitute
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Single‐centre results from multicentre study (Marston 2003) | |
| No outcome data before cross‐over point | |
| The use of a recombinant human platelet‐derived growth factor in the control group | |
| Single‐centre results from multicentre study (Veves 2001) | |
| Single‐centre results from multicentre study (Veves 2001) |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Trial of dehydrated human amnion/chorion membrane (dHACM) In the management of diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | EpiFix® and standard of care |
| Outcomes | Percentage of subjects with complete closure of the study ulcer |
| Starting date | July 2012 |
| Contact information | William Tettelbach, Intermountain Medical Center, Myrray, Utah, USA |
| Notes | Recruiting |
| Trial name or title | Study of ReCell® treating for diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | ReCell® with skin graft (experiment group) versus skin graft (control group) |
| Outcomes | Healing rate by week 4, recurrent rate at 6 months, complication rate at week 4 |
| Starting date | March 2013 |
| Contact information | Hu Zhicheng, First Affiliated Hospital, Sun Yat‐Sen University |
| Notes | Recruiting |
| Trial name or title | A randomized comparison of AmnioClear™ human allograft amniotic membrane versus moist wound dressing in the treatment of diabetic wounds |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | AmnioClear™ human allograft amniotic membrane versus standard moist wound dressing |
| Outcomes | Reduction in wound size at week 12 |
| Starting date | May 2014 |
| Contact information | Cameron Howes, Duke University |
| Notes | Not yet recruiting |
| Trial name or title | Allogenic dermis versus standard care in the management of diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Application of human allogenic dermis with dressing application |
| Outcomes | Proportion of ulcers completely healed ulcers at 6 weeks |
| Starting date | December 2014 |
| Contact information | Charles M Zelen, Professional Education and Research Institute, Roanoke, Viginia, USA |
| Notes | Recruiting |
| Trial name or title | Study of amniotic membrane graft in the management of diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Amniotic membrane/amnioband |
| Outcomes | Proportion healed wounds at 4 and 12 weeks. Mean time to healing and cost‐effectiveness |
| Starting date | March 2015 |
| Contact information | Lawrence Didomenico, Lower Extremity Institute of Research and Therapy, Canfield, Ohio, USA |
| Notes | Recruiting |
ABI: ankle brachial index
ADA: American Diabetes Association
UT: University of Texas Diabetic Wound Classification
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 13 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.30, 1.85] |
| Analysis 1.1  Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 1 Incidence of complete closure of the foot ulcer. | ||||
| 1.1 Apligraf® or Graftskin® | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.17, 2.04] |
| 1.2 Dermagraft® | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.85, 2.65] |
| 1.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 11.08 [1.69, 72.82] |
| 1.4 Graftjacket® | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.97, 3.71] |
| 1.5 Hyalograft 3D® | 3 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.06, 2.33] |
| 1.6 Kaloderm® | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.90, 1.92] |
| 1.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.61, 5.05] |
| 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis Show forest plot | 6 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.21, 1.85] |
| Analysis 1.2  Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis. | ||||
| 3 Incidence of lower limb amputations Show forest plot | 2 | 522 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.01] |
| Analysis 1.3  Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 3 Incidence of lower limb amputations. | ||||
| 3.1 Graftskin® | 1 | 208 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.18, ‐0.01] |
| 3.2 Dermagraft® | 1 | 314 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Ulcer recurrence Show forest plot | 4 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.22] |
| Analysis 1.4  Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 4 Ulcer recurrence. | ||||
| 4.1 Apligraf® or Graftskin® | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.18, 2.35] |
| 4.2 Dermagraft® | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 13.68] |
| 5 Incidence of infection Show forest plot | 9 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.98] |
| Analysis 1.5  Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 5 Incidence of infection. | ||||
| 5.1 Apligraf® or Graftskin® | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.76] |
| 5.2 Dermagraft® | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 5.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.52] |
| 5.4 Graftjacket® | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.18, 2.04] |
| 5.5 Hyalograf 3D® | 1 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.90] |
| 5.6 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.12, 3.24] |
| 5.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Meshed skin graft compared with split‐skin graft, Outcome 1 Incidence of complete closure of the foot ulcer. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Dermagraft® compared with OASIS®, Outcome 1 Incidence of complete closure of the foot ulcer. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Apligraf® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Dermagraft® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer. | ||||

Study flow diagram of the number of records identified, included and excluded, and the reasons for exclusion

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Skin grafts or tissue replacements compared with standard care, outcome: 1.1 Incidence of complete closure of the foot ulcer.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 1 Incidence of complete closure of the foot ulcer.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 3 Incidence of lower limb amputations.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 4 Ulcer recurrence.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 5 Incidence of infection.

Comparison 2 Meshed skin graft compared with split‐skin graft, Outcome 1 Incidence of complete closure of the foot ulcer.
Comparison 3 Dermagraft® compared with OASIS®, Outcome 1 Incidence of complete closure of the foot ulcer.
Comparison 4 Apligraf® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer.

Comparison 5 Dermagraft® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer.
| Skin grafts and tissue replacements compared to placebo or standard care for treating foot ulcers in people with diabetes | ||||||
| Patient or population: People with diabetes who have foot ulcers | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Skin grafts and tissue replacement products | |||||
| Incidence of complete closure of the ulcer (healing rate) Follow‐up: 6 to 16 weeks | 273 per 1000 | 423 per 1000 (354 to 504) | RR 1.55 (1.30 to 1.85) | 1472 | ⊕⊕⊝⊝ | Downgraded to low quality of evidence due to lack of blinding, industry involvement and possible publication bias. Furthermore we found wide confidence intervals for a number of comparisons (imprecision). |
| Time to complete closure of the ulcer | N/A | N/A | N/A | 0 (0 studies) | N/A | Time to compete healing of the ulcer was reported very heterogeneously. The majority of studies did not used survival analysis and reported hazard ratios, so meta‐analysis was not possible for this outcome and grading the quality of the evidence was not applicable |
| Total incidence of lower limb amputations Follow‐up: 12 weeks | 109 per 1000 | 47 per 1000 (25 to 89) | RR 0.43 (0.23 – 0.81) | 522 | ⊕⊝⊝⊝ | Downgraded by three levels because only two studies reported on this outcome (imprecision) and possible publication bias is present. Furthermore, a longer follow‐up period is necessary to estimate the effect more precisely. |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 13 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.30, 1.85] |
| 1.1 Apligraf® or Graftskin® | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.17, 2.04] |
| 1.2 Dermagraft® | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.85, 2.65] |
| 1.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 11.08 [1.69, 72.82] |
| 1.4 Graftjacket® | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.97, 3.71] |
| 1.5 Hyalograft 3D® | 3 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.06, 2.33] |
| 1.6 Kaloderm® | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.90, 1.92] |
| 1.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.61, 5.05] |
| 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis Show forest plot | 6 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.21, 1.85] |
| 3 Incidence of lower limb amputations Show forest plot | 2 | 522 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.01] |
| 3.1 Graftskin® | 1 | 208 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.18, ‐0.01] |
| 3.2 Dermagraft® | 1 | 314 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Ulcer recurrence Show forest plot | 4 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.22] |
| 4.1 Apligraf® or Graftskin® | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.18, 2.35] |
| 4.2 Dermagraft® | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 13.68] |
| 5 Incidence of infection Show forest plot | 9 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.98] |
| 5.1 Apligraf® or Graftskin® | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.76] |
| 5.2 Dermagraft® | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 5.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.52] |
| 5.4 Graftjacket® | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.18, 2.04] |
| 5.5 Hyalograf 3D® | 1 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.90] |
| 5.6 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.12, 3.24] |
| 5.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |

