Dimetilfumarato para la esclerosis múltiple
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011076.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 abril 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esclerosis múltiple y enfermedades raras del sistema nervioso central
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All correspondence: Dian He
Drafting of review versions: Zhu Xu and Dian He
Search for trials: Feng Zhang and FangLi Sun
Obtaining copies of trial reports: KeFeng Gu and Shuai Dong
Selection of trials for inclusion/exclusion: Zhu Xu and Dian He
Extraction of data: Zhu Xu and Dian He
Entry of data: Zhu Xu and Dian He
Interpretation of data analyses: Dian He
Sources of support
Internal sources
-
Guiyang Medical College, China.
External sources
-
The Science and Technology Department of Guizhou Province [No. LH(2014)7134], China.
Declarations of interest
HD ‐ none
XZ ‐ none
ZF ‐ none
SFL ‐ none
GKF‐ none
DS ‐ none
Acknowledgements
We thank Andrea Fittipaldo, Trials Search Co‐ordinator, and Liliana Coco, Managing Editor of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group, for their help and support in developing this review. We thank all peer reviewers, and Graziella Filippini, the Co‐ordinating editor of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group, for their constructive comments and suggestions for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Apr 22 | Dimethyl fumarate for multiple sclerosis | Review | Zhu Xu, Feng Zhang, FangLi Sun, KeFeng Gu, Shuai Dong, Dian He | |
| 2014 Apr 18 | Dimethyl fumarate for multiple sclerosis | Protocol | Zhu Xu, Feng Zhang, FangLi Sun, KeFeng Gu, Shuai Dong, Dian He | |
Differences between protocol and review
In the current review, we rephrased "disability progression" using "disability worsening". Only the primary benefit outcomes, the proportion of patients who discontinued study drug because of AEs and the proportion of patients with lymphopenia for low‐dose dimethyl fumarate administration were selected to create the 'Summary of findings' table because these outcomes are the most relevant to clinical practice. The restriction of "double‐blind studies" pre‐set in the protocol was removed. This change did not influence the conclusion of the review because all the trials eligible for the revised criteria were double‐blind. In addition, we removed duration of follow‐up and administration frequency as covariates for subgroup analysis in future updates.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.
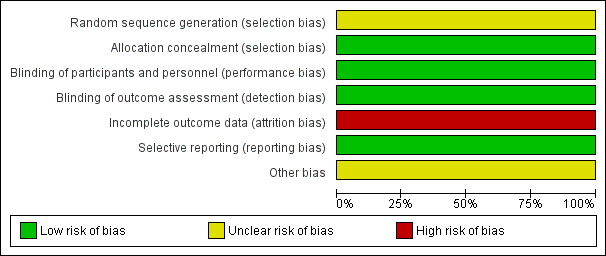
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
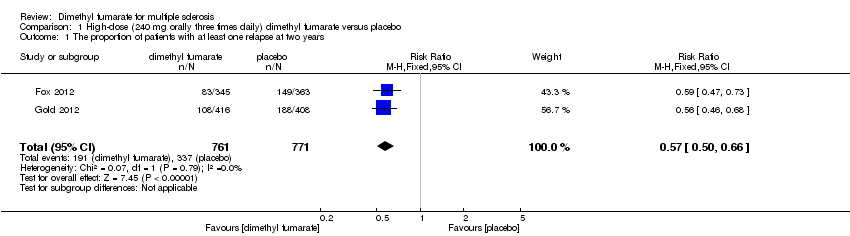
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 1 The proportion of patients with at least one relapse at two years.
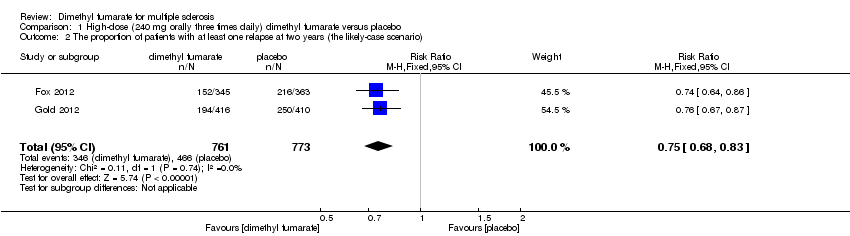
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 2 The proportion of patients with at least one relapse at two years (the likely‐case scenario).
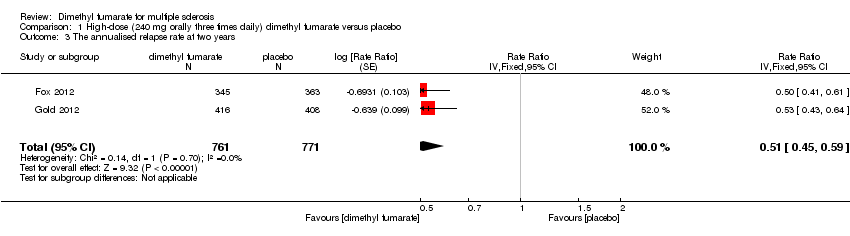
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 3 The annualised relapse rate at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 4 The proportion of patients with disability worsening at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 5 The proportion of patients with disability worsening at two years (the likely‐case scenario).
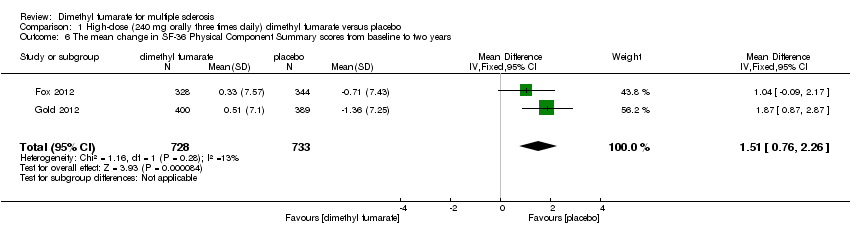
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 6 The mean change in SF‐36 Physical Component Summary scores from baseline to two years.
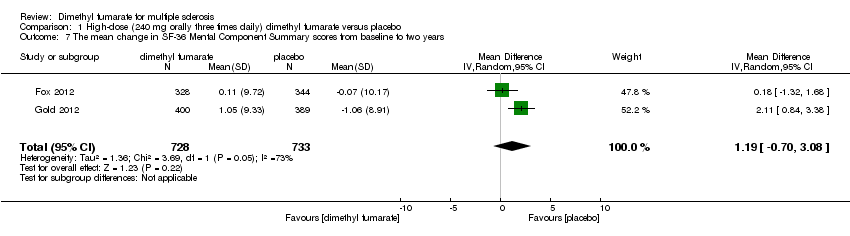
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 7 The mean change in SF‐36 Mental Component Summary scores from baseline to two years.
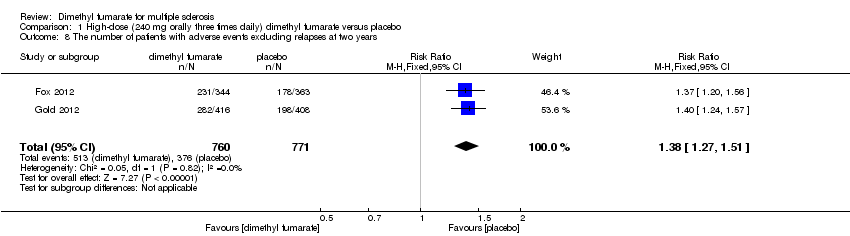
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 8 The number of patients with adverse events excluding relapses at two years.
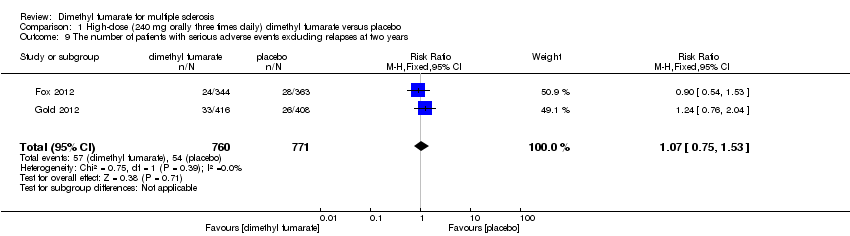
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 9 The number of patients with serious adverse events excluding relapses at two years.
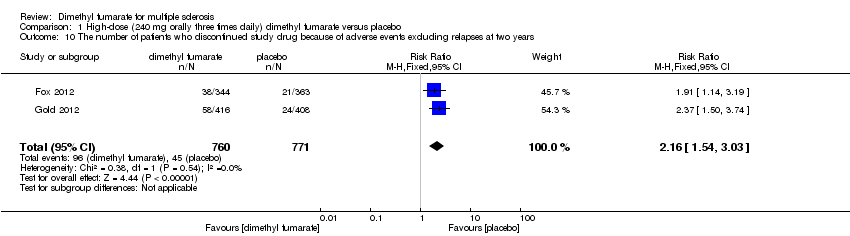
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 10 The number of patients who discontinued study drug because of adverse events excluding relapses at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 11 The number of patients with flushing at two years.
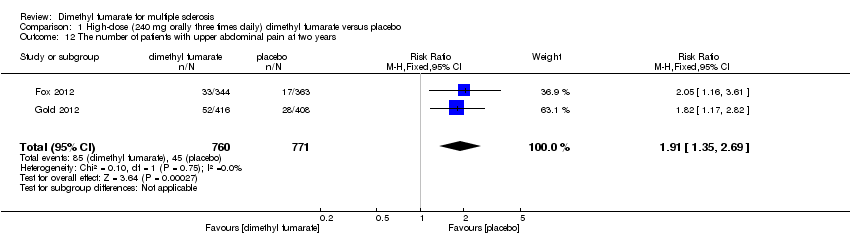
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 12 The number of patients with upper abdominal pain at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 13 The number of patients with nausea at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 14 The number of patients with diarrhoea at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 15 The number of patients with proteinuria at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 16 The number of patients with a decreased lymphocyte count of less than 0.5×109 per litre at two years.
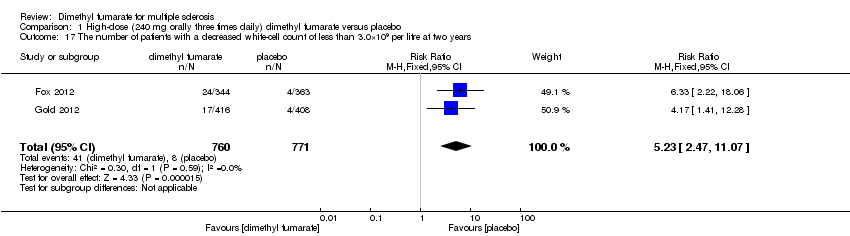
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 17 The number of patients with a decreased white‐cell count of less than 3.0×109 per litre at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 18 The number of patients with an increased alanine aminotransferase level at least three times the upper limit of the normal range at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 19 The number of patients with gastrointestinal events at two years.
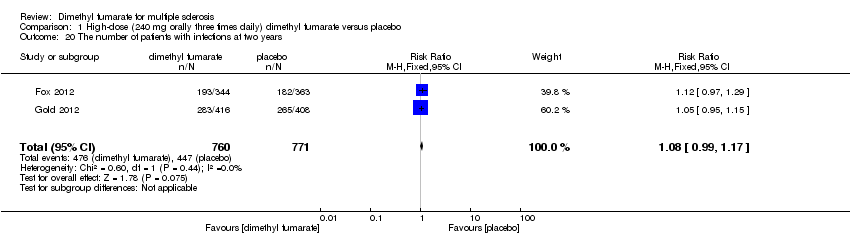
Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 20 The number of patients with infections at two years.

Comparison 1 High‐dose (240 mg orally three times daily) dimethyl fumarate versus placebo, Outcome 21 The number of patients with serious infections at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 1 The proportion of patients with at least one relapse at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 2 The proportion of patients with at least one relapse at two years (the likely‐case scenario).
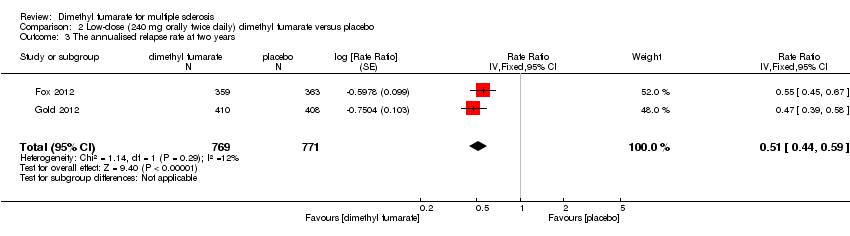
Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 3 The annualised relapse rate at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 4 The proportion of patients with disability worsening at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 5 The proportion of patients with disability worsening at two years (the likely‐case scenario).
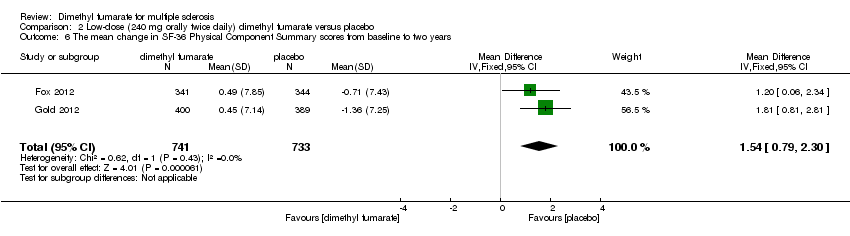
Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 6 The mean change in SF‐36 Physical Component Summary scores from baseline to two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 7 The mean change in SF‐36 Mental Component Summary scores from baseline to two years.
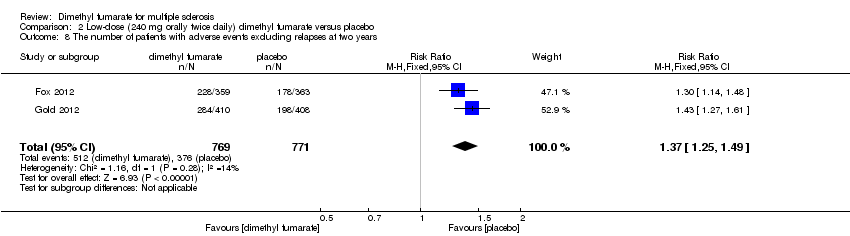
Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 8 The number of patients with adverse events excluding relapses at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 9 The number of patients with serious adverse events excluding relapses at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 10 The number of patients who discontinued study drug because of adverse events excluding relapses at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 11 The number of patients with flushing at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 12 The number of patients with upper abdominal pain at two years.
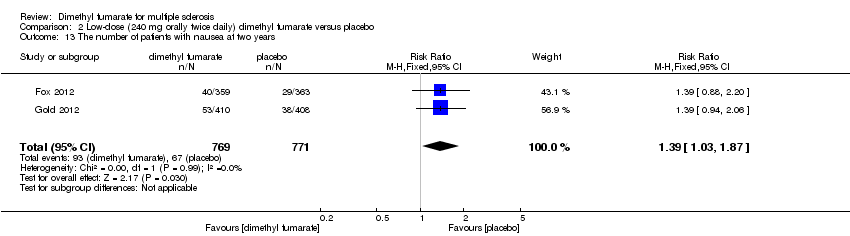
Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 13 The number of patients with nausea at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 14 The number of patients with diarrhoea at two years.
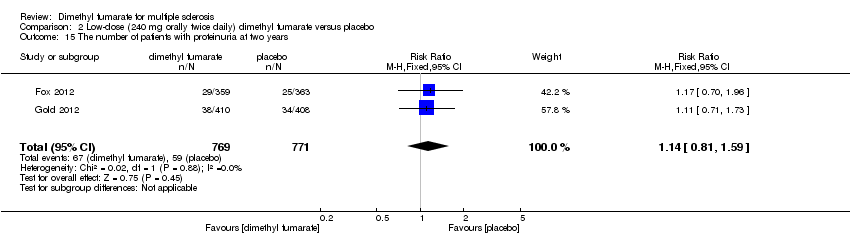
Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 15 The number of patients with proteinuria at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 16 The number of patients with a decreased lymphocyte count of less than 0.5×109 per litre at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 17 The number of patients with a decreased white‐cell count of less than 3.0×109 per litre at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 18 The number of patients with an increased alanine aminotransferase level at least three times the upper limit of the normal range at two years.
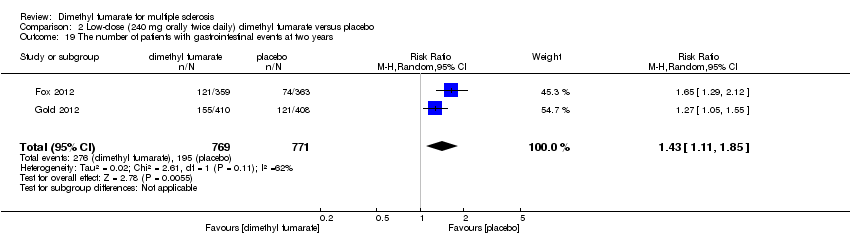
Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 19 The number of patients with gastrointestinal events at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 20 The number of patients with infections at two years.

Comparison 2 Low‐dose (240 mg orally twice daily) dimethyl fumarate versus placebo, Outcome 21 The number of patients with serious infections at two years.
| Dimethyl fumarate for multiple sclerosis | ||||||
| Patient or population: Patients with relapsing‐remitting multiple sclerosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Dimethyl fumarate | |||||
| The proportion of patients with at least one relapse at two years | Study population | RR 0.64 | 1540 | ⊕⊕⊕⊝ | ||
| 437 per 1000 | 280 per 1000 | |||||
| The proportion of patients with disability worsening at two years | Study population | RR 0.65 | 1539 | ⊕⊕⊝⊝ | ||
| 223 per 1000 | 145 per 1000 | |||||
| The proportion of patients who discontinued study drug because of adverse events excluding relapses at two years | Study population | RR 2.18 | 1540 | ⊕⊕⊕⊝ | ||
| 58 per 1000 | 127 per 1000 | |||||
| The proportion of patients with lymphopenia at two years Follow‐up: 2 years | Study population | RR 5.69 | 1540 | ⊕⊕⊕⊝ | ||
| 8 per 1000 | 44 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median placebo group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 A high rate of dropouts existed and reasons of dropouts were unbalanced between arms | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The proportion of patients with at least one relapse at two years Show forest plot | 2 | 1532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.50, 0.66] |
| 2 The proportion of patients with at least one relapse at two years (the likely‐case scenario) Show forest plot | 2 | 1534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| 3 The annualised relapse rate at two years Show forest plot | 2 | 1532 | Rate Ratio (Fixed, 95% CI) | 0.51 [0.45, 0.59] |
| 4 The proportion of patients with disability worsening at two years Show forest plot | 2 | 1532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.87] |
| 5 The proportion of patients with disability worsening at two years (the likely‐case scenario) Show forest plot | 2 | 1534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 6 The mean change in SF‐36 Physical Component Summary scores from baseline to two years Show forest plot | 2 | 1461 | Mean Difference (IV, Fixed, 95% CI) | 1.51 [0.76, 2.26] |
| 7 The mean change in SF‐36 Mental Component Summary scores from baseline to two years Show forest plot | 2 | 1461 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐0.70, 3.08] |
| 8 The number of patients with adverse events excluding relapses at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.27, 1.51] |
| 9 The number of patients with serious adverse events excluding relapses at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.53] |
| 10 The number of patients who discontinued study drug because of adverse events excluding relapses at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.54, 3.03] |
| 11 The number of patients with flushing at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.57 [4.62, 9.35] |
| 12 The number of patients with upper abdominal pain at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.35, 2.69] |
| 13 The number of patients with nausea at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.19, 2.12] |
| 14 The number of patients with diarrhoea at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.20, 2.01] |
| 15 The number of patients with proteinuria at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.06, 2.00] |
| 16 The number of patients with a decreased lymphocyte count of less than 0.5×109 per litre at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [2.20, 12.51] |
| 17 The number of patients with a decreased white‐cell count of less than 3.0×109 per litre at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.23 [2.47, 11.07] |
| 18 The number of patients with an increased alanine aminotransferase level at least three times the upper limit of the normal range at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.61, 2.94] |
| 19 The number of patients with gastrointestinal events at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.31, 2.12] |
| 20 The number of patients with infections at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.99, 1.17] |
| 21 The number of patients with serious infections at two years Show forest plot | 2 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.64, 2.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The proportion of patients with at least one relapse at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.54, 0.77] |
| 2 The proportion of patients with at least one relapse at two years (the likely‐case scenario) Show forest plot | 2 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.89] |
| 3 The annualised relapse rate at two years Show forest plot | 2 | 1540 | Rate Ratio (Fixed, 95% CI) | 0.51 [0.44, 0.59] |
| 4 The proportion of patients with disability worsening at two years Show forest plot | 2 | 1539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.53, 0.81] |
| 5 The proportion of patients with disability worsening at two years (the likely‐case scenario) Show forest plot | 2 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.73, 0.94] |
| 6 The mean change in SF‐36 Physical Component Summary scores from baseline to two years Show forest plot | 2 | 1474 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [0.79, 2.30] |
| 7 The mean change in SF‐36 Mental Component Summary scores from baseline to two years Show forest plot | 2 | 1474 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐0.06, 1.93] |
| 8 The number of patients with adverse events excluding relapses at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.25, 1.49] |
| 9 The number of patients with serious adverse events excluding relapses at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.74] |
| 10 The number of patients who discontinued study drug because of adverse events excluding relapses at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.56, 3.06] |
| 11 The number of patients with flushing at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.01 [5.66, 11.34] |
| 12 The number of patients with upper abdominal pain at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.19, 2.41] |
| 13 The number of patients with nausea at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.03, 1.87] |
| 14 The number of patients with diarrhoea at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.91, 1.87] |
| 15 The number of patients with proteinuria at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.81, 1.59] |
| 16 The number of patients with a decreased lymphocyte count of less than 0.5×109 per litre at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.69 [2.40, 13.46] |
| 17 The number of patients with a decreased white‐cell count of less than 3.0×109 per litre at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.53 [3.13, 13.64] |
| 18 The number of patients with an increased alanine aminotransferase level at least three times the upper limit of the normal range at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.07] |
| 19 The number of patients with gastrointestinal events at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.11, 1.85] |
| 20 The number of patients with infections at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 21 The number of patients with serious infections at two years Show forest plot | 2 | 1540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.73, 3.28] |

