Tratamiento farmacológico del estreñimiento relacionado con los antipsicóticos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011128.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Susanna Every‐Palmer wrote the protocol and review
Giles Newton‐Howes and Mike Clarke reviewed and amended parts of the protocol and review
Susanna Every‐Palmer and Giles Newtown‐Howes screened all identified studies and extracted data. Mike Clarke screened and extracted data from 20% of the studies.
Sources of support
Internal sources
-
Te Korowai Whariki, Capital and Coast District Health Board, New Zealand.
Institution at which researcher (SEP) is employed
-
Wellington School of Medicine, University of Otago, New Zealand.
Institution at which researcher (GNH) is employed
External sources
-
No sources of support supplied
Declarations of interest
None declared.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
The search strategy was developed by the Information Specialist (formerly the Trial Search Co‐ordinator) of the Cochrane Schizophrenia Group.
We would like to thank Marilyn H. Bamford and Mathildah Maria Malaza for peer reviewing the protocol.
Thank you to Jun Xia and Chunhu Shi for assisting with the screening and data extraction of the studies written in Mandarin, and to Donna Tiejtens in the Wellington Medical Library for assistance with obtaining reference material.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 24 | Pharmacological treatment for antipsychotic‐related constipation | Review | Susanna Every‐Palmer, Giles Newton‐Howes, Mike J Clarke | |
| 2014 May 23 | Pharmacological treatment for antipsychotic‐related constipation | Protocol | Susanna Every‐Palmer, Giles Newton‐Howes, Mike J Clarke | |
Differences between protocol and review
Elobixibat was added as an eligible pharmacological treatment for antipsychotic‐related constipation. We also added cholinergic agents (e.g. bethanecol, donepezil) and acetylcholinesterase inhibitors (e.g. physostigmine) as potentially eligible treatments, recognising that antipsychotic‐induced constipation is considered primarily mediated by anticholinergic mechanisms.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Rheum;
- Acupuncture Therapy;
- Antipsychotic Agents [*adverse effects];
- Constipation [chemically induced, *drug therapy];
- Glycerol [*therapeutic use];
- Laxatives [*therapeutic use];
- Mannitol [*therapeutic use];
- Massage [methods];
- Phenolphthalein [*therapeutic use];
- Randomized Controlled Trials as Topic;
- Suppositories;
Medical Subject Headings Check Words
Humans;
PICO
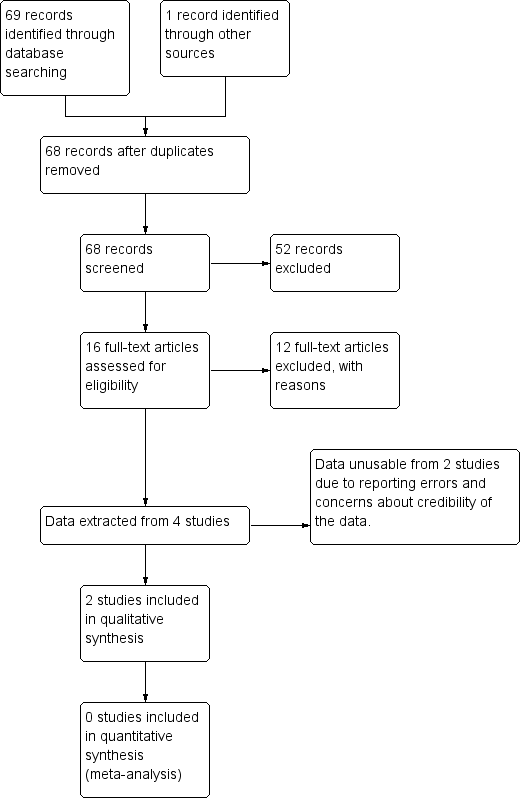
Study flow diagram.
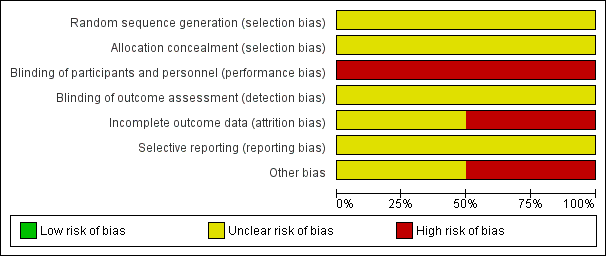
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
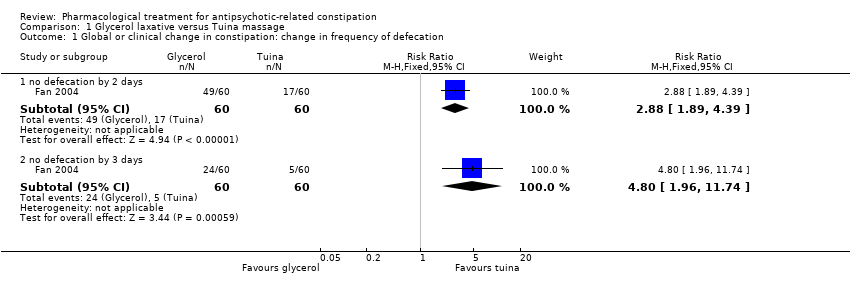
Comparison 1 Glycerol laxative versus Tuina massage, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
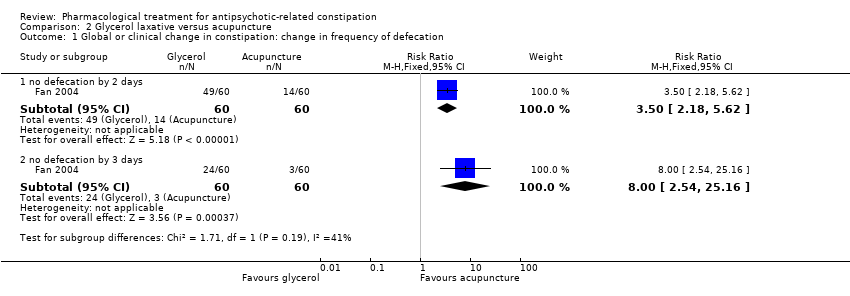
Comparison 2 Glycerol laxative versus acupuncture, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
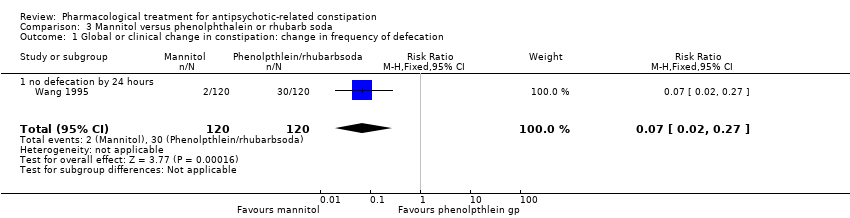
Comparison 3 Mannitol versus phenolphthalein or rhubarb soda, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
| Methods | Allocation: centralised, randomised, sequence generation described. Blinding: participants, personnel recruiting and assigning participants, and assessors. Blinding can be tested by asking participants and raters to guess the assigned treatment. Study duration: 12 weeks. Setting: Inpatients and outpatients |
| Participants | Diagnosis: antipsychotic‐related constipation or antipsychotic induced gastrointestinal hypomotility N = sample size obtained through power calculation* Age: any Sex: both |
| Intervention | Any of the interventions listed in Appendix 1 compared against any other intervention or placebo, |
| Outcomes | Primary Outcomes 1. Global or clinical change in constipation 1.1 Change in the frequency of defecation (e.g. complete spontaneous bowel movements per week) Secondary outcomes
|
| Notes | * Size of study with sufficient power to detect a approximate 10% difference between the two groups for the primary outcome with 80% certainty. |
| Glycerol laxative versus tuina massage | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Tuina | Risk with Glycerol | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 2.88 | 120 | ⊕⊝⊝⊝ | ||
| 283 per 1000 | 816 per 1000 | |||||
| Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 3 days | Study population | RR 4.80 | 120 | ⊕⊝⊝⊝ | ||
| 83 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Glycerol laxative versus acupuncture | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with acupuncture | Risk with glycerol laxative | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 3.50 | 120 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 817 per 1000 | |||||
| Still constipated (no defecation) at 3 days | Study population | RR 8.00 | 120 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Mannitol versus phenolphthalein or rhubarb soda | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with phenolphthalein or rhubarb soda | Risk with Mannitol | |||||
| 1. Global or clinical change in constipation as defined by the study a) Still constipated (no defecation) at 24 hours | Study population | RR 0.07 | 240 | ⊕⊝⊝⊝ | Results from the phenolphthalein and rhubarb soda groups were combined by the study authors. | |
| 250 per 1000 | 18 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | 0 per 1000 | 0 per 1000 | Not estimable | 240 (1 RCT) | ‐ | It is highly questionable how well adverse effects were monitored and recorded. The study simply notes "No side‐effects were detected in the two groups after treatment". |
| 6. Leaving the study early | No studies reported these important outcomes | |||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no defecation by 2 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.89, 4.39] |
| 1.2 no defecation by 3 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.8 [1.96, 11.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no defecation by 2 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [2.18, 5.62] |
| 1.2 no defecation by 3 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [2.54, 25.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.27] |
| 1.1 no defecation by 24 hours | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.27] |

