Videolaringoscopia versus laringoscopia directa para pacientes adultos que requieren intubación traqueal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011136.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 noviembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Sharon R Lewis (SL), Andrew R Butler (AB), Joshua Parker (JP), Tim M Cook (TC), Andrew F Smith (AS).
Conceiving the review: AS.
Co‐ordinating the review: SL.
Undertaking manual searches: SL.
Screening search results: SL, AB.
Organizing retrieval of papers: SL.
Screening retrieved papers against inclusion criteria: SL, AB.
Appraising quality of papers: SL, AB, JP.
Abstracting data from papers: SL, AB, JP.
Writing to authors of papers for additional information: SL.
Managing data for the review: SL.
Entering data into Review Manager (RevMan 5.3): SL.
Analysing RevMan statistical data: SL.
Interpreting data: SL, AB, AS, TC.
Making statistical inferences: SL, TC, AS.
Writing the review: SL, AB, AS, TC.
Securing funding for the review: AS.
Performing previous work that was the foundation of the present study: N/A
Serving as guarantor for the review (one review author): AS.
Taking responsibility for reading and checking the review before submission: SL.
Sources of support
Internal sources
-
No sources of support provided
External sources
-
NIHR Cochrane Collaboration Programme Grant: Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04, UK. This grant funded the work of SRL, AN, AB, AFS and PA performed for this review, UK
Declarations of interest
Sharon R Lewis: see Sources of support.
Andrew R Butler: see Sources of support.
Joshua Parker: none known.
Tim M Cook was paid for lecturing, several years ago (> 36 months), by Intavent Orthofix and the LMA Company. This company manufactures and distributes several supraglottic airway devices and one videolaryngoscope: AP Venner. Dr Cook's department has received free or at cost airway equipment from numerous 'airway' companies for evaluation or research. He and his family have no financial investments and no ownership of any such company of which he is aware. Dr Cook has reported no other conflicts of interest. He spoke at a Storz educational meeting in 2015, and the company paid the costs of travel to this meeting and accommodations. He received no financial benefit from the meeting and was not paid to speak.
Andrew Bulter: See Sources of support.
Andrew F Smith: See Sources of support.
Acknowledgements
We would like to thank Rodrigo Cavallazzi (content editor), Marialena Trivella) (statistical editor), Davide Cattano, Shirley Zhao, Melissa Rethlefsen, Joshua Atkins (peer reviewers), Odie Geiger (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Rodrigo Cavallazzi (Content Editor); Cathal Walsh (Statistical Editor); and Davide Cattano and Joshua Atkins (Peer Reviewers) for help and editorial advice provided during preparation of the protocol (Lewis 2014) for this systematic review.
We would like to thank Amanda Nicholson, who was an author of the protocol (Lewis 2014) (see Sources of support).
We would like to thank study authors who responded to requests for further study information, in particular, Dr Waleed Riad, Dr Daniel Cordovani and Dr Aki Suzuki.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Apr 04 | Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation | Review | Jan Hansel, Andrew M Rogers, Sharon R Lewis, Tim M Cook, Andrew F Smith | |
| 2016 Nov 15 | Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation | Review | Sharon R Lewis, Andrew R Butler, Joshua Parker, Tim M Cook, Andrew F Smith | |
| 2014 May 28 | Videolaryngoscopy versus direct laryngoscopy for adult surgical patients requiring tracheal intubation for general anaesthesia | Protocol | Sharon R Lewis, Amanda Nicholson, Tim M Cook, Andrew F Smith | |
Differences between protocol and review
We made the following changes to the protocol (Lewis 2014).
Title
We changed the title from "Videolaryngoscopy versus direct laryngoscopy for adult surgical patients requiring tracheal intubation for general anaesthesia" to "Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation" because this better reflects the focus of the review.
Review authors
Amanda Nicholson contributed to the protocol but not to the review.
Objectives
We stated inclusion of participants with a known or predicted difficult airway, which reflected our intended subgroup analysis.
Searching of other resources
We did not contact investigators known to be involved in previous studies to enquire about ongoing or unpublished studies.
Types of outcome measures
We edited the definition of our secondary outcome, serious respiratory complications, which stated "including aspiration" to "pulmonary aspiration of gastric contents and lower respiratory tract infection". This added greater detail to the definition.
Selection of studies; data extraction and management
We did not use paper eligibility and data extraction forms as previously indicated in the protocol. We used on‐line software (www.covidence.org) for this stage of the review.
Measures of treatment effect
We did not collect time‐to‐event data for mortality. Only two studies reported mortality, and we did not combine these results.
Unit of analysis issues
We were not able to amalgamate data into a single pair‐wise comparison without creating a unit of analysis issue. Therefore, we made the decision during the review to include data from the VLS group that would be closest to a result of 'no effect', and to assess this decision in sensitivity analysis.
Dealing with missing data
We did not perform sensitivity analysis for missing data to compare effects of complete case scenario, worst case scenario and last observation carried forward.
Assessment of reporting bias
We did not conduct further assessment of publication bias with the Eggers test.
Effects of interventions
We altered time points for the sore throat outcome to reflect the time points commonly reported in the included studies.
Subgroup analysis and investigation of heterogeneity
We did not carry out subgroup analysis on outcomes other than our primary outcome of failed intubation. We added a sentence to the review to explain how we had defined intubator experience by number of uses.
Summary of findings
We did not include the outcome 'Number of attempts' in the 'Summary of findings table' but replaced it with the outcome 'Proportion of successful first attempts'. We added data for the outcome 'Sore throat'. We altered the definition of hypoxia in the 'Summary of findings table' to match that provided in the 'Primary outcomes' section. We altered the order of outcomes in the 'Summary of findings' section to reflect the order in the sections Types of outcome measures and Effects of interventions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
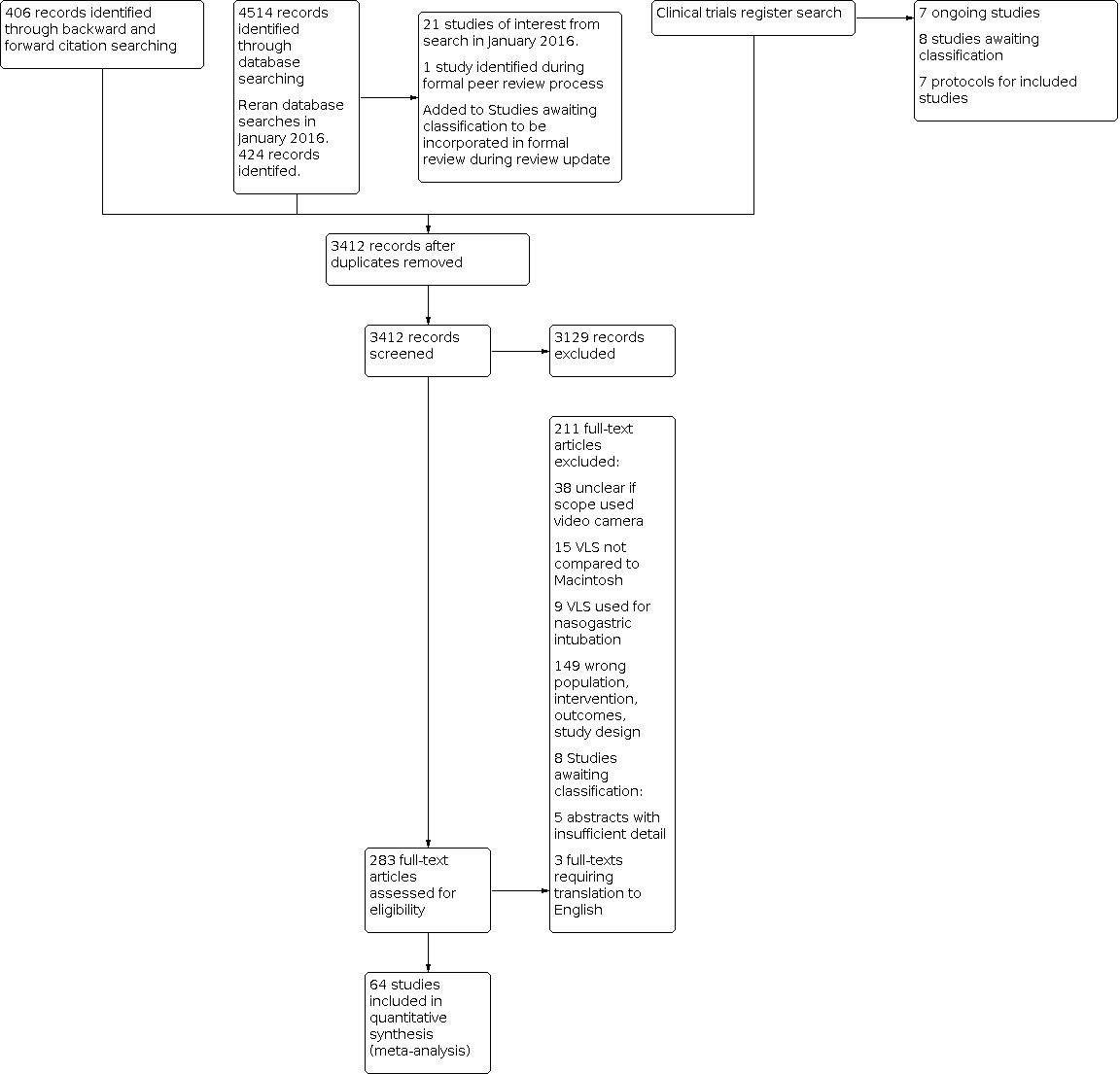
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Failed intubation, outcome: 1.1 Failed intubation.

Comparison 1: VLS versus Macintosh, Outcome 1: Failed intubation

Comparison 2: VLS versus Macintosh, Outcome 1: Hypoxia

Comparison 3: VLS versus Macintosh, Outcome 1: Mortality

Comparison 4: VLS versus Macintosh, Outcome 1: Laryngeal/airway trauma

Comparison 5: VLS versus Macintosh, Outcome 1: Patient‐reported sore throat

Comparison 6: VLS versus Macintosh, Outcome 1: Hoarseness

Comparison 7: VLS versus Macintosh, Outcome 1: Successful first attempt

Comparison 8: VLS versus Macintosh, Outcome 1: Number of attempts

Comparison 9: VLS versus Macintosh, Outcome 1: Time for tracheal intubation

Comparison 10: VLS versus Macintosh, Outcome 1: Intubation difficult score (IDS)

Comparison 11: VLS versus Macintosh, Outcome 1: Improved visualization Cormack & Lehane (CL) 1

Comparison 12: VLS versus Macintosh, Outcome 1: Improved visualization Cormack & Lehane (CL) 1 to 4

Comparison 13: VLS versus Macintosh, Outcome 1: Improved visualization POGO

Comparison 14: VLS versus Macintosh, Outcome 1: Failed intubation by scope

Comparison 15: VLS versus Macintosh, Outcome 1: Failed intubation by airway difficulty

Comparison 16: VLS versus Macintosh, Outcome 1: Failed intubation by experience of personnel
| Videolaryngoscopy compared with direct laryngoscopy for tracheal intubation | ||||||
| Patient or population: patients requiring tracheal intubation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Direct laryngoscopy | Videolaryngoscopy | |||||
| Failed intubation | Study population | OR 0.35 | 4127 | ⊕⊕⊕⊝ | Downgraded by 1 level. See footnote. | |
| 94 per 1000 | 35 per 1000 | |||||
| Moderate | ||||||
| Hypoxia | Study population | OR 0.39 | 1319 | ⊕⊝⊝⊝ | Downgraded by 3 levels. See footnotes. | |
| 58 per 1000 | 23 per 1000 | |||||
| Moderate | ||||||
| Serious respiratory complications | See comment | See comment | Not estimable | 78 | ⊕⊝⊝⊝ | Insufficient data to complete meta‐analysis. Downgraded by 2 levels. See footnotes. |
| Mortality | Study population | OR 1.09 | 663 | ⊕⊝⊝⊝ | Downgraded by 3 levels. See footnotes. | |
| 106 per 1000 | 114 per 1000 | |||||
| Very low | ||||||
| Proportion of successful first attempts | Study population | OR 0.79 | 4731 | ⊕⊕⊕⊝ | Downgraded by 1 level. See footnotes. | |
| 831 per 1000 | 795 per 1000 | |||||
| Moderate | ||||||
| Sore throat | Study population | OR 1.00 | 1548 | ⊕⊕⊕⊝ | Downgraded by 1 level. See footnotes. | |
| 250 per 1000 | 289 per 1000 | |||||
| Moderate | ||||||
| Time for tracheal intubation | See comment | See comment | Not estimable | 4488 | ⊕⊝⊝⊝ | High level of statistical heterogeneity between studies; therefore meta‐analysis not completed. Downgraded by 3 levels. See footnotes. |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aNot possible to blind intubator to device. Downgraded for study limitations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Failed intubation Show forest plot | 38 | 4127 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.19, 0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Hypoxia Show forest plot | 8 | 1319 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mortality Show forest plot | 2 | 663 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Laryngeal/airway trauma Show forest plot | 29 | 3110 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.48, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Patient‐reported sore throat Show forest plot | 17 | 2392 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.56, 1.19] |
| 5.1.1 Postanaesthesia care unit | 10 | 1548 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 5.1.2 Postoperative day 1 | 8 | 844 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.27, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Hoarseness Show forest plot | 6 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Successful first attempt Show forest plot | 36 | 4731 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.77, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Number of attempts Show forest plot | 28 | 6692 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.68, 1.66] |
| 8.1.1 1 attempt | 28 | 3346 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.68, 2.31] |
| 8.1.2 2 to 4 attempts | 28 | 3346 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.47, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Time for tracheal intubation Show forest plot | 37 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Intubation difficult score (IDS) Show forest plot | 7 | 568 | Odds Ratio (M‐H, Random, 95% CI) | 7.13 [3.12, 16.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Improved visualization Cormack & Lehane (CL) 1 Show forest plot | 22 | 2240 | Odds Ratio (M‐H, Random, 95% CI) | 6.77 [4.17, 10.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Improved visualization Cormack & Lehane (CL) 1 to 4 Show forest plot | 22 | 4480 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.87] |
| 12.1.1 CL 1 to 2 | 22 | 2240 | Odds Ratio (M‐H, Random, 95% CI) | 5.42 [3.70, 7.95] |
| 12.1.2 CL 3 to 4 | 22 | 2240 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.13, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Improved visualization POGO Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Failed intubation by scope Show forest plot | 33 | 3916 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.75] |
| 14.1.1 GlideScope | 16 | 1306 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.25, 1.32] |
| 14.1.2 Pentax AWS | 11 | 1086 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.20] |
| 14.1.3 McGrath | 5 | 466 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.06, 23.92] |
| 14.1.4 C‐MAC | 8 | 1058 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.15, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Failed intubation by airway difficulty Show forest plot | 34 | 3383 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.65] |
| 15.1.1 Predicted not difficult | 19 | 1743 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.22, 1.67] |
| 15.1.2 Predicted difficult | 6 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.15, 0.55] |
| 15.1.3 Simulated difficult | 9 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Failed intubation by experience of personnel Show forest plot | 22 | 2273 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.13, 0.67] |
| 16.1.1 Personnel experienced with both devices | 17 | 1927 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.13, 0.75] |
| 16.1.2 Personnel less experienced with VLS | 5 | 346 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 2.56] |

