Cuidados paliativos tempranos en pacientes adultos con cáncer avanzado
Appendices
Appendix 1. Search strategy for MEDLINE (OvidSP)
1. exp Palliative Care/
2. palliat*.tw.
3. "advanced disease*".tw.
4. ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage").tw.
5. Terminally Ill/
6. Terminal Care/
7. (terminal* adj6 care*).tw.
8. ((terminal* adj6 ill*) or terminal‐stage* or dying or (close adj6 death)).tw.
9. (terminal* adj6 disease*).tw.
10. (end adj6 life).tw.
11. hospice*.tw.
12. or/1‐11
13. exp Neoplasms/
14. (neoplasm* or cancer* or tumo?r*).tw.
15. or/13‐14
16. 12 and 15
17. randomized controlled trial.pt.
18. controlled clinical trial.pt.
19. randomized.ab.
20. placebo.ab.
21. clinical trials as topic.sh.
22. randomly.ab.
23. trial.ti.
24. 17 or 18 or 19 or 20 or 21 or 22 or 23
25. exp animals/ not humans.sh.
26. 24 not 25
27. 16 and 26
Appendix 2. Seach strategy for CENTRAL (the Cochrane Library)
#1 MESH DESCRIPTOR Terminal Care EXPLODE ALL TREES
#2 palliat*
#3 ("advanced disease*")
#4 (("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage"))
#5 MESH DESCRIPTOR Terminally Ill
#6 MESH DESCRIPTOR Terminal Care
#7 ((terminal* near/6 care*))
#8 (((terminal* near/6 ill*) or terminal‐stage* or dying or (close near/6 death)))
#9 ((terminal* near/6 disease*))
#10 ((end near/6 life))
#11 hospice*
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#13 MESH DESCRIPTOR Neoplasms EXPLODE ALL TREES
#14 ((neoplasm* or cancer* or tumo?r*))
#15 #13 OR #14
#16 #12 AND #15
Appendix 3. Seach strategy for Embase (OvidSP)
1. exp Palliative Care/
2. palliat*.tw.
3. Terminally Ill/
4. Terminal Care/
5. (terminal* adj6 care*).tw.
6. ((terminal* adj6 ill*) or terminal‐stage* or dying or (close adj6 death)).tw.
7. (terminal* adj6 disease*).tw.
8. (end adj6 life).tw.
9. hospice*.tw.
10. ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage").tw.
11. "advanced disease*".tw.
12. or/1‐11
13. exp Neoplasms/
14. (neoplasm* or cancer* or tumo?r*).tw.
15. or/13‐14
16. 12 and 15
17. random$.tw.
18. factorial$.tw.
19. crossover$.tw.
20. cross over$.tw.
21. cross‐over$.tw.
22. placebo$.tw.
23. (doubl$ adj blind$).tw.
24. (singl$ adj blind$).tw.
25. assign$.tw.
26. allocat$.tw.
27. volunteer$.tw.
28. Crossover Procedure/
29. double‐blind procedure.tw.
30. Randomized Controlled Trial/
31. Single Blind Procedure/
32. or/17‐31
33. (animal/ or nonhuman/) not human/
34. 32 not 33
35. 16 and 34
36. limit 35 to embase
Appendix 4. Seach strategy for PsycINFO (OvidSP)
1. exp Palliative Care/
2. palliat*.tw.
3. Terminally Ill Patients/
4. (terminal* adj6 care*).tw.
5. ((terminal* adj6 ill*) or terminal‐stage* or dying or (close adj6 death)).tw.
6. (terminal* adj6 disease*).tw.
7. (end adj6 life).tw.
8. hospice*.tw.
9. ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage").tw.
10. "advanced disease*".tw.
11. exp Neoplasms/
12. (neoplasm* or cancer* or tumo?r*).tw.
13. or/11‐12
14. or/1‐10
15. 13 and 14
16. clinical trials/
17. (randomis* or randomiz*).tw.
18. (random$ adj3 (allocat$ or assign$)).tw.
19. ((clinic$ or control$) adj trial$).tw.
20. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
21. (crossover$ or "cross over$").tw.
22. random sampling/
23. Experiment Controls/
24. Placebo/
25. placebo$.tw.
26. exp program evaluation/
27. treatment effectiveness evaluation/
28. ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw.
29. or/16‐28
30. 15 and 29
Appendix 5. Search strategy for CINAHL (EBSCO)
| S6 | S1 AND (S2 OR S3) AND S4 AND S5 |
| S5 | (cancer OR neoplasm* OR tumor* OR tumour* OR malignan*) |
| S4 | (early AND OR timely OR proactive OR (early AND care) OR (early AND treatment*) OR (early AND medicine* OR (early AND surgery) OR (early AND therapy)) |
| S3 | (best AND support*) OR (optim* AND support*) OR (best AND care) OR (best AND treatment*) OR *supportive care* |
| S2 | ((palliate* OR (terminal* AND ill*) OR (terminal* AND caring) OR (terminal* AND care*) OR bereave* OR hospice*) OR euthanas* OR (attitude* AND death*) OR (assist* AND death*) OR (assist* AND die*) OR (assist* AND suicide*) OR (help* AND death*) OR (help* AND die*) OR (help* AND suicide*) OR (aid* AND death*) OR (aid* AND die*) OR (aid* AND suicide*) OR (right* AND die*) OR (respite AND care*) OR (respite AND caring) OR (living AND will*) OR (advance* AND directive*) OR (advance* AND care AND plan) OR (“end of life” AND care) OR (“end of life” AND caring)) |
| S1 | (randomized controlled Trial* OR controlled clinical trial* OR placebo OR randomly OR Trial*) |
Appendix 6. Search strategy for OpenGrey (EXALEAD)
(randomized controlled trial OR controlled clinical trial OR placebo OR randomly OR trial) AND ((palliate* OR (terminal* AND ill*) OR (terminal* AND caring) OR (terminal* AND care*) OR bereave* OR hospice*) OR euthanas* OR (attitude* AND death*) OR (assist* AND death*) OR (assist* AND die*) OR (assist* AND suicide*) OR (help* AND death*) OR (help* AND die*) OR (help* AND suicide*) OR (aid* AND death*) OR (aid* AND die*) OR (aid* AND suicide*) OR (right* AND die*) OR (respite AND care*) OR (respite AND caring) OR (living AND will*) OR (advance* AND directive*) OR (advance* AND care AND plan) OR (“end of life” AND care) OR (“end of life” AND caring) OR ((chemoth* AND (induced AND vomiting)) OR (chemoth* AND (induced AND sickness))) OR (chemoth* AND (related AND sickness)) OR (chemoth* AND (related AND vomiting))) OR ((induced AND hypersalivation) OR (induced AND hyposalivation)) OR (induced AND xerostomi*) OR ((induced AND cachexi*) OR (related AND cachexi*)) OR ("terminal* ill*"AND "symptom* management"))) OR (((anorexi* AND cancer*) OR (anorexi* AND carcinoma*)) OR ((anorexi* AND radiotherap*)))) OR Search (((cancer AND weight‐loss) OR (cancer AND weight AND loss) OR (cancer AND weight AND losing) OR (carcinoma* AND weight‐loss) OR (carcinoma* AND weight AND loss) OR (carcinoma* AND weight AND losing))) OR ((((cancer AND weight‐gain*) OR (cancer AND weight AND gain*) OR (carcinoma* AND weight‐gain) OR (carcinoma* AND weight AND gain*)))) OR (((cancer AND appetite AND stimulat*) OR (carcinoma* AND appetite AND stimulat*))) OR (((appetite AND stimulat*) OR ((cancer AND hot AND flush*) OR (cancer AND hot AND flash*)) OR (related AND cachexi*) OR (neoplastic AND cachexi*) OR ((induced AND constipat*) OR (induced AND emesis)) OR (opioid AND induced) OR (morphine AND induced) OR (methadone AND induced) OR (cancer or carcinoma* AND music AND therapy) OR ((cancer or carcinoma*) AND ((aroma AND therapy) OR aromatherapy))) OR ((dysphag* AND cancer) OR ((symptom AND control AND (cancer OR carcinoma*)) OR (radiotherap* AND induced) OR (chemotherap* AND induced) OR (radiotherap* AND related) OR (chemotherap* AND related) OR ((cancer AND related) OR (carcinoma* AND related)) OR (anorexi* AND radiochemotherap*))) AND ((best AND support*) OR (optim* AND support*) OR (best AND care) OR (best AND treatment*) OR “supportive care”)((best AND support*) OR (optim* AND support*) OR (best AND care) OR (best AND treatment*) OR “supportive care”) AND (early AND OR timely OR proactive OR (early AND care) OR (early AND treatment*) OR (early AND medicine* OR (early AND surgery) OR (early AND therapy)) AND (cancer OR neoplasm* OR tumor* OR tumour* OR malignan*)
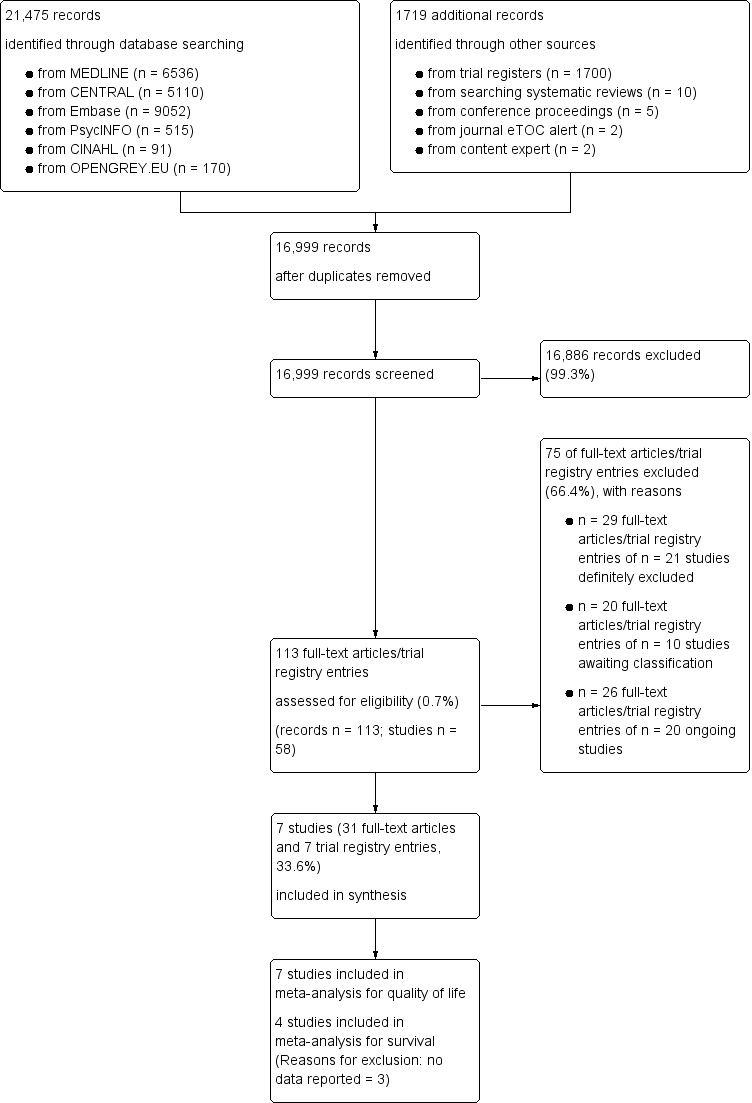
Screening process diagram (as recommended by the PRISMA statement).
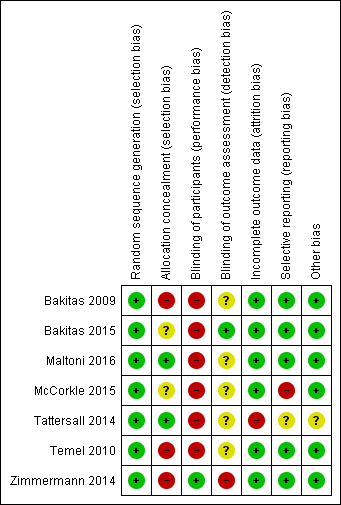
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
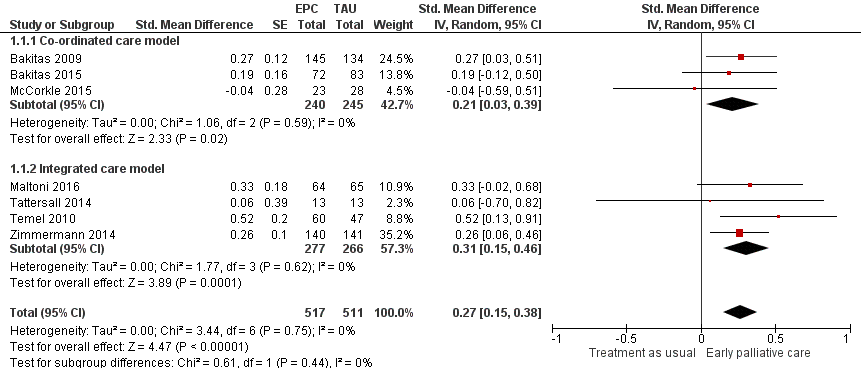
Forest plot of comparison: 1 Health‐related quality of life, outcome: 1.1 Health‐related quality of life.

Forest plot of comparison: 1 Early palliative care vs TAU, outcome: 1.2 Survival.

Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.2 Depression.
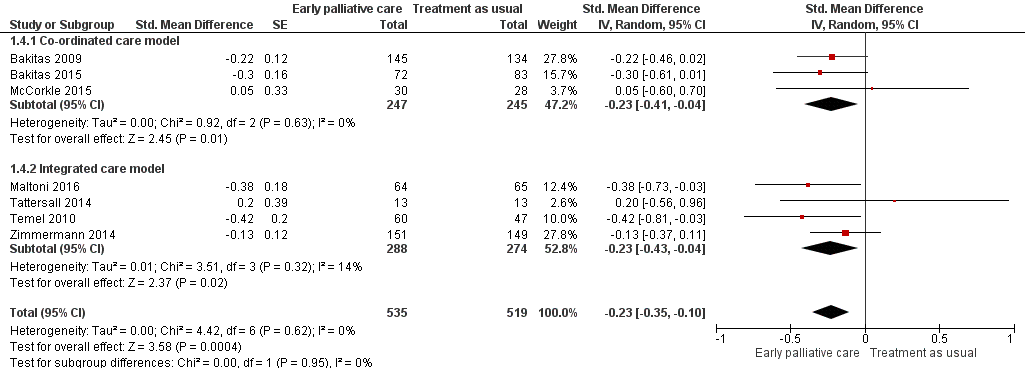
Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.4 Symptom intensity.

Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.5 Health‐related quality of life (sensitivity analysis for study design including RCTs only).
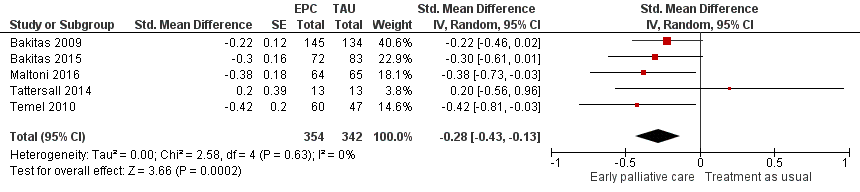
Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.6 Symptom intensity (sensitivity analysis for study design including RCTs only).

Comparison 1 Early palliative care vs standard oncological care, Outcome 1 Health‐related quality of life.
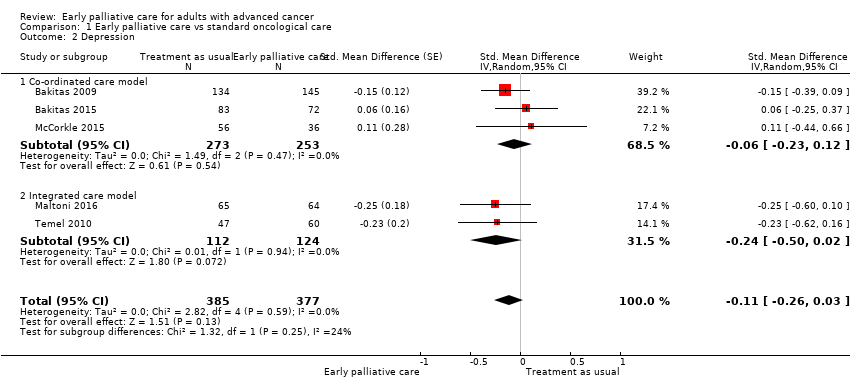
Comparison 1 Early palliative care vs standard oncological care, Outcome 2 Depression.
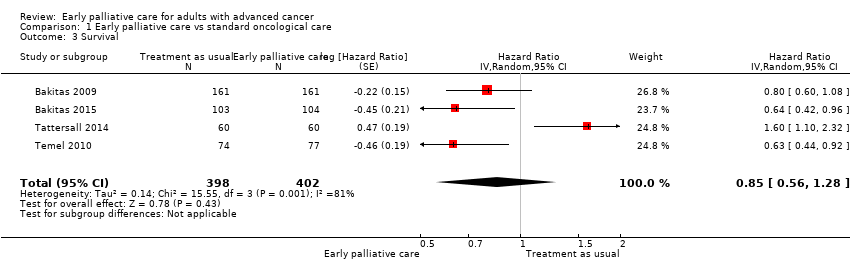
Comparison 1 Early palliative care vs standard oncological care, Outcome 3 Survival.

Comparison 1 Early palliative care vs standard oncological care, Outcome 4 Symptom intensity.
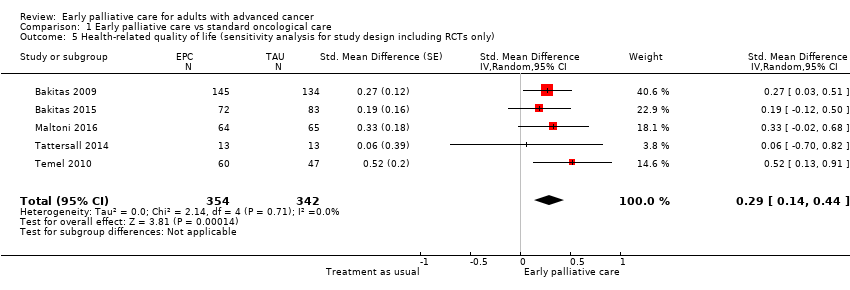
Comparison 1 Early palliative care vs standard oncological care, Outcome 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only).

Comparison 1 Early palliative care vs standard oncological care, Outcome 6 Symptom intensity (sensitivity analysis for study design including RCTs only).
| Clinical question: Should early palliative care be preferred over treatment as usual for improving health‐related quality of life, depression, and symptom intensity in patients with advanced cancer? | ||||||
| Patient or population: patients with advanced cancer Settings: mainly outpatient care in Australia, Canada, Italy, and the USA Comparison: treatment as usual Follow‐up: at 12 weeks or mean difference in repeated measurement results for longitudinal designs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with treatment as usual | Risk with early palliative care | |||||
| Health‐related quality of life (HRQOL), SD units: measured on FACIT‐Pal, TOI of FACT‐Hep, TOI of FACT‐L, FACT‐G, McGill Quality of Life, FACIT‐Sp. Higher scores indicate better HRQOL. Follow‐up: range 12 weeks to 52 weeks | HRQOL score improved on average 0.27 (95% CI 0.15 to 0.38) SDs more in early palliative care participants than in control participants | ‐ | 1028 | ⊕⊕⊝⊝ | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Health‐related quality of life (HRQOL), natural units: measured on FACT‐G (from 0 to 108) | Baseline control group mean score at 70.5 pointsa | HRQOL score improved on average 4.59 (95% CI 2.55 to 6.46) points more in early palliative care participants than in control participants | ‐ | 1028 | ⊕⊕⊝⊝ | Calculated by transforming all scales to the FACT‐G in which the minimal clinically important difference is approximately 5 and the SD in the cancer validation sample was 17.0 (Brucker 2005) |
| Survival: estimated with the unadjusted death hazard ratio | Study populationb | HR 0.85, 95% CI 0.56 to 1.28 | 800 | ⊕⊝⊝⊝ | ||
| 61 per 100 | 56 per 100 (41‐71) | |||||
| Depression, SD units: measured on CES‐D, HADS‐D, PHQ‐9. Higher scores indicate higher depressive symptom load. Follow‐up: range 12 weeks to 52 weeks | Depression score improved on average ‐0.11 (95% CI ‐0.26 to 0.03) SDs more in early palliative care participants than in control participants | ‐ | 762 | ⊕⊝⊝⊝ VERY LOW1,2,4 | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Depression, natural units: measured on CES‐D (from 0 to 60). Higher scores indicate higher depressive symptom load | Baseline control group mean score at 13.8 pointsc | Depressive symptoms score improved on average ‐0.98 (95% CI ‐2.31 to 0.27) points more in early palliative care participants than in control participants | ‐ | 762 | ⊕⊝⊝⊝ VERY LOW1,2,4 | Calculated by transforming all scales to CES‐D and applying an SD of 8.9 from baseline control group score in Bakitas 2009 |
| Symptom intensity, SD units: measured on ESAS, QUAL‐E Symptom Impact Subscale, SDS, RSC, LCS of FACT‐L, HCS of FACT‐Hep. Higher scores indicate higher symptom intensity. Follow‐up: range 12 weeks to 52 weeks | Symptom intensity score improved on average ‐0.23 (95% CI ‐0.35 to ‐0.1) SDs more in early palliative care participants than in control participants | ‐ | 1054 | ⊕⊕⊝⊝ | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Symptom intensity, natural units: measured on ESAS (from 0 to 900). Follow‐up: range 12 weeks to 52 weeks | Baseline control group mean score at 286.3 pointsc | Symptom intensity symptoms score improved on average ‐35.4 (95% CI ‐53.9 to ‐15.4) points more in early palliative care participants than in control participants | ‐ | 1054 | ⊕⊕⊝⊝ | Calculated by transforming all scales to the ESAS and applying an SD of 154.0 from baseline control group score in Bakitas 2009 |
| Adverse events | See comment | See comment | Not estimable | 1614 | See comment | Most often, study authors did not address assessment or findings on adverse events in their study publications. However, on request, authors of 6 studies described the tolerability of early palliative care as very good. A single study mentioned adverse events only in the early palliative care group, i.e. higher percentage of participants with severe scores for pain and poor appetite along with higher level of unmet needs (Tattersall 2014) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) aApproximate average of baseline control group FACT‐G scores across 4 included studies (Bakitas 2009; Bakitas 2015; Maltoni 2016; Temel 2010) b12‐Month follow‐up control group risk in the largest study reporting on survival (Bakitas 2009) cBaseline control group CES‐D score in the largest study reporting on depression (Bakitas 2009) CI: confidence interval; GRADE: Grading of Recommendations Assessment; HR: unadjusted death hazard ratio; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded 2 points owing to very serious limitations in study quality (high risk of bias across studies) 2We decided against downgrading for indirectness, although 2 studies were conducted exclusively in patients with metastatic pancreatic and advanced lung cancer, respectively (Maltoni 2016; Temel 2010). We decided against downgrading for inconsistency, as we did not detect significant heterogeneity 3We decided against downgrading for imprecision, as the optimal information size (OIS) criterion was met, and the 95% confidence interval around the difference in effect between intervention and control excludes zero 4We downgraded 1 point for imprecision, as the optimal information size (OIS) criterion was met, but the 95% confidence interval around the difference in effect between intervention and control includes zero. The 95% confidence interval fails to exclude harm 5We decided against downgrading for important inconsistency (large I2) because we had downgraded by 3 points already 6We decided against downgrading for indirectness, as only a single study was conducted exclusively in patients with advanced lung cancer (Temel 2010) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life Show forest plot | 7 | 1028 | Std. Mean Difference (Random, 95% CI) | 0.27 [0.15, 0.38] |
| 1.1 Co‐ordinated care model | 3 | 485 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.39] |
| 1.2 Integrated care model | 4 | 543 | Std. Mean Difference (Random, 95% CI) | 0.31 [0.15, 0.46] |
| 2 Depression Show forest plot | 5 | 762 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| 2.1 Co‐ordinated care model | 3 | 526 | Std. Mean Difference (Random, 95% CI) | ‐0.06 [‐0.23, 0.12] |
| 2.2 Integrated care model | 2 | 236 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.50, 0.02] |
| 3 Survival Show forest plot | 4 | 800 | Hazard Ratio (Random, 95% CI) | 0.85 [0.56, 1.28] |
| 4 Symptom intensity Show forest plot | 7 | 1054 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.35, ‐0.10] |
| 4.1 Co‐ordinated care model | 3 | 492 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.41, ‐0.04] |
| 4.2 Integrated care model | 4 | 562 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.43, ‐0.04] |
| 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | 0.29 [0.14, 0.44] |
| 6 Symptom intensity (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] |

