Cuidados paliativos tempranos en pacientes adultos con cáncer avanzado
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011129.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MWH drafted the protocol, developed and ran the search strategy, obtained copies of studies, selected studies for inclusion, extracted data from studies, entered data into RevMan, carried out and interpreted the analysis, and drafted the review. SE ran the search strategy, obtained copies of studies, selected studies for inclusion, extracted data from studies, and entered data into RevMan. GR drafted the protocol and assisted in carrying out and interpreting the analyses, and in drafting the final review. HCF and MT drafted the protocol, interpreted the analyses, and drafted the final review. MV interpreted the analyses from a clinical point of view and drafted the final review. MH drafted the protocol, developed the search strategy, selected studies for inclusion (as arbiter), supervised in carrying out and interpreting the analyses, and drafted the review.
Sources of support
Internal sources
-
Heidelberg University Hospital, Germany.
Employer of MWH, SE, MV, MT, and MH
-
University Medical Center Freiburg, Germany.
Employer of GR
-
University of Duesseldorf, Germany.
Employer of HCF
External sources
-
No sources of support supplied
Declarations of interest
MWH: none known; MWH is an internal medicine physician (internist) and a junior research group leader in mental health services research.
SE: none known.
GR: none known.
HCF: none known; HCF is a specialist in psychosomatic medicine and internal medicine, and manages psychiatric comorbidity in patients with somatic illnesses.
MV: none known; MV is a specialist oncology and palliative care physician and manages patients with advanced cancer.
MT is a department head of thoracic oncology (Thoraxklinik, University of Heidelberg) and manages patients with malignant thoracic diseases and lung metastases. MT received personal consulting fees from Lilly, Novartis, Roche, AstraZeneca, Pfizer, Boehringer, and BMS in 2014; from Lilly, Novartis, Roche, AstraZeneca, BMS, MSD, Pfizer, Boehringer, and Celgene in 2015; and from Lilly, Novartis, Roche, AstraZeneca, BMS, MSD, Pfizer, Boehringer, and Celgene in 2016 for attending boards. MT received lecture fees from Lilly, Novartis, Roche, AstraZeneca, Pfizer, and Boehringer in 2014; from Lilly, Novartis, Roche, AstraZeneca, BMS, MSD, Pfizer, and Boehringer in 2015; and from Lilly, Novartis, AstraZeneca, BMS, MSD, Pfizer, Boehringer, and Celgene in 2016.
MH: none known.
Acknowledgements
We would like to thank Sabine Sommerfeldt for her contribution to development of this protocol. We would also like to thank Maria‐Inti Metzendorf of the Library for the Medical Faculty of Mannheim, Heidelberg University, for contributing to the search strategies, and Joanne Abbott, Information Specialist at the Cochrane PaPaS Group, for running database searches.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS).
Disclaimer: The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 12 | Early palliative care for adults with advanced cancer | Review | Markus W Haun, Stephanie Estel, Gerta Rücker, Hans‐Christoph Friederich, Matthias Villalobos, Michael Thomas, Mechthild Hartmann | |
| 2014 May 26 | Early palliative care for improving quality of life and survival time in adults with advanced cancer | Protocol | Markus W Haun, Stephanie Estel, Gerta Rücker, Hans‐Christoph Friederich, Michael Thomas, Mechthild Hartmann | |
Differences between protocol and review
We updated and added references to the Background and Methods sections. In the Background section, we updated our definition of 'early palliative care' on the basis of current literature and introduced the recently conceptualised classification of models for early palliative care provided by Hui 2015a. In the Methods section, we documented our decision to conduct a subgroup analysis for two different models. In the 'Types of interventions' section, we specified as an additional inclusion criterion 'An early palliative care intent had to be stated explicitly or be reflected in the sample composition, i.e. most participants had to be enrolled shortly after diagnosis of advanced disease.' In the 'Assessment of risk of bias in included studies' section, we now state that we included blinding of participants and outcome assessment as sixth and seventh domains in the risk of bias assessment. Also in this section, we updated our justification for not excluding small studies from the review. We refrained from compiling funnel plots because of the small number of included studies. In light of new recommendations by the GRADE Working Group (Alonso‐Coello 2016), we have replaced the term "quality of the evidence", which we had used in the protocol, with the term "certainty of the evidence". For reasons of completeness, we reported results on outcomes in the review that had not been prespecified in the protocol (i.e. place of death, problems with medical interactions and satisfaction with care, and illness and prognosis understanding). Furthermore, we now explicitly state that we based survival analysis on unadjusted death hazard ratios. To enhance comprehensibility, we decided to refrain from additionally converting SMDs to odds ratios. We have explained in the Methods section all post‐protocol decisions concerning methods.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
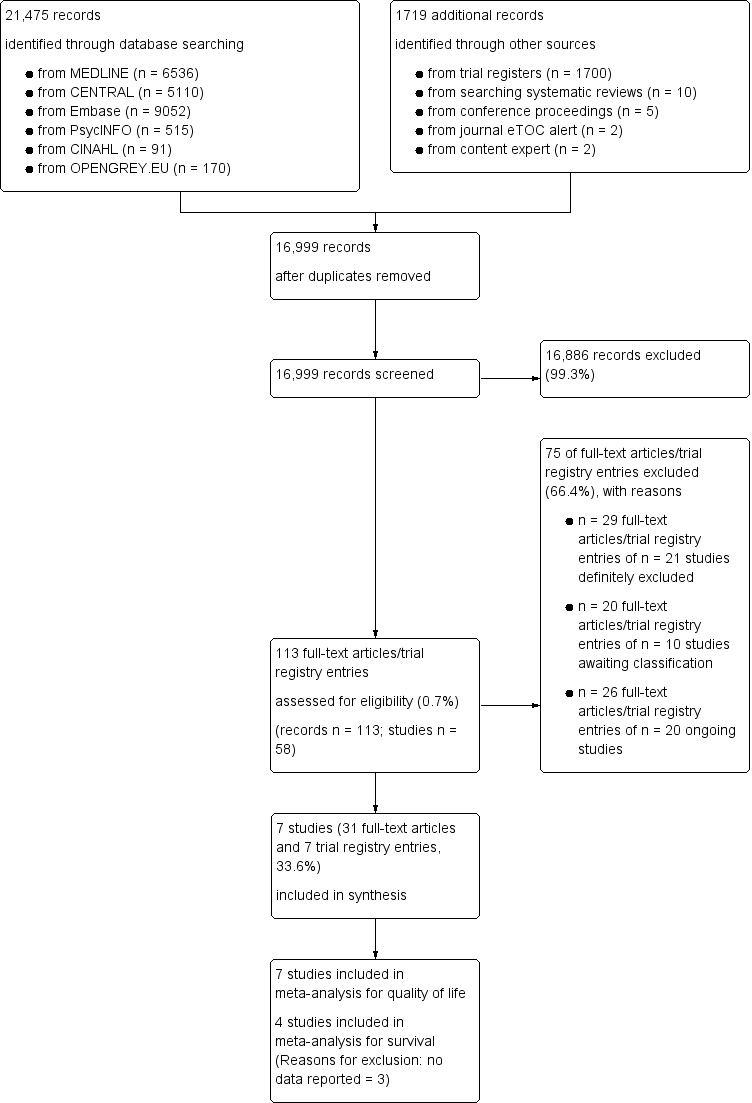
Screening process diagram (as recommended by the PRISMA statement).
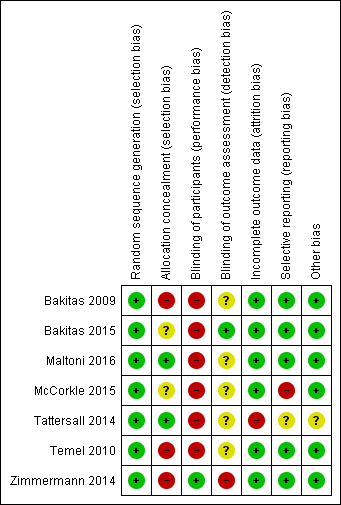
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
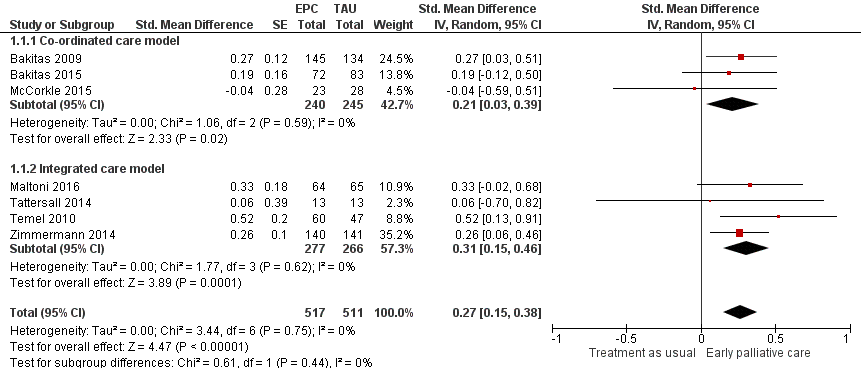
Forest plot of comparison: 1 Health‐related quality of life, outcome: 1.1 Health‐related quality of life.

Forest plot of comparison: 1 Early palliative care vs TAU, outcome: 1.2 Survival.

Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.2 Depression.
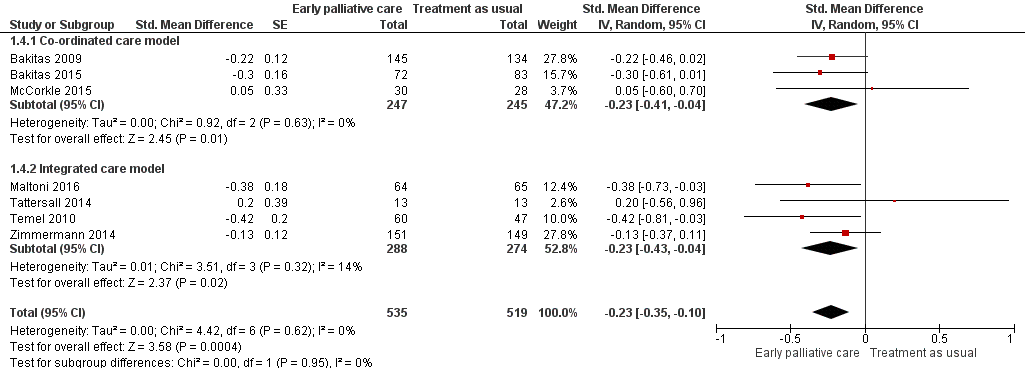
Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.4 Symptom intensity.

Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.5 Health‐related quality of life (sensitivity analysis for study design including RCTs only).
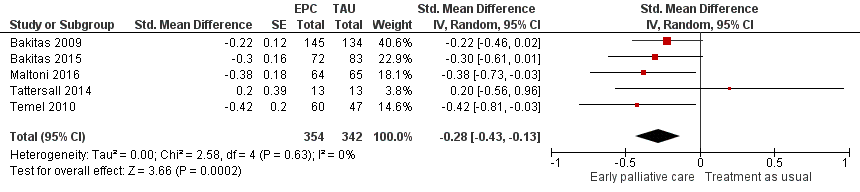
Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.6 Symptom intensity (sensitivity analysis for study design including RCTs only).

Comparison 1 Early palliative care vs standard oncological care, Outcome 1 Health‐related quality of life.
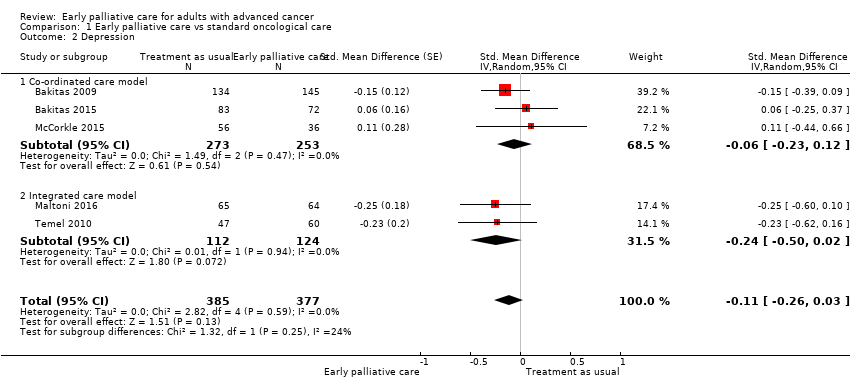
Comparison 1 Early palliative care vs standard oncological care, Outcome 2 Depression.
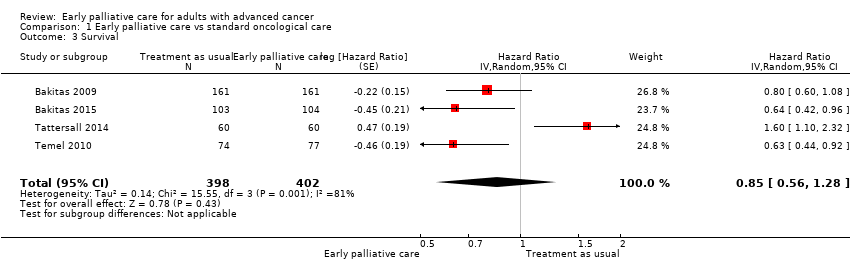
Comparison 1 Early palliative care vs standard oncological care, Outcome 3 Survival.

Comparison 1 Early palliative care vs standard oncological care, Outcome 4 Symptom intensity.
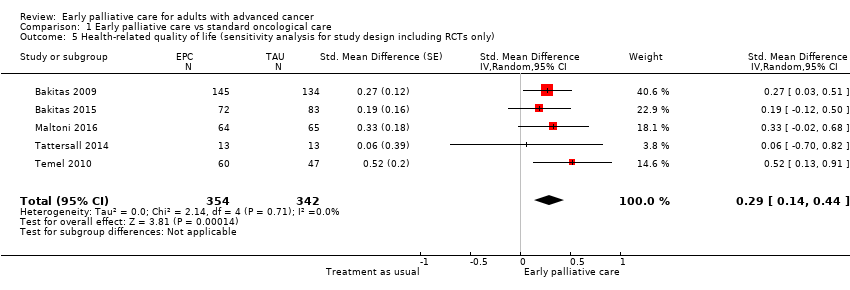
Comparison 1 Early palliative care vs standard oncological care, Outcome 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only).

Comparison 1 Early palliative care vs standard oncological care, Outcome 6 Symptom intensity (sensitivity analysis for study design including RCTs only).
| Clinical question: Should early palliative care be preferred over treatment as usual for improving health‐related quality of life, depression, and symptom intensity in patients with advanced cancer? | ||||||
| Patient or population: patients with advanced cancer Settings: mainly outpatient care in Australia, Canada, Italy, and the USA Comparison: treatment as usual Follow‐up: at 12 weeks or mean difference in repeated measurement results for longitudinal designs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with treatment as usual | Risk with early palliative care | |||||
| Health‐related quality of life (HRQOL), SD units: measured on FACIT‐Pal, TOI of FACT‐Hep, TOI of FACT‐L, FACT‐G, McGill Quality of Life, FACIT‐Sp. Higher scores indicate better HRQOL. Follow‐up: range 12 weeks to 52 weeks | HRQOL score improved on average 0.27 (95% CI 0.15 to 0.38) SDs more in early palliative care participants than in control participants | ‐ | 1028 | ⊕⊕⊝⊝ | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Health‐related quality of life (HRQOL), natural units: measured on FACT‐G (from 0 to 108) | Baseline control group mean score at 70.5 pointsa | HRQOL score improved on average 4.59 (95% CI 2.55 to 6.46) points more in early palliative care participants than in control participants | ‐ | 1028 | ⊕⊕⊝⊝ | Calculated by transforming all scales to the FACT‐G in which the minimal clinically important difference is approximately 5 and the SD in the cancer validation sample was 17.0 (Brucker 2005) |
| Survival: estimated with the unadjusted death hazard ratio | Study populationb | HR 0.85, 95% CI 0.56 to 1.28 | 800 | ⊕⊝⊝⊝ | ||
| 61 per 100 | 56 per 100 (41‐71) | |||||
| Depression, SD units: measured on CES‐D, HADS‐D, PHQ‐9. Higher scores indicate higher depressive symptom load. Follow‐up: range 12 weeks to 52 weeks | Depression score improved on average ‐0.11 (95% CI ‐0.26 to 0.03) SDs more in early palliative care participants than in control participants | ‐ | 762 | ⊕⊝⊝⊝ VERY LOW1,2,4 | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Depression, natural units: measured on CES‐D (from 0 to 60). Higher scores indicate higher depressive symptom load | Baseline control group mean score at 13.8 pointsc | Depressive symptoms score improved on average ‐0.98 (95% CI ‐2.31 to 0.27) points more in early palliative care participants than in control participants | ‐ | 762 | ⊕⊝⊝⊝ VERY LOW1,2,4 | Calculated by transforming all scales to CES‐D and applying an SD of 8.9 from baseline control group score in Bakitas 2009 |
| Symptom intensity, SD units: measured on ESAS, QUAL‐E Symptom Impact Subscale, SDS, RSC, LCS of FACT‐L, HCS of FACT‐Hep. Higher scores indicate higher symptom intensity. Follow‐up: range 12 weeks to 52 weeks | Symptom intensity score improved on average ‐0.23 (95% CI ‐0.35 to ‐0.1) SDs more in early palliative care participants than in control participants | ‐ | 1054 | ⊕⊕⊝⊝ | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Symptom intensity, natural units: measured on ESAS (from 0 to 900). Follow‐up: range 12 weeks to 52 weeks | Baseline control group mean score at 286.3 pointsc | Symptom intensity symptoms score improved on average ‐35.4 (95% CI ‐53.9 to ‐15.4) points more in early palliative care participants than in control participants | ‐ | 1054 | ⊕⊕⊝⊝ | Calculated by transforming all scales to the ESAS and applying an SD of 154.0 from baseline control group score in Bakitas 2009 |
| Adverse events | See comment | See comment | Not estimable | 1614 | See comment | Most often, study authors did not address assessment or findings on adverse events in their study publications. However, on request, authors of 6 studies described the tolerability of early palliative care as very good. A single study mentioned adverse events only in the early palliative care group, i.e. higher percentage of participants with severe scores for pain and poor appetite along with higher level of unmet needs (Tattersall 2014) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) aApproximate average of baseline control group FACT‐G scores across 4 included studies (Bakitas 2009; Bakitas 2015; Maltoni 2016; Temel 2010) b12‐Month follow‐up control group risk in the largest study reporting on survival (Bakitas 2009) cBaseline control group CES‐D score in the largest study reporting on depression (Bakitas 2009) CI: confidence interval; GRADE: Grading of Recommendations Assessment; HR: unadjusted death hazard ratio; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded 2 points owing to very serious limitations in study quality (high risk of bias across studies) 2We decided against downgrading for indirectness, although 2 studies were conducted exclusively in patients with metastatic pancreatic and advanced lung cancer, respectively (Maltoni 2016; Temel 2010). We decided against downgrading for inconsistency, as we did not detect significant heterogeneity 3We decided against downgrading for imprecision, as the optimal information size (OIS) criterion was met, and the 95% confidence interval around the difference in effect between intervention and control excludes zero 4We downgraded 1 point for imprecision, as the optimal information size (OIS) criterion was met, but the 95% confidence interval around the difference in effect between intervention and control includes zero. The 95% confidence interval fails to exclude harm 5We decided against downgrading for important inconsistency (large I2) because we had downgraded by 3 points already 6We decided against downgrading for indirectness, as only a single study was conducted exclusively in patients with advanced lung cancer (Temel 2010) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life Show forest plot | 7 | 1028 | Std. Mean Difference (Random, 95% CI) | 0.27 [0.15, 0.38] |
| 1.1 Co‐ordinated care model | 3 | 485 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.39] |
| 1.2 Integrated care model | 4 | 543 | Std. Mean Difference (Random, 95% CI) | 0.31 [0.15, 0.46] |
| 2 Depression Show forest plot | 5 | 762 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| 2.1 Co‐ordinated care model | 3 | 526 | Std. Mean Difference (Random, 95% CI) | ‐0.06 [‐0.23, 0.12] |
| 2.2 Integrated care model | 2 | 236 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.50, 0.02] |
| 3 Survival Show forest plot | 4 | 800 | Hazard Ratio (Random, 95% CI) | 0.85 [0.56, 1.28] |
| 4 Symptom intensity Show forest plot | 7 | 1054 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.35, ‐0.10] |
| 4.1 Co‐ordinated care model | 3 | 492 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.41, ‐0.04] |
| 4.2 Integrated care model | 4 | 562 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.43, ‐0.04] |
| 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | 0.29 [0.14, 0.44] |
| 6 Symptom intensity (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] |

