Tratamiento farmacológico del estreñimiento relacionado con los antipsicóticos
Resumen
Antecedentes
El estreñimiento relacionado con los antipsicóticos es un efecto adverso común y grave, especialmente para las personas que toman clozapina. Se ha demostrado que la clozapina impide la motilidad gastrointestinal, lo que provoca estreñimiento, y se ha informado de que hasta el 60% de los pacientes que reciben clozapina. En casos raros, las complicaciones pueden ser fatales. Se deben prescribir laxantes apropiados para tratar el estreñimiento en las personas que toman antipsicóticos, pero falta orientación sobre la efectividad y los daños comparativos de los diferentes agentes en esta población. La comprensión de la efectividad y la seguridad del tratamiento del estreñimiento relacionado con los antipsicóticos es importante tanto para los médicos como para los pacientes.
Objetivos
Evaluar la efectividad y la seguridad del tratamiento farmacológico (frente al placebo o comparado con otro tratamiento) para el estreñimiento relacionado con los antipsicóticos (definido como los pacientes estreñidos de cualquier edad, que son tratados con antipsicóticos, independientemente de la dosis, en los que el estreñimiento se considera un efecto secundario relacionado con los antipsicóticos).
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos del Grupo Cochrane de Esquizofrenia (Cochrane Schizophrenia Group) (15 de junio de 2015), que se basa en búsquedas regulares en MEDLINE, Embase, CINAHL, BIOSIS, AMED, PubMed, PsycINFO, y en registros de ensayos clínicos, literatura gris y actas de congresos. No hay limitaciones de idioma, fecha, tipo de documento o estado de publicación para la inclusión de los archivos en el registro. También se realizaron búsquedas manuales en las bibliografías y se estableció contacto con los autores pertinentes para obtener información adicional.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorizados (ECA) publicados y no publicados que investigaban la eficacia de los tratamientos farmacológicos en pacientes con estreñimiento relacionado con los antipsicóticos. Los tratamientos farmacológicos incluían laxantes y otros medicamentos que podían utilizarse razonablemente para combatir el estreñimiento en esta población (p.ej., agentes anticolinérgicos, como el betanecol).
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, extrajeron los datos de los estudios incluidos y evaluaron su riesgo de sesgo. Un tercer autor revisó el 20% de los ensayos. Los datos dicotómicos se analizaron mediante los riesgos relativos (RR) y los intervalos de confianza (IC) del 95%. Se evaluó el riesgo de sesgo de los estudios incluidos y se utilizó GRADE para crear la tabla "Resumen de los hallazgos". Se discutió cualquier desacuerdo, se documentó las decisiones e se intentó contactar con los autores del estudio cuando fue necesario.
Resultados principales
Se identificaron dos estudios chinos pertinentes (N = 480) que aportaron datos a esta revisión. Ambos estudios tenían más de diez años de antigüedad y se informaron de manera deficiente, ya que carecían de descripciones de los requisitos previos contemporáneos para la presentación de informes CONSORT, como la generación de secuencias, la ocultación de la asignación, el cegamiento, el flujo de participantes, la forma en que se determinó el tamaño de la muestra o la forma en que se midieron los resultados. Los estudios tampoco informaron sobre el registro de los ensayos, los protocolos preestablecidos, los procesos de consentimiento, la revisión ética o la fuente de financiación. No se pudo establecer contacto con los autores para aclarar los detalles que faltaban. Ambos estudios se clasificaron como de alto riesgo de sesgo.
Un estudio comparó el supositorio de glicerol con los enfoques de la medicina tradicional china (MTC) de masaje tuina y acupuntura. En comparación con el masaje con tuina, el laxante de glicerol fue menos eficaz para aliviar el estreñimiento tanto a los dos días después del tratamiento (1 ECA; N = 120; RR 2,88, IC del 95%: 1,89 a 4,39; evidencia de calidad muy baja), como a los tres días (1 ECA; N = 120; RR 4,80, IC del 95%: 1,96 a 11,74, evidencia de calidad muy baja). También se observaron resultados favorables para la acupuntura a los dos días (1 ECA; N = 120; RR 3,50; IC del 95%: 2,18 a 5,62; evidencia de calidad muy baja), y a los tres días (1 ECA; N = 120; RR 8,00; IC del 95%: 2,54 a 25,16; evidencia de calidad muy baja.
El otro estudio comparó el manitol, un laxante osmótico, con la soda de ruibarbo o la fenolftaleína. El manitol fue más eficaz que la soda de ruibarbo o la fenolftaleína para aliviar el estreñimiento en las 24 horas siguientes al tratamiento (1 ECA; N = 240; RR 0,07; IC del 95%: 0,02 a 0,27, evidencia de calidad muy baja).
No se informaron datos sobre otros resultados importantes: necesidad de medicación de rescate, obstrucción intestinal (una complicación del estreñimiento relacionado con los antipsicóticos), calidad de vida, eventos adversos, abandono temprano del estudio y costes económicos.
Conclusiones de los autores
Se esperaba encontrar evidencia clínicamente útil que evaluara los méritos relativos de las intervenciones que se utilizan habitualmente para tratar el estreñimiento relacionado con los antipsicóticos, un efecto adverso común y potencialmente grave del uso de estas drogas. Los resultados fueron decepcionantes. No había datos que compararan las intervenciones farmacológicas comunes para el estreñimiento, como la lactulosa, el polietilenglicol, los ablandadores de heces, los laxantes lubricantes o los tratamientos novedosos como la linaclotida. Los datos disponibles eran de muy mala calidad y los ensayos tenían un alto riesgo de sesgo. Los datos de esos estudios sesgados indicaban que el manitol, un laxante osmótico, era más eficaz que la soda de ruibarbo y la fenolftaleína para aliviar el estreñimiento, y que un ciclo de dos semanas de supositorios de glicerol era menos eficaz que los enfoques de la MTC del masaje tuina y la acupuntura.
En general, no hay suficiente evidencia basada en ensayos para evaluar la efectividad y la seguridad de las intervenciones farmacológicas para el tratamiento del estreñimiento relacionado con los antipsicóticos, debido a los datos limitados y de baja calidad (pocos estudios con alto riesgo de sesgo y ningún metanálisis). Las limitaciones metodológicas de los estudios incluidos eran evidentes, y cualquier conclusión basada en sus resultados debe hacerse con cautela. Se necesitan ECA metodológicamente rigurosos que evalúen las intervenciones para tratar el estreñimiento relacionado con los antipsicóticos.
PICOs
Resumen en términos sencillos
Tratamientos farmacológicos para el estreñimiento causado por los medicamentos antipsicóticos
Antecedentes
El estreñimiento es un efecto secundario común para las personas que toman medicamentos antipsicóticos, especialmente la clozapina. Se ha demostrado que la clozapina reduce la motilidad intestinal, y las consecuencias de esto son a veces graves. Por cada mil pacientes tratados con clozapina, se cree que entre 300 y 600 sufrirán estreñimiento; al menos cuatro desarrollarán graves complicaciones gastrointestinales (como obstrucción intestinal), de las cuales al menos una morirá.
Búsqueda
En junio de 2015, se buscaron ensayos que compararan los medicamentos utilizados para tratar el estreñimiento relacionado con los antipsicóticos (como los laxantes) con cualquier otro tratamiento. Sin embargo, se encontró poca información útil. Se identificaron dos estudios relevantes en la literatura china, pero ninguno en la literatura occidental.
Resultados principales
Los estudios identificados fueron de corta duración, muy probablemente sesgados, y proporcionaron evidencia de calidad muy baja. Un estudio sugirió que un supositorio de glicerol era menos eficaz que la acupuntura o el masaje tuina, y el otro sugirió que el manitol, un laxante osmótico, era más eficaz que la soda de ruibarbo o la fenolftaleína para tratar el estreñimiento.
Conclusiones
Sobre la base de los resultados, se llegó a la conclusión de que en la actualidad no se dispone de evidencia de buena calidad basada en ensayos que puedan orientar a los médicos y los pacientes en el tratamiento farmacológico del estreñimiento relacionado con los antipsicóticos.
Authors' conclusions
Summary of findings
| Glycerol laxative versus tuina massage | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Tuina | Risk with Glycerol | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 2.88 | 120 | ⊕⊝⊝⊝ | ||
| 283 per 1000 | 816 per 1000 | |||||
| Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 3 days | Study population | RR 4.80 | 120 | ⊕⊝⊝⊝ | ||
| 83 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Glycerol laxative versus acupuncture | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with acupuncture | Risk with glycerol laxative | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 3.50 | 120 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 817 per 1000 | |||||
| Still constipated (no defecation) at 3 days | Study population | RR 8.00 | 120 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Mannitol versus phenolphthalein or rhubarb soda | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with phenolphthalein or rhubarb soda | Risk with Mannitol | |||||
| 1. Global or clinical change in constipation as defined by the study a) Still constipated (no defecation) at 24 hours | Study population | RR 0.07 | 240 | ⊕⊝⊝⊝ | Results from the phenolphthalein and rhubarb soda groups were combined by the study authors. | |
| 250 per 1000 | 18 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | 0 per 1000 | 0 per 1000 | Not estimable | 240 (1 RCT) | ‐ | It is highly questionable how well adverse effects were monitored and recorded. The study simply notes "No side‐effects were detected in the two groups after treatment". |
| 6. Leaving the study early | No studies reported these important outcomes | |||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
Background
Description of the condition
Antipsychotic medications are effective agents in the treatment of schizophrenia and other psychotic disorders, but their adverse effect profiles can be considerable. Constipation due to gastrointestinal hypomotility is one commonly reported and potentially serious adverse effect. Antipsychotics such as clozapine, which have high affinity for muscarinic cholinergic receptors, are the most frequently implicated agents in antipsychotic‐related gastrointestinal hypomotility (Nielsen 2012; Talley 2003). For example, in a study using ingested radiopaque markers to measure gastrointestinal motility, colonic transit times were over four times longer in clozapine‐treated patients compared with both those on other antipsychotics and with population normative values (P < 0.0001). Gastrointestinal hypomotility was demonstrated in 80% of clozapine‐treated patients (Every‐Palmer 2016).
The earliest manifestation of gastrointestinal hypomotility is usually constipation. Constipation is common with many antipsychotics, and especially so with clozapine (Ozbilen 2009). A recent systematic review found that people taking clozapine had significantly higher rates of constipation compared with those taking other antipsychotics (odds ratio (OR) 3.02, 95% confidence interval (CI) 1.91 to 4.77 (Shirazi 2016)).
For clozapine‐treated patients, iatrogenic mortality is higher from constipation or gastrointestinal hypomotility complications than from agranulocytosis (Cohen 2012). For every thousand patients treated with clozapine, it is estimated that 300 to 600 will suffer constipation, and at least four will develop serious gastrointestinal complications (for example bowel obstruction), from which one or more may die (Every‐Palmer 2016). Progression from constipation to ileus, intestinal obstruction, bowel ischaemia, megacolon, and death is not uncommon in antipsychotic‐treated patients, particularly those on clozapine (Drew 1997; Hibbard 2009; Leung 2008; Nielsen 2012; Palmer 2008; Rousseau 2007; Shammi 1997). The mechanism is usually obstruction caused by an impacted stool bolus (sometimes classified as a pseudo‐obstruction), which can increase intraluminal pressure proximal to the impaction, consequently causing ischaemia (Hass 2007), and distension (Boley 1969). This distension may result in perforation, especially if the resultant bowel diameter exceeds 12 cm. Aspiration pneumonia may result from inhalation of faeculent vomitus or as a result of dysphagia (Palmer 2008). However, the underlying pharmacophysiology of these gastrointestinal complications is poorly understood. Primary responsibility is usually attributed to the anticholinergic inhibition of gastrointestinal smooth muscle contraction and peristalsis (Ozbilen 2009; Sirois 2005), but it is likely that antipsychotic medications’ antagonism of various serotonin receptor subtypes is contributory (Palmer 2008), because serotonin plays a crucial role in gastrointestinal motility (Crowell 2001).
Antipsychotic‐treated patients with serious gastrointestinal hypomotility often under‐report symptoms and present late (Palmer 2008). The delayed presentation may arise, at least in part, from patients with psychosis experiencing attenuated pain sensitivity (Dworkin 1994; Fishbain 1982; Rosenthal 1990; Singh 2006), difficulty in communicating discomfort (Bickerstaff 1988), diminished awareness of pain due to the sedative and analgesic effects of psychotropic drugs, such as antidepressants, neuroleptics, and anticonvulsants (Palmer 2008), or a failure by health professionals to recognise gastrointestinal hypomotility as a potentially life‐threatening condition. Consequently, gastrointestinal pathology may be considerably more advanced than the patient’s presentation would suggest.
It is important to recognise that while not all patients with objective gastrointestinal hypomotility are aware of constipation symptoms, laxatives may still have a role in improving bowel motility in those without subjective distress (Every‐Palmer 2016; Baptista 2015). Prophylactic laxatives for all people starting clozapine are recommended by the Porirua Protocol, to prevent clozapine‐related constipation (Every‐Palmer 2017).
Description of the intervention
The treatment of chronic constipation generally includes both non‐pharmacological and pharmacological interventions. This review will consider pharmacological treatment of constipation in treated antipsychotic patients.
The most commonly used pharmacological intervention for treating constipation is the class of medication commonly referred to as laxatives. The subtypes of conventional laxatives are bulk‐forming, emollient, osmotic, and stimulant aperients (see Appendix 1). Other agents that have been researched for their efficacy in treating constipation include: colchicine (Taghavi 2010; Verne 1997; Verne 2003); misoprostol (Roarty 1997; Soffer 1994); cisapride (Vandenplas 1999); lubiprostone (Johanson 2005; Johanson 2005a; Johanson 2007; Johanson 2008); tegaserod (Johanson 2004; Johanson 2004a; Kamm 2005); renzapride (Tack 2006); prucalopride (Camilleri 2008; Camilleri 2009); linaclotide (Andresen 2007; Lembo 2010); and elobixibat (Chey 2011; Wong 2011). In this review, we also included cholinergic agents, such as bethanecol or donepezil, or anticholinesterase inhibitors, such as physostigmine, given the suspected pharmacological mechanism of antipsychotic‐related constipation. The usual dose ranges, mechanisms of action and adverse effects are also summarised in Appendix 1.
Some of these medications have significant, serious, or controversial side‐effect profiles (e.g. cisapride and tegaserod), and their use has been discontinued, or restricted to certain subgroups of patients. However, these restricted medications have been included within the ambit of this review, given the potential for future relaxation of restrictions, and because their prokinetic properties are of particular interest in treating hypomotility disorders. The potential harms and benefits of all treatments were to be analysed and addressed in the discussion.
The treatment of gastrointestinal hypomotility disorders is an evolving field, and subsequent updates of this review will also consider the evidence for any new treatments.
How the intervention might work
Antipsychotic medications can cause bowel motility problems and reduce bowel transit times, resulting in constipation and more serious sequelae. The goals of constipation management include improving symptoms, restoring normal gastrointestinal function, increasing bowel transit, and facilitating defecation (Bleser 2005).
The mechanisms of actions of the different pharmacological treatments for constipation are summarised in Appendix 1.
Why it is important to do this review
Antipsychotics are widely prescribed throughout the world. They currently comprise the largest class of pharmaceuticals by sales in the United States, and their international market was estimated at $19.6 billion in 2010 (BCC Research 2010). Until recently, the morbidity and mortality associated with antipsychotic‐related constipation has been under‐recognised. In a review of 164 English language, randomised controlled trials of clozapine, only 14 of 164 (8.5%) had reported figures on constipation amongst the side‐effect data (Meek 2008). Until recently, clinicians have regarded antipsychotic‐related constipation as an irksome, but not particularly serious complication of treatment. However, it is now evident that progression from constipation to ileus, intestinal obstruction, bowel ischaemia, megacolon, and death is not uncommon, particularly in patients who have been prescribed clozapine. In Australasia, there have been at least 15 deaths related to clozapine‐induced gastrointestinal hypomotility (Palmer 2008). Apart from the life‐threatening consequences, constipation has been consistently shown to have a significant impact on the quality of life of those suffering from it (Damon 2004; Dennison 2005; Glia 1997; Wald 2007).
Surprisingly, given the prevalence of chronic constipation and the widespread use of laxatives, reliable evidence‐based data regarding their effectiveness are lacking, even in the general population (Brandt 2005; Jones 2002; Ramkumar 2005; Rao 2011). Two systematic reviews comparing medical therapies for chronic idiopathic constipation found good evidence for the efficacy of polyethylene glycol (PEG; Brandt 2005; Ramkumar 2005). However, both of these reviews were restricted to published English language studies indexed in Medline, and both listed drug‐induced constipation amongst the exclusion criteria. A Cochrane review similarly endorsed PEG over lactulose for chronic constipation, but also excluded patients with medication‐related constipation (Lee‐Robichaud 2010). Systematic reviews have considered different aspects of the pharmacological treatment for chronic constipation (Brandt 2005; Evans 2008; Lee‐Robichaud 2010; Ramkumar 2005), constipation in adults with central neurological disease (Coggrave 2009), constipation in pregnancy (Jewell 2009; Rungsiprakarn 2015), and constipation in palliative care (Candy 2015). However, there are no systematic reviews collating and examining the strength of the evidence for the effectiveness and safety of pharmacological treatment in antipsychotic‐related constipation.
To date, there is little evidence‐based research on the management of constipation in antipsychotic‐treated patients. Although guidance exists to minimise antipsychotic medications’ adverse haematological (Honigfeld 1998; Lieberman 1989), metabolic (Lambert 2004), and cardiac effects (Berk 2007), these guidelines do not emphasise the need to monitor or treat constipation and its more serious sequelae. Of the few international guidelines that do address antipsychotic‐related constipation (Hasan 2012), recommendations are based on a scarce database (Hasan 2016). There is uncertainty about the management of constipation in this group of patients.
Objectives
To evaluate the effectiveness and safety of pharmacologic treatment (versus placebo, or compared against another treatment) for antipsychotic‐related constipation (defined as constipated patients of any age, who are treated with antipsychotics, regardless of dose, in which constipation is considered to be an antipsychotic‐related side effect).
Methods
Criteria for considering studies for this review
Types of studies
The inclusion criteria were all randomised controlled trials investigating the efficacy of pharmacotherapy for antipsychotic‐related constipation.
If a trial was described as double blind and implied randomisation, we had planned to include it in a sensitivity analysis (see Sensitivity analysis). If participants had been given treatments additional to the pharmacological treatment of constipation, we would only have included data if the adjunct treatment was evenly distributed between groups, and it was only the pharmacological treatment of constipation that was randomised.
We excluded non‐randomised studies, quasi‐randomised studies (such as allocation by day of the week), case reports, clinical observations (open studies), or studies of laxatives used to treat constipation due to other causes.
We included all eligible studies, regardless of language.
Types of participants
Eligible participants were male or female patients of any age who had developed constipation (diagnosed by any method) while prescribed any antipsychotic medications (any dose) with constipation attributed to a side effect of the antipsychotic.
Types of interventions
1. Any pharmacological treatment of antipsychotic related constipation
Any type of the following medication:
-
bulking agents such as psyllium, methylcellulose, calcium polycarbophil, and wheat dextrin;
-
faecal softeners, such as mineral oil, docusate;
-
stimulant laxatives, such as senna and bisacodyl;
-
osmotic agents, such as lactulose, sorbitol, magnesium hydroxide, glycerine, magnesium sulphate, polyethylene glycol;
-
alternative agents, such as lubiprostone, misoprostol, colchicine, tegaserod, cisapride, renzapride, prucalopride, linaclotide, elobixibat, orlistat;
-
cholinergic agents, such as bethanecol or donepezil, or anticholinesterase inhibitors, such as physostigmine.
2. Any other intervention, placebo or no intervention
Types of outcome measures
Primary outcomes
1. Global or clinical change in constipation as defined by the identified studies, including, but not limited to, the following:
1.1 Change in the frequency of defecation;
1.2 Change in straining at defecation;
1.3 Change in the frequency of lumpy or hard stools;
1.4 Change in the frequency of manual manoeuvres to facilitate defecation.
Secondary outcomes
1. Global state
1.1 Significant change in mental or global state (as defined by identified studies);
1.2 Average endpoint score on mental or global state scales;
1.3 Average change scores on mental or global state scales.
2. Need for rescue medication for constipation
3. Constipation‐related outcomes
3.1 Gastrointestinal transit time measurement (determined by gastrointestinal motility studies);
3.2. Presence of antipsychotic‐related constipation complications such as bowel obstruction.
4. Satisfaction with treatment
5. Quality of life
6. Adverse effects
6.1 At least one adverse effect;
6.2 Specific effects;
6.3 Death.
7. Leaving the study early
7.1 Due to adverse effects;
7.2 Due to non‐compliance;
7.3 Any reason.
8. Economic Costs
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011), and the GRADE profiler (GRADEpro GDT) to import data from Review Manager (Review Manager), to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. In our protocol, we selected the following main outcomes for the 'Summary of findings' table.
-
Global or clinical change in constipation (as defined by the studies, including, but not limited to, a change in the frequency of: defecation; straining; hard or lumpy stools; or manual manoeuvres to facilitate defecation);
-
Need for rescue medication;
-
Presence of antipsychotic‐related constipation complications such as bowel obstruction;
-
Quality of life (changed to any extent);
-
Adverse effects;
-
Leaving the study early (the numbers of participants withdrawing before completion of the study due to non‐compliance, adverse effects, or both);
-
Economic costs.
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group’s Trials Register
The Information Specialist (formally the Trials Search Co‐ordinator) searched the Cochrane Schizophrenia Group’s Registry of Trials (5 June 2014 and 15 June 2015), using the following phrase:
*constipat*:ti,ab,kw of REFERENCE or *constipat*:SCO of STUDY
The Cochrane Schizophrenia Group’s Registry of Trials is compiled by systematic searches of major resources (including MEDLINE, Embase, AMED, BIOSIS, CINAHL, PsycINFO, PubMed, and registries of clinical trials), and their monthly updates, hand‐searches, grey literature, and conference proceedings (see Group Module). There are no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies (Horsley 2011).
2. Personal contact
We attempted to contact the first author of each included study for information regarding unpublished trials (Young 2011). Any responses were to be noted in 'Description of Studies' and the 'Characteristics of Included Studies' table.
Data collection and analysis
Selection of studies
Two review authors (SEP and GNH) independently inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by the third author (MC) to ensure reliability. Where uncertainty remained, the full report was acquired for more detailed scrutiny. Full reports of all abstracts meeting the review criteria were obtained and independently inspected by SEP and GNH. Again, a random 20% of reports were re‐evaluated by MC in order to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Two review authors (SEP and GNH) independently extracted data from all included studies. In addition, to ensure reliability, the third author (MC) independently extracted data from a 20% random sample of these studies. Disagreements were discussed, decisions documented, and when necessary, we contacted the authors of studies for clarification. With any remaining problems, MC helped clarify issues, and these final decisions were documented. Data presented only in graphs and figures were extracted where possible, but included only if two review authors independently had the same result. When necessary, we attempted to contact authors through an open‐ended request in order to obtain missing information, or for clarification. No included studies were multicentre, but if they had been, we would have attempted to extract data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple pre‐specified forms. If available, we obtained the following information for each of the eligible studies:
-
study methods (trial design, duration, sequence generation, allocation sequence concealment, blinding, setting, study eligibility criteria);
-
participants (number, age, gender, ethnicity, psychiatric diagnosis, how constipation was defined, dose, type and duration of antipsychotic medication, co‐morbidities, drop‐outs, or withdrawals);
-
pharmacological intervention(s) (type, dose(s), duration, route of administration, integrity of intervention, control used);
-
outcome data (outcome measures used, definition of thresholds, change in frequency of defecation, straining, stool consistency, manual manoeuvres to facilitate defecation, global improvement in symptoms, patient satisfaction, need for rescue medication, transit time measurement);
-
tolerance and adverse effects.
2.2 Scale‐derived data
We would have included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should have either been (i) a self‐report or (ii) completed by an independent rater or relative (not the treatment provider).
2.3 End point versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions. We decided to use primarily end point data, and only use change data if the former were not available. End point and change data were to be combined in the analysis, since, if such data had been available, we would have used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011).
2.4. Skewed data
While no continuous data were eligible for inclusion in this review, for future revisions, recognising that continuous data on clinical outcomes are often not normally distributed, we plan to apply the following standards to all data before inclusion, to avoid the pitfall of applying parametric tests to non‐parametric data:
-
for example, we will enter data from studies of at least 200 participants in the analysis, regardless of the following rules, because skewed data pose less of a problem in large studies. We will also enter change data when continuous data are presented on a scale that includes a possibility of negative values (such as change data), and it is difficult to tell whether data are skewed or not. We will present and enter change data into statistical analyses.
For end point data:
-
when a scale starts from the finite number zero, we will subtract the lowest possible value from the mean, and divide this by the standard deviation (SD). If this value is less than one, it strongly suggests a skew, and the study will be excluded. If this ratio is higher than one but less than two, there is suggestion of skew. We will enter the study and test whether its inclusion or exclusion would change the results substantially. Finally, if the ratio is larger than two, the study will be included, because skew is less likely (Altman 1996; Higgins 2011);
-
if a scale starts from a positive value, the calculation described above will be modified to take the scale starting point into account. In these cases, skew is present if 2 SD > (S ‐ S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
To facilitate comparison between trials, we had intended to convert variables that could be reported in different metrics, such as the number of complete spontaneous bowel motions (mean bowel motions per day, per week or per month), to a common metric (e.g. mean bowel motions per week).
2.6 Conversion of continuous to binary
If relevant, we would have made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into clinically improved or not clinically improved. If data based on these thresholds had not been available, we would have used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data so that the area to the left of the line of no effect indicated a favourable outcome for the treatment of constipation. If keeping to this had made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. Not improved), we would have reported data so that the left of the line indicated an unfavourable outcome, but this would have been noted in the relevant graphs.
Assessment of risk of bias in included studies
Again, two review authors (SEP and GNH) independently assessed risk of bias by using criteria described in the Cochrane Handbook for Systemic Reviews of Interventions to assess trial quality (Higgins 2011a). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article, such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting. The third author (MC) assessed the risk of bias for a 20% random sample of these studies.
If the raters disagreed, the final rating was made by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted the authors of the studies in order to obtain further information. Non‐concurrence in quality assessment was reported, but if disputes arose about which category a trial was to be allocated, again, we resolved by discussion.
We assessed seven main sources of systematic bias for each included study.
-
Randomisation sequence generation (selection bias);
-
Concealment of allocation sequence (selection bias);
-
Blinding of participants and personnel (performance bias);
-
Blinding of outcome assessors (detection bias);
-
Level of completeness of outcome data (attrition bias);
-
Selective outcome reporting (reporting bias);
-
Other bias.
We assessed each domain by determining whether the criteria for that domain had been met (i.e. low risk of bias), whether they had not (i.e. high risk of bias), or whether it was judged 'unclear' because of inadequate reporting.
We did not exclude studies based on a high risk of bias.
Measures of treatment effect
1. Binary data
The outcome measures reported in all four included trials were binary. For these outcomes, where possible, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RRs are more intuitive than odds ratios (Boissel 1999), and that odds ratios tend to be interpreted as RRs by clinicians (Deeks 2000). The number needed to treat for an additional beneficial outcome and the number needed to treat foran additional harmful outcome with their confidence intervals are intuitively attractive to clinicians, but are problematic, both in the accurate calculation in meta‐analyses and interpretation (Hutton 2009). If binary data had been presented in the 'Summary of findings' table(s), we would have calculated illustrative comparative risks.
2. Continuous data
None of the usable data were continuous. For continuous outcomes, we had planned to estimate mean difference (MD) between groups. We prefer not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of considerable similarity had been used, we would have presumed there was a small difference in measurement, and we would have calculated the effect size and transformed it to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Cluster trials were eligible for inclusion, although no such trials were identified. Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice), but analysis and pooling of clustered data pose problems. First, authors often fail to account for intra‐class correlation in clustered studies, leading to a unit of analysis error, whereby P values are spuriously low, confidence intervals unduly narrow, and statistical significance overestimated (Divine 1992). This causes type I errors (Bland 1997; Gulliford 1999).
If cluster trials had been identified, where clustering was not accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. If such trials are included in subsequent versions of this review, we will contact first authors of studies to request intra‐class correlation coefficients (ICCs) for their clustered data, and will adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We have sought statistical advice, and have been advised that the binary data presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the ICC (Design effect = 1 + (m ‐ 1) * ICC; Donner 2002). If the ICC had not been reported, it would have been assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed, taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
While none of our eligible studies were cross‐over trials, for future revisions, we will only use data of the first phase of cross‐over studies. Our concern with cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state, despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002).
3. Studies with multiple treatment groups
We included trials with multiple treatment groups in the analyses. When the study involved more than two treatment groups, and the additional treatment groups were relevant, they were presented in comparisons. Binary data were added and combined within the two‐by‐two table. No continuous outcomes were presented, but if this is the case in future studies, we will combine data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011). Where the additional treatment groups were not relevant, we did not use these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss to follow‐up, data must lose credibility (Xia 2009). No loss to follow‐up was reported in the four eligible studies included in this review, which in itself raises issues of credibility, which are discussed in the Incomplete outcome data (attrition bias) section.
For future versions of this review, we have specified that if more than 50% of the data for any particular outcome are unaccounted for, we will not reproduce these data, or use them in analyses. However, if more than 50% are lost from one randomised group of a study, but the total loss is less than 50%, we will address this in the 'Summary of findings' table(s) by downgrading the quality of the evidence. Finally, we will also downgrade the quality of the evidence if the total loss is between 25% and 50%.
2. Binary
For future reviews, when attrition for a binary outcome is between 0% and 50%, and when these data are not clearly described, we intend to present data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early will be all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcomes of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ will be used for those who did not. We will undertake a sensitivity analysis to test how prone the primary outcomes are to change when data obtained only from people who completed the study to that point are compared to the ITT analysis, using the above assumptions.
3. Continuous
3.1 Attrition
For future revisions, if studies are included where attrition for a continuous outcome is between 0% and 50%, and only data from people who complete the study to that point are reported, we will reproduce these.
3.2 Standard deviations
If standard deviations (SDs) had not been reported, we would have first tried to obtain the missing values from the authors. If not available, and there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either P or Tau² available for differences in mean, we could have calculated them according to the rules described in the Cochrane Handbook for Systemic Reviews of Interventions: "When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n)". Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systemic Reviews of Interventions present detailed formulae for estimating SDs from P, Tau², or F values, confidence intervals, ranges, or other statistics (Deeks 2011). If these formulae did not apply, we would have calculated the SDs according to a validated imputation method, which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus lose information. Nevertheless, we would have examined the validity of the imputations in a sensitivity analysis, excluding imputed values.
3.3. Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who leave trials early or are lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently, methods, such as multiple imputation or mixed‐effects models for repeated measurements (MMRM), have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem. Therefore, in future, we will not exclude studies based on the statistical approach used. However, we will preferably use the more sophisticated approaches, e.g. MMRM, or multiple‐imputation will be preferred to LOCF, and completer analyses will only be presented if some kind of ITT data are not available at all. Moreover, we will address this issue in the Incomplete outcome data item of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered the included studies without seeing comparison data, to judge clinical heterogeneity. We inspected all studies for clearly outlying people or situations, which we had not predicted would arise.
2. Methodological heterogeneity
We considered all included studies without seeing comparison data, to judge methodological heterogeneity. We inspected studies for clearly outlying methods, which we had not predicted would arise.
3. Statistical heterogeneity
3.1 Visual inspection
If meta‐analysis had been possible, we would have visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We had planned to investigate heterogeneity between studies by considering the I² statistic alongside the P value of the Chi² test. The I² provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I² depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (e.g. P value from Chi², or a confidence interval for I²). An I² estimate of at least 50% accompanied by a statistically significant Chi² statistic would have been interpreted as evidence of substantial levels of heterogeneity (Deeks 2011). If substantial levels of heterogeneity were found in the primary outcome, we would have explored reasons for heterogeneity (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systemic Reviews of Interventions (Sterne 2011). We tried to locate the protocols of the included randomised trials. We had planned to compare outcomes in the protocol and in the published report. The protocols were not available for any of our included studies, and so we compared the outcomes listed in the methods section of the trial report with the results actually reported.
2. Funnel plot
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). Again, these are described in Section 10 of the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating publication biases but are of limited use to detect small‐study effects. We had planned to use funnel plots for outcomes if there were 10 or more studies, or where all studies were of similar sizes. When funnel plots were possible, we had planned to seek statistical advice in their interpretation.
Data synthesis
No meta‐analysis was possible due to the small number of studies and quality issues. We had planned to combine data in a meta‐analysis to provide a pooled effect estimate when study data were of sufficient quality and similarity (in diagnostic criteria, intervention, outcome measure, length of follow‐up, and type of analysis). We would have used a fixed‐effect model in the first instance. We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We had elected to use fixed‐effect model for analyses. However, the reader would have been able to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
If a sufficient number of randomised trials had been identified, we had intended to explore the following potential sources of heterogeneity, using subgroup analyses or meta‐regression.
-
Different doses, classes, types of antipsychotics (clozapine versus other antipsychotics; highly anticholinergic antipsychotics versus other antipsychotics; high dose antipsychotics versus low dose antipsychotics).
-
Treatment setting (inpatient or outpatient treatment).
-
Treatment duration (less than or more than two weeks).
-
Intervention ‐ different types, class of laxative used.
2. Investigation of heterogeneity
If inconsistency was high, this would have been reported. First, we would have investigated whether data had been entered correctly. Second, if data were correct, we would have visually inspected the graph, and outlying studies would have been successively removed to see if homogeneity was restored. For this review, we had decided that this should be done with data that contributed no more than 10% of the total weight of the pooled data. If data contributed more than 10%, they would not have been pooled, and issues would have been discussed.
When unanticipated clinical or methodological heterogeneity were obvious, we simply stated hypotheses for future reviews or versions of this review. We did not formally analyse them.
Sensitivity analysis
Had we identified sufficient data, we would have conducted these sensitivity analyses to determine their impact on the primary outcomes.
1. Implication of randomisation
We had planned to inlcude trials that implied randomisation in a sensitivity analysis. We would have included data for primary outcomes in the analyses. If the inclusion of these data did not result in a substantive difference, the study would have remained in the analyses. If inclusion did result in important clinically significant but not necessarily statistically significant differences, we would not have added these data to the results of the better trials, but would have presented such data within a subcategory. All included trials for this review implied randomisation so we did not carry out this sensitivy analysis.
2. Assumptions for lost binary data
We had anticipated that people would be lost to follow‐up. If assumptions were needed about people lost to follow‐up (see Dealing with missing data), we would have compared the primary outcomes' findings when we used our assumption(s), and when we only used data from people who had completed the study to that point. If there had been a substantial difference, we would have reported and discussed results, but would have continued with our assumption.
If assumptions were needed about missing SDs (see Dealing with missing data), we would have compared the primary outcomes' findings when we used our assumption(s), and when we only used data from people who had completed the study to that point. We would have completed the analyses to test how prone the results were to change when completer‐only data were compared to the imputed data, using the above assumption. If there had been a substantial difference, we would have reported results and discussed them, but continued with our assumption.
3. Risk of bias
We had planned to analyse the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding, and outcome reporting. If excluding these trials did not substantially alter the direction or precision of the effect estimates, then data from these trials would have been included in the analysis. However, as we judged all studiesto be at high risk of bias, we did not conduct this analysis.
4. Imputed values
If we had identified eligible cluster‐randomised trials, we would have assessed the effects of including data from trials in which we had used imputed values for ICC when calculating the design effect.
If substantial differences had been noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not have pooled data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
5. Fixed and random effects
We had planned to synthesise all data using a fixed‐effect model, but we had also planned to synthesise data for the primary outcome using a random‐effects model, to evaluate whether this altered the significance of the results.
Results
Description of studies
For substantive descriptions of studies, please see Characteristics of included studies, Characteristics of studies awaiting classification, and Characteristics of excluded studies.
Results of the search
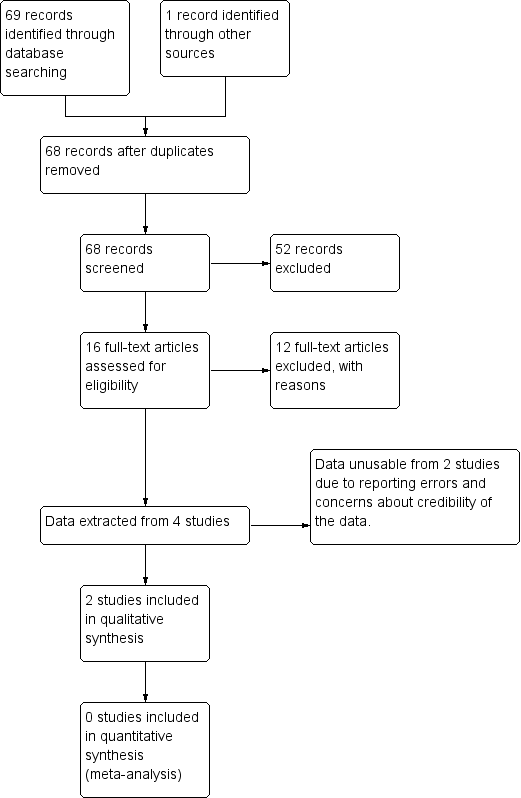
The search was completed on 15 June 2015. The database searches identified 69 potentially relevant papers. One further paper was identified through handsearching reference lists, making a total of 70 studies for screening, 25 of which required translation from Chinese, and one from French. We screened all trials by reviewing their abstracts. We obtained 16 full‐text articles. Of these, four studies were eligible for inclusion, but only two could be included in this version (Fan 2004; Wang 1995); the other two are in Characteristics of studies awaiting classification. We excluded 12 studies. Figure 1 charts the project progress from screening to inclusion.
Study flow diagram.
Included studies
The two studies included a total of 480 participants. Both studies were of parallel design, were undertaken in Chinese hospitals, and reported in Chinese journals. One of the studies required translation from Mandarin (Wang 1995), while the other was published in Mandarin, with an accompanying English translation (Fan 2004).
For detailed characteristics of the included studies see Characteristics of included studies.
Neither study was reported according to CONSORT guidelines.
1. Study design
Both studies were described as randomised controlled trials (RCTs), however, it was unclear whether they were RCTs or quasi‐RCTs because the methods of randomisation and sequence generation were not described. No contact details for the authors (e.g. postal, email address, or telephone number) were provided in the articles, and attempts to contact the authors by writing to them at their host institution to clarify the methodology were unsuccessful.
Study durations were short, with outcomes recorded at 24 hours in one of the studies (Wang 1995), and after two weeks in the other study (Fan 2004).
There was no loss to follow‐up reported.
2. Participants
Both studies reported sample sizes of 240. All 480 participants were inpatients suffering from antipsychotic‐related constipation, defined in both studies as the absence of defecation for four days preceding the trial. Neither study reported whether the absence of defecation was prospectively or retrospectively measured, or was self‐reported or clinician‐rated. The type of antipsychotic was specified, but not the dose. Overall, 64% (N = 306) of the participants were taking clozapine. The other antipsychotics were chlorpromazine (N = 146, 20 of whom were also prescribed clozapine), sulpiride (N = 12), perphenazine (N = 30), and haloperidol (N = 6). There were 145 male participants and 335 females; one study only recruited female participants (Wang 1995).
3. Interventions
Fan 2004 compared the effect of a glycerol suppository compared with acupuncture alone; tuina massage alone; and combined with acupuncture and tuina massage (Fan 2004). Tuina is a form of Traditional Chinese Medicine (TCM) that aims to ‘open the body's defensive (wei) chi and get energy moving in the meridians’ (Beal 2000). The specific technique is described in the paper. Acupuncture is another form of TCM that involves penetration of the skin with needles to stimulate certain points on the body (called acupoints). The selected points are described in the paper. The treatments were given every day for 14 days, and then responses to the laxative were recorded over the following three days (days 14 to 17).
Wang 1995 compared a single 250 mL 10% mannitol dose versus two doses of either (a) rhubarb soda or (b) six to eight phenolphthalein tablets. The results from the rhubarb soda and phenolphthalein groups were not presented separately, meaning we could only analyse the data for mannitol versus the combined group. It was not clear how, why, or how many patients were allocated to phenolphthalein (as opposed to rhubarb soda).
4. Outcomes
Our primary outcome of interest was defined broadly as global or clinical change in constipation, as defined by the identified studies including, but not limited to the following: change in the frequency of defecation; change in straining at defecation; change in the frequency of lumpy or hard stools; change in the frequency of manual manoeuvres to facilitate defecation. Neither study used a validated measure (such as the ROME criteria) to diagnose the presence or resolution of constipation. Fan 2004 defined a constipation "cure" as "defecate once within two days with soft stool and free movement of bowels". "Effectiveness" was defined as "defecate once within three days with soft stool and still no free movement of bowels". "Ineffectiveness" was defined as "no symptoms improved after treatment". Wang 1995 defined defecation within 24 hours as an effective response. It is not clear in either of the studies how response to the laxative was measured, i.e. whether it was self‐reported or observed.
Wang 1995 mentioned that no participant reported adverse effects, but these are referred to generically, without any description of whether they screened for adverse effects (and if so, how), or relied on spontaneous complaints.
Neither study reported continuous data, nor presented data on our other pre‐defined secondary outcomes including: changes in mental state; need for rescue medication; presence of antipsychotic‐related constipation complications, such as bowel obstruction; quality of life; leaving the study early (the numbers of participants withdrawing before completion of the study due to non‐compliance, adverse effects, or both); or economic costs.
Excluded studies
We excluded 12 studies after assessing the full‐text publications. Reasons for exclusion included the study not being a randomised trial, the population not suffering from antipsychotic‐related constipation, or the intervention not constituting a pharmacological treatment for constipation. See Characteristics of excluded studies table for details.
Most exclusion decisions were straightforward and unanimous, but discussion was required between the authors in order to make a decision on Chukhin 2013. This study compared orlistat, a peripherally acting anti‐obesity agent, with placebo in overweight clozapine‐treated patients in a placebo‐controlled add‐on study. The proportion of constipation in each group was compared, using the Bristol Stool Chart as a measurement tool. This study was excluded because the study population selected did not all suffer antipsychotic‐related constipation; of the forty participants, only half were identified as constipated at the start of the trial, and separate data were not provided for this cohort.
Ongoing studies
Searches of trial registries did not identify any ongoing trials in this area.
Studies awaiting classification
After extracting data from the four potentially eligible studies, it became evident that two of these studies, both of which compared senna to TCM remedies, could not be included without further clarification from the authors (Liang 2002; Zhang 2004). We had concerns about discrepancies in methodology and data analysis, and serious concerns that the papers were virtually identical, although credited to different authors, raising concerns about authenticity and credibility. To date, we have been unsuccessful in making contact with the authors to clarify these issues. These two studies remain in the Characteristics of studies awaiting classification section.
Risk of bias in included studies
Both trials under‐reported key design features. The majority of information across all the quality domains was at high or unclear risk of bias. Attempts to contact the authors to clarify these areas of bias were unsuccessful. See Characteristics of included studies for full details of risk of bias judgements for each study. Graphical representations of the overall risk of bias for included studies are presented in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Neither study adequately described how random allocation was generated, nor the methods used to conceal random allocation. In both studies, the number of participants were evenly balanced in each group. It is possible that one or both of these studies were in fact not randomised, or were quasi‐randomised. A poor researcher understanding of the difference between randomisation and quasi‐randomisation in older TCM studies has been demonstrated in other medical fields. In a systematic review considering TCM for ectopic pregnancy, the reviewers communicated by telephone with the original authors of 243 potentially eligible studies that had been described as randomised (Qu 2011). Of these, all 243 were excluded. More than 67% of study authors were shown to have misunderstood the concepts of randomisation and allocation concealment.
Unfortunately we did not receive responses to our letters to study authors requesting further details, so could not clarify whether the studies were randomised or quasi‐randomised. We have rated this domain as unclear risk of bias for both studies.
Blinding
No information regarding blinding was provided in either of the studies. Owing to differences in the physical characteristics of the pharmacological treatment and the comparison, blinding was thought highly unlikely to have occurred in the participants and personnel. Asessor blinding was methodologically possible, although was not described in either study.
Incomplete outcome data
Neither study provided any information about losses to follow‐up, or whether intention‐to‐treat or per‐protocol analysis was used. Prima facie across both studies, it appears that all 480 recruited participants completed the study with 0% dropouts or exclusions.
For example, in Fan 2004, 240 psychiatric inpatients were recruited, and for 14 days underwent either daily rectal enemas, daily periods of tuina massage, half an hour of acupuncture, or a combination of all three treatments. It seems improbable, given the nature of the population and interventions that there would be no attrition from either discharges, withdrawal of consent due to inability (or unwillingness) to adhere to the intensive experimental regime, or protocol deviations due to the need for rescue medications or missed treatments. However, on paper, this study has 100% adherence, no dropouts, no missing data, no protocol violations and no adverse events. We are sceptical about this.
We suspect both studies used a per‐protocol (as treated) analysis and only reported outcomes on those who completed the study. Exclusions have been found to be poorly reported in studies from the pre‐CONSORT era (Moher 2001). The Cochrane Handbook of Systematic Reviews of Interventions speculates that an apparent lack of exclusions may be indicative of poor trial conduct rather than the true lack of exclusions (Higgins 2011).
Selective reporting
It is unclear if the studies were at risk of reporting bias because protocols were not available, therefore, we could not compare pre‐specified outcomes with those outcomes reported in the published reports.
Other potential sources of bias
Neither study reported their funding source. This is important, given the strong evidence that industry‐funded studies produce results that differ from independently‐funded studies, with industry‐sponsored trials exaggerating treatment effects in favour of the products preferred by their sponsor (Bekelman 2003; Song 2010; Lundh 2012). This may be particularly important in mental health; although industry influence has been pervasive across medicine, psychiatry has been at the epicentre of much of the controversy about funding source bias and conflict of interest (Every‐Palmer 2014). We see the lack of transparency regarding funding source as another potential source of bias.
Both included studies were from the People's Republic of China and involved TCM remedies for constipation compared against a pharmacological intervention. It is unclear if this represented a racial or cultural bias when applied to other regions.
Effects of interventions
See: Summary of findings for the main comparison Glycerol laxative versus tuina massage for antipsychotic‐related constipation; Summary of findings 2 Glycerol laxative versus acupuncture for antipsychotic‐related constipation; Summary of findings 3 Mannitol versus phenolphthalein or rhubarb soda for antipsychotic‐related constipation
1. Comparison 01: Glycerol laxative versus tuina massage
See also summary of findings Table for the main comparison.
1.1 Global or clinical change in constipation: change in frequency of defecation
One study, involving 120 participants in these two arms, provided data for this comparison (Fan 2004). Categorical outcomes were reported for global improvement in constipation as defined by the authors, and categorised as a cure, which was defined as defecating once within two days with soft stool and free movement of bowels effectiveness, which was defined as defecating once within three days with soft stool and still no free movement of bowels, or ineffectiveness, which was no symptoms improved after treatment.
Glycerol was shown to be less effective than tuina massage in promoting defecation by two days (1 RCT; N = 120; RR 2.88, 95% CI 1.89 to 4.39), compared with being not cured, and by three days (1 RCT; N = 120; RR 4.80, 95% CI 1.96 to 11.74; Analysis 1.1).
Efficacy time was also reported as a continuous measure, in hours. We inferred that this was the mean time‐to‐defecation for the subset of participants in the cure group, but because this was not clearly defined, they were not usable data.
No data were identified for any of the other pre‐specified primary outcomes (change in the frequency of defecation, change in straining at defecation, change in the frequency of lumpy or hard stools, change in the frequency of manual manoeuvres to facilitate defecation), or any of the secondary outcomes.
Adverse effect data were not provided.
2. Comparison 02: Glycerol laxative versus acupuncture
See summary of findings Table 2.
The same study also provided data for the second comparison (Fan 2004).
2.1 Global or clinical change in constipation: change in frequency of defecation
Glycerol was shown to be less effective than acupuncture in promoting defecation by two days (1 RCT; N = 120; RR 3.50, 95% CI 2.18 to 5.62), and by three days (1 RCT; N = 120; RR 8.00, 95% CI 2.54 to 25.16; Analysis 2.1).
Adverse effect data were not provided.
3. Comparison 03: Mannitol versus phenolphthalein or rhubarb soda
See summary of findings Table 3.
One study, involving 240 women, provided data for this comparison (Wang 1995).
3.1 Global or clinical change in constipation: change in frequency of defecation
Data were identified for global improvement in constipation as defined by the authors, defined in this study as defecation within 24 hours of treatment. No data were identified for any of this review's other pre‐specified primary outcomes (change in the frequency of defecation, change in straining at defecation, change in the frequency of lumpy or hard stools, change in the frequency of manual manoeuvres to facilitate defecation).
The risk of remaining constipated within 24 hours of treatment was lower in women who took mannitol compared with those who took rhubarb soda or phenolphthalein (1 RCT; N = 240; RR 0.07, 95% CI 0.02 to 0.27; Analysis 3.1).
Average time‐to‐defecation was also reported for a subset of participants, but because it was not clear how many participants comprised this subset, we could not use these data.
The authors reported that no adverse effects were detected in either group. They provided no description of what adverse effects they monitored, or how they assessed these.
Discussion
Summary of main results
There was an overall paucity of data, with only two studies contributing data to the review. The included studies were judged to be at high risk of bias. We were only able to analyse three comparisons, with one study each.
Fan 2004 found a glycerol suppository to be less effective than the traditional Chinese medicine (TCM) approach of tuina massage for relieving constipation at two days (RR 2.88, 95% CI 1.89 to 4.39), and at three days (RR 4.80, 95% CI 1.96 to 11.74). In the same study, a glycerol suppository was also less effective than acupuncture at two days (RR 3.50, 95% CI 2.18 to 5.62), and at three days (RR 8.00, 95% CI 2.54 to 25.16). In Wang 1995, a single dose of mannitol, an osmotic laxative, was more effective than rhubarb soda or phenolphthalein in relieving constipation within 24 hours of treatment (RR 0.07, 95% CI 0.02 to 0.27).
Insufficient data were provided for any subgroup analyses.
None of the included studies reported information on the secondary outcomes identified (need for rescue medication, transit time measurement, measures of satisfaction or patient preference, measures of quality of life, cost‐effectiveness, or the presence of antipsychotic‐related gastrointestinal hypomotility complications, such as bowel obstruction).
Overall completeness and applicability of evidence
1. Completeness
Our review findings are limited. The studies were few and study durations were very short. The data on phenolphthalein could not be presented separately, as it had been combined with results from another intervention (rhubarb soda; Wang 1995). Usable data related only to mannitol and glycerol enemas, neither of which are commonly used for managing constipation in contemporary psychiatric care.
There were no data available on the safety and efficacy of commonly used pharmacological interventions, such as bulking agents (e.g. psyllium, methylcellulose, calcium polycarbophil, and wheat dextrin), faecal softeners (e.g. mineral oil, docusate), or commonly used osmotic agents (e.g. lactulose, polyethylene glycol). Neither were there any studies on novel agents, such as lubiprostone, prucalopride, linaclotide, or elobixibat, on cholinergic agents, such as bethanecol or donepezil, or on anticholinesterase inhibitors, such as physostigmine. The pharmacological intervention was so poorly described in the two senna trials we identified, that the data were unusable (Liang 2002; Zhang 2004).
Within the included studies, many important outcomes were not reported. Both of the included studies used idiosyncratic, invalidated, and poorly described methods to measure constipation, for example the passing of a bowel movement over a specified timeframe (e.g. one or two days), as resolution of constipation.
Simply passing a bowel motion does not equate to a cure for constipation. Constipated people still pass stool, although this may be infrequent, uncomfortable, or difficult. Even severe constipation leading to faecal impaction may still present with overflow diarrhoea. Assessing stool frequency (usually averaged over a period of time), consistency, amount, and ease of passing are critical in assessing constipation. The Rome criteria for constipation have been the benchmark over the last two decades, and an updated version has just been released (Drossman 2016). It has been recommended that the Rome definition should form the basis of any tool used to assess constipation (Panchal 2007). However, the included studies did not use any standardised tools.
Neither study adequately reported any of our pre‐specified secondary outcome measures. Adverse event data were very poorly reported. We were able to extract data from one study, stating they did not collect any data on adverse effects (Fan 2004). ,The other simply stated that "no side effects were detected in the two groups after treatment", without any description of how adverse effects were defined and monitored (Wang 1995). There were no data on any of the other secondary outcome measures.
There were also insufficient data to perform the intended subgroup analyses, for example comparing constipation treatment efficacy by different doses, classes, and types of antipsychotics, in different treatment settings, or for short‐term versus long‐term treatment.
2. Applicability
Both studies were conducted in China, in psychiatric inpatients. The primary focus was non‐pharmacological TCM techniques, with pharmacological treatment as the comparator. Studies were of very short duration. There are difficulties in applying this evidence to psychiatric practice elsewhere.
Quality of the evidence
The included studies had serious methodological flaws, and overall the quality of the evidence was very low.
Neither of the included studies provided information on whether they were following an a priori protocol. Neither had been registered. There was also a lack of information about any ethical review or any informed consent process. Both trials provided very limited information about trial design and methodology. Neither trial reported a pre‐trial estimation of sample size, nor methods of randomisation or allocation concealment. Neither appeared to use any form of blinding. The trials were extremely short, with one running for just 24 hours, and one running for 17 days. Given that antipsychotic‐related constipation is a chronic condition, longer trials are clearly needed.
The studies presented only limited outcome data, without the raw data sets, and with confusing data presentation with some obvious mistakes and contradictions.
The studies were both at high or unclear risk of bias in all domains. Please see Figure 2 for 'Risk of bias' graph and Figure 3 for 'Risk of bias' summary. Potential bias may have been present in participant selection, treatment administration, and outcome reporting; all results should be interpreted with caution. The very low‐quality evidence means there is currently insufficient evidence to adequately assess the effectiveness and safety of pharmacological interventions for antipsychotic‐related constipation.
Potential biases in the review process
None of the review authors could read Mandarin, and studies required translation by third parties.
We identified only a few trials, all from the Chinese literature. It is possible other small Chinese trials exist, which may not have been identified.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews assessing the effectiveness and harms of interventions for antipsychotic‐related constipation.
One naturalistic study has shown a significant reduction in the colonic transit times of clozapine‐treated patients, when treated with docusate and senna, and augmented by polyethylene glycol, in accordance with the Porirua Protocol (Every‐Palmer 2017). Another study found the proportion of patients with constipation reduced with orlistat compared to placebo (Chukhin 2013). Neither of these studies were eligible for this review, but they are noted as they do specifically address the treatment of antipsychotic‐related constipation.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Glycerol laxative versus Tuina massage, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.

Comparison 2 Glycerol laxative versus acupuncture, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.

Comparison 3 Mannitol versus phenolphthalein or rhubarb soda, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
| Methods | Allocation: centralised, randomised, sequence generation described. Blinding: participants, personnel recruiting and assigning participants, and assessors. Blinding can be tested by asking participants and raters to guess the assigned treatment. Study duration: 12 weeks. Setting: Inpatients and outpatients |
| Participants | Diagnosis: antipsychotic‐related constipation or antipsychotic induced gastrointestinal hypomotility N = sample size obtained through power calculation* Age: any Sex: both |
| Intervention | Any of the interventions listed in Appendix 1 compared against any other intervention or placebo, |
| Outcomes | Primary Outcomes 1. Global or clinical change in constipation 1.1 Change in the frequency of defecation (e.g. complete spontaneous bowel movements per week) Secondary outcomes
|
| Notes | * Size of study with sufficient power to detect a approximate 10% difference between the two groups for the primary outcome with 80% certainty. |
| Glycerol laxative versus tuina massage | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Tuina | Risk with Glycerol | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 2.88 | 120 | ⊕⊝⊝⊝ | ||
| 283 per 1000 | 816 per 1000 | |||||
| Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 3 days | Study population | RR 4.80 | 120 | ⊕⊝⊝⊝ | ||
| 83 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Glycerol laxative versus acupuncture | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with acupuncture | Risk with glycerol laxative | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 3.50 | 120 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 817 per 1000 | |||||
| Still constipated (no defecation) at 3 days | Study population | RR 8.00 | 120 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Mannitol versus phenolphthalein or rhubarb soda | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with phenolphthalein or rhubarb soda | Risk with Mannitol | |||||
| 1. Global or clinical change in constipation as defined by the study a) Still constipated (no defecation) at 24 hours | Study population | RR 0.07 | 240 | ⊕⊝⊝⊝ | Results from the phenolphthalein and rhubarb soda groups were combined by the study authors. | |
| 250 per 1000 | 18 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | 0 per 1000 | 0 per 1000 | Not estimable | 240 (1 RCT) | ‐ | It is highly questionable how well adverse effects were monitored and recorded. The study simply notes "No side‐effects were detected in the two groups after treatment". |
| 6. Leaving the study early | No studies reported these important outcomes | |||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no defecation by 2 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.89, 4.39] |
| 1.2 no defecation by 3 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.8 [1.96, 11.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no defecation by 2 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [2.18, 5.62] |
| 1.2 no defecation by 3 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [2.54, 25.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.27] |
| 1.1 no defecation by 24 hours | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.27] |

