Injerto de piel y reemplazo tisular para el tratamiento de las úlceras del pie en personas con diabetes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011255.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 febrero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Katrien Santema: conceived, designed and co‐ordinated the review; extracted data and checked quality of data extraction; undertook and checked quality assessment; interpreted and analysed data; performed statistical analysis and checked quality of statistical analysis; wrote and edited the review, including writing the first draft; approved the final review prior to submission; and wrote to study authors/experts/companies.
-
Paul Poyck: extracted data and checked quality of data extraction; undertook and checked quality assessment; interpreted and analysed data; performed statistical analysis and checked quality of statistical analysis; performed part of writing and editing the review; and approved the final review prior to submission.
-
Dirk Ubbink: extracted data and checked quality of data extraction; undertook and checked quality assessment; interpreted and analysed data; performed statistical analysis and checked quality of statistical analysis; performed part of writing and editing the review; and approved the final review prior to submission.
Contributions of editorial base
-
Joan Webster (Editor): edited the protocol and review, advised on methodology, interpretation and content and approved the final protocol prior to submission.
-
Sally Bell‐Syer and Gill Rizzello (Managing Editors): co‐ordinated the editorial process; advised on interpretation and content; edited the protocol and review.
-
Amanda Briant: designed the search strategy and edited the search methods section. Rocio Rodriguez ran the searches.
Sources of support
Internal sources
-
Academic Medical Centre at the University of Amsterdam, Amsterdam, Netherlands.
Salary
External sources
-
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
-
Katrien Santema: none known.
-
Paul Poyck: none known.
-
Dirk Ubbink: none known.
Acknowledgements
The authors would like to thank editors Andrea Nelson and Andrew Jull and peer reviewers Debra Fayter, Joyce Black, Devi Prasad Mohapatra, Sharon Van Wicklin, Duncan Chambers, Malcolm Brewster and Gill Worthy. Thanks also to copy editors Elizabeth Royle and Clare Dooley.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Feb 11 | Skin grafting and tissue replacement for treating foot ulcers in people with diabetes | Review | Trientje B Santema, Paul PC Poyck, Dirk T Ubbink | |
| 2014 Aug 24 | Skin grafting and tissue replacement for treating foot ulcers in people with diabetes | Protocol | Trientje B Santema, Paul PC Poyck, Dirk T Ubbink | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram of the number of records identified, included and excluded, and the reasons for exclusion

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Skin grafts or tissue replacements compared with standard care, outcome: 1.1 Incidence of complete closure of the foot ulcer.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 1 Incidence of complete closure of the foot ulcer.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 3 Incidence of lower limb amputations.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 4 Ulcer recurrence.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 5 Incidence of infection.

Comparison 2 Meshed skin graft compared with split‐skin graft, Outcome 1 Incidence of complete closure of the foot ulcer.
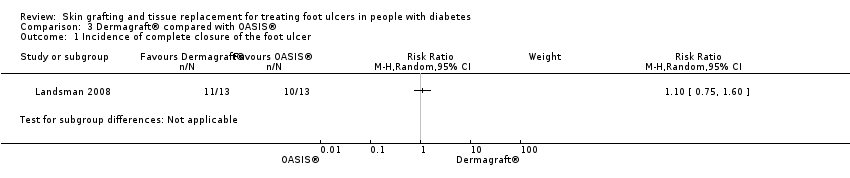
Comparison 3 Dermagraft® compared with OASIS®, Outcome 1 Incidence of complete closure of the foot ulcer.
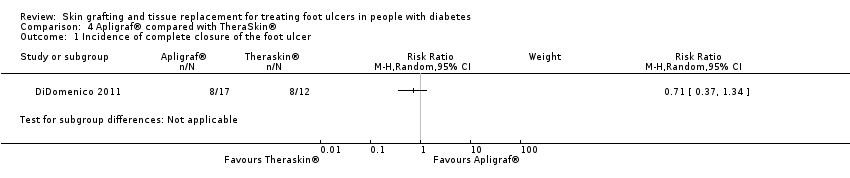
Comparison 4 Apligraf® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer.

Comparison 5 Dermagraft® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer.
| Skin grafts and tissue replacements compared to placebo or standard care for treating foot ulcers in people with diabetes | ||||||
| Patient or population: People with diabetes who have foot ulcers | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Skin grafts and tissue replacement products | |||||
| Incidence of complete closure of the ulcer (healing rate) Follow‐up: 6 to 16 weeks | 273 per 1000 | 423 per 1000 (354 to 504) | RR 1.55 (1.30 to 1.85) | 1472 | ⊕⊕⊝⊝ | Downgraded to low quality of evidence due to lack of blinding, industry involvement and possible publication bias. Furthermore we found wide confidence intervals for a number of comparisons (imprecision). |
| Time to complete closure of the ulcer | N/A | N/A | N/A | 0 (0 studies) | N/A | Time to compete healing of the ulcer was reported very heterogeneously. The majority of studies did not used survival analysis and reported hazard ratios, so meta‐analysis was not possible for this outcome and grading the quality of the evidence was not applicable |
| Total incidence of lower limb amputations Follow‐up: 12 weeks | 109 per 1000 | 47 per 1000 (25 to 89) | RR 0.43 (0.23 – 0.81) | 522 | ⊕⊝⊝⊝ | Downgraded by three levels because only two studies reported on this outcome (imprecision) and possible publication bias is present. Furthermore, a longer follow‐up period is necessary to estimate the effect more precisely. |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 13 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.30, 1.85] |
| 1.1 Apligraf® or Graftskin® | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.17, 2.04] |
| 1.2 Dermagraft® | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.85, 2.65] |
| 1.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 11.08 [1.69, 72.82] |
| 1.4 Graftjacket® | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.97, 3.71] |
| 1.5 Hyalograft 3D® | 3 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.06, 2.33] |
| 1.6 Kaloderm® | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.90, 1.92] |
| 1.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.61, 5.05] |
| 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis Show forest plot | 6 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.21, 1.85] |
| 3 Incidence of lower limb amputations Show forest plot | 2 | 522 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.01] |
| 3.1 Graftskin® | 1 | 208 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.18, ‐0.01] |
| 3.2 Dermagraft® | 1 | 314 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Ulcer recurrence Show forest plot | 4 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.22] |
| 4.1 Apligraf® or Graftskin® | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.18, 2.35] |
| 4.2 Dermagraft® | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 13.68] |
| 5 Incidence of infection Show forest plot | 9 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.98] |
| 5.1 Apligraf® or Graftskin® | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.76] |
| 5.2 Dermagraft® | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 5.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.52] |
| 5.4 Graftjacket® | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.18, 2.04] |
| 5.5 Hyalograf 3D® | 1 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.90] |
| 5.6 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.12, 3.24] |
| 5.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure of the foot ulcer Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |

