Risperidona versus otros antipsicóticos para pacientes con enfermedad mental grave y abuso de sustancias concomitante
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: Structured Clinical Interview for DSM (SCID‐I) schizophrenia or schizoaffective disorder and either current cannabis or cocaine abuse or dependence. Age: mean ˜36 years. Ethnicity: 54% African American, 32% Hispanic, 14% Caucasian | |
| Interventions | 1. Risperidone: fixed dose escalation of 3 mg/day for 3 days followed by 6 mg for 4 days and then 9 mg for remainder of study. N = 14 2. Olanzapine: fixed dose escalation of 5 mg/day for 3 days then 10 mg/day for 4 days and then 15 mg/day for 5 days followed by 20 mg/day for remainder of study. N = 14 All participants received weekly psychotherapy over the study period and were asked to nominate a "significant other" to assist with attendance and follow‐up. | |
| Outcomes | Mental state: change scores HAM‐D scale Adverse effects: parkinsonism endpoint score SAS. Leaving the study early: any reason Unable to use: Mental state: PANSS positive and PANSS negative subscales (no means or SD, longitudinal data), CGI (no data reported). Substance use: proportion of positive urine tests for cannabis and cocaine weekly over 10‐week study period (no means or SD, longitudinal data), days of self‐reported substance use (no SD); Quantitative Substance Use Inventory (psychometric properties of instrument not validated). Craving for substances: Marijuana Craving Report, Cocaine Craving Report (no means or SD, longitudinal data). Adherence to antipsychotic medication: number of medication doses missed (no means or SD). Adverse effects: tardive dyskinesia (AIMS)(not reported by group), sedation (no data provided). | |
| Notes | Funding: support for this study was provided in part by grants from the National Institute on Drug Abuse and the National Alliance for Research on Schizophrenia and Depression (NARSAD, currently known as The Brain and Behavior Research Foundation) and Eli Lilly and Co. Declarations of interest made by researchers conducting this study include support from a number of pharmaceutical companies, i.e. Ortho‐McNeil Pharmaceuticals, Eli Lilly & Company, UCB Pharma and consultancy to Shire, Pharmaceuticals Inc, AstraZeneca Pharmaceutical, Eli Lilly and Company. Contact of authors: we contacted the study primary and co‐authors by e‐mail to clarify items of study design and to obtain study data. The authors did not respond to these attempts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but there was insufficient information on the method used to randomise the participants. Quote: "Randomization was not stratified, but was a 50=50 uniform distribution of groups of 4". |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information provided to determine if study medication allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No description of blinding is provided in the study report. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention is made of whether the outcome assessors were indeed blinded and independent. |
| Incomplete outcome data (attrition bias) | High risk | Twice as many people withdrew from the olanzapine group (8/14; 57%) compared to the risperidone group (4/14; 29%). The most common reasons for withdrawal were that the participants were not interested (N = 10) or that they were admitted to inpatient units (N = 3). There were no other significant differences between the groups with respect to demographic and baseline clinical characteristics. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available for this study. Subgroups are reported as primary outcomes. |
| Other bias | High risk | Funding for study provided in part by industry (Eli Lilly and Co). No other sources of bias were identified. |
| Methods | Allocation: randomised Funding: National Institute on Drug Abuse | |
| Participants | Diagnosis: Structured Clinical Interview for DSM (SCID‐I) diagnosis of schizophrenia or schizoaffective disorder and a current cannabis use disorder (abuse or dependence). Age: range 18 to 65 years; mean ˜36 years. Ethnicity: 26 (83.9%) were Caucasian. Inclusion: outpatient status prior to randomisation and on current antipsychotic treatment other than clozapine. | |
| Interventions | Clozapine: titrated to 400 mg daily in 4 weeks. n = 15 Treatment as usual (TAU): i.e. continue on existing antipsychotic treatment. n = 16; (n = 5 on risperidone) All participants received weekly individual substance abuse and mental health counselling and attended weekly Alcoholics Anonymous meetings. | |
| Outcomes | Unable to use: Mental state: (BPRS, CGI, SANS) (no data reported) Substance use: cannabis use (TLFB) number of "average joints" used per week, assessed weekly for 12 weeks; (no data on subgroup with risperidone) Substance Abuse Treatment Scale (SATS) (1 to 8) measuring treatment involvement, Single‐Item Contemplation ladder (0 to 10) motivation to stop using cannabis (no data on subgroup with risperidone) Adverse effects: SAS scale, BARS scale, AIMS scale (no data reported) | |
| Notes | Further data requested. Author responded and indicated that 5/16 patients in TAU group were on risperidone. Authors decided not to provide requested data as they were advised by their methodological consultant that using subgroup data will in effect interfere with the randomisation given the specific design of this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was blocked by site. No description of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was blocked by site. No description of how sequence was generated or how allocation concealment was maintained. |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded clinicians prescribed and adjusted study medications weekly. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded raters assessed patients weekly, independent of study physicians. |
| Incomplete outcome data (attrition bias) | Unclear risk | Longitudinal random‐effects modelling was used that could have accounted for missing data; however no indication as to the extent of missing data; |
| Selective reporting (reporting bias) | Low risk | Study outcomes are identical to protocol‐defined outcomes. |
| Other bias | High risk | Protocol indicates study was sponsored by Janssen, Novartis. |
| Methods | Allocation: randomised. Funding: Dutch Health Research Council. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophreniform, schizophrenia or schizoaffective disorder. CIDI diagnosis of cannabis use disorder (abuse or dependence, N = 35). Age: range 18 to 50 years; mean ˜22.4 years (risperidone), mean ˜22.3 years (clozapine). Ethnicity: not stated. Inclusion: males, aged 18 to 30 with DSM‐IV diagnosis of schizophreniform, schizophrenia or schizoaffective disorder. | |
| Interventions | Risperidone: titrated to initial dose of 3.5 mg/day, then according to treatment response. N = 16 Clozapine: titrated to initial dose of 350 mg/day, then according to treatment response. N = 15 Participants had "supportive treatment as usual". | |
| Outcomes | Mental state: positive psychotic symptoms (average endpoint score, PANSS positive sub‐scale), negative symptoms (average endpoint score, PANSS negative sub‐scale), general psychopathology (average endpoint score, PANSS general sub‐scale). Substance use: number discontinuing cannabis use. Subjective well‐being: Subjective well‐being under neuroleptics scale (SWN scale) Craving for substances: Marijuana Craving Questionnaire (MCQ), Obsessive Compulsive Drug Use Scale (OCDUS). Adherence to medication: discontinuing medication. Adverse effects: any extrapyramidal side‐effects (no data for subgroups with specific extrapyramidal side‐effects). Leaving the study early. | |
| Notes | Authors e‐mailed for additional information: response was given to questions about randomisation, and a flow diagram of study attrition was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator software was used through the ALEA program: randomisation has been performed on‐line via a secure internet facility by the TENALEA Clinical Trial Data Management System. Randomisation has been performed in a 1:1 ratio, using randomly permuted blocks with maximum blocksize of 4, within strata formed by use of drugs (Cannabis use, no drugs use). |
| Allocation concealment (selection bias) | Low risk | The physician states the patient’s date of birth and the stratification factor and receives treatment allocation when submitting this information to the website from central trial office. |
| Blinding of participants and personnel (performance bias) | High risk | Due to feasibility and ethical considerations this was an open label study over a relatively short period of time in which dosage of medication could be adjusted in case of side‐effects or lack of efficacy. Clozapine required blood monitoring which differs from risperidone requirements. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors provided flow diagram on request reporting differential drop‐out (with reasons stated) in two treatment arms (20%, 3/15 cannabis users in clozapine arm, 0/16 in risperidone arm). The impact of not including these participants in the endpoint analysis is unclear. |
| Selective reporting (reporting bias) | Low risk | Outcomes in pre‐published protocol are identical to study reported outcomes. |
| Other bias | Low risk | No clear evidence for bias. |
| Methods | Allocation: randomised Funding: Sponsors and collaborators stated as Dartmouth–Hitchcock Medical Center and National Institute of Mental Health. | |
| Participants | Diagnosis: Structured Clinical Interview for DSM (SCID‐I) schizophrenia or schizoaffective disorder and either current cannabis abuse or dependence. Sex: 8 M, 6 F Ethnicity: Caucasian Exclusion: Medical contraindications to treatment with clozapine or risperidone, including previous paralytic ileus. Cumulative treatment with antipsychotic medication in excess of 16 weeks prior to hospital admission (or case identification if an outpatient), unless waived by the medication adjustment group (MAG). History of allergic reaction to clozapine or risperidone. History of seizure disorder or blood dyscrasia. Note: if participants had a history of seizures, but not a diagnosed seizure disorder, they could be admitted to the study if approved by the medication adjustment group. Current treatment with clozapine. Currently pregnant, planning to become pregnant, or unwilling to use an acceptable form of birth control. Currently residing in a residential programme designed to treat substance use disorders. Participants who required treatment at baseline with a psychotropic agent proposed to curtail substance use (e.g. disulfiram, naltrexone, valproic acid, topiramate, acamprosate or benzodiazepines) were reviewed by the medication adjustment group before entering into the study. Participants who, in the opinion of the investigator, are judged unsuitable to participate in the study (for example, are actively homicidal or have a pending incarceration that would prevent them from participating in the study). History of, or current breast cancer. People who are doing well on current therapy. Lack of an identifiable primary family/support person, and unable to come to a study site for weekly visits. Treatment with serotonin re‐uptake inhibitors did not mean exclusion but required a review by the MAG prior to randomisation. Participants with current cocaine dependence required review by the MAG to determine stability for the study.Treatment with multiple antipsychotics or long‐acting injectable antipsychotic at baseline not excluded, but reviewed by the MAG to assess appropriateness for the study. | |
| Interventions | 1. Clozapine: tablets ‒ 12.5 mg to maximum 100 mg daily for 24 weeks. N = 7 2. Risperidone: tablets ‒ 0.5 mg to maximum 5 mg daily for 24 weeks. N = 7 All participants received a Lifestyle Intervention to manage metabolic side‐effects and to assist with recovery. | |
| Outcomes | Mental state: worsening of psychotic symptoms, emergence of anxiety symptoms reported as trial adverse events. Substance use: cannabis use (TLFB), urine tests, collateral reports, and monthly clinician ratings, final expert clinician rating ‒ dichotomised Adverse effects: movement disorder, various adverse effects. Leaving the study early. Unable to use: Mental state: (BPRS, CGI, SANS ‒ no data reported). | |
| Notes | Contact of authors: no response from authors to e‐mails sent requesting clarification on study design and to obtain missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given as to how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details given as to whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Study described as single‐blind with outcome assessors blind. Knowledge of treatment allocation and monitoring procedures of clozapine could have influenced participants or personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors described as masked, but no mention is made of whether the outcome assessors were indeed blinded and independent. |
| Incomplete outcome data (attrition bias) | High risk | One participant in both groups did not receive treatment but were randomised and were not included in the outcome report. "About half of participants in the clozapine group have discontinued treatment early, a rate similar to previous first episode schizophrenia studies in the US. There were no discontinuations due to lack of efficacy in either group, but several discontinuations due to inability to tolerate medication side‐effects in clozapine group". It is also mentioned that 2 participants in risperidone group terminated early and 2 study completers in the risperidone group elected to discontinue medication and 1 to switch to a different antipsychotic at the end of the study. |
| Selective reporting (reporting bias) | High risk | The manner in which the primary outcome was determined changed in later versions of the protocol (from 2007 to following completion of data collection in 2011, with a change in 2013). The initial outcome was marijuana use measured weekly by means of TLFB method, but this changed to improvement as judged by experts at a particular cut‐point of 20% improvement and then dichotomised, assessed at the end of the study. |
| Other bias | Unclear risk | Study did not receive funding from pharmaceutical industry and principal Investigators are not employed by the organization sponsoring the study. Declarations of interest made by researchers conducting this study: "Principal Investigators are not employed by the organization sponsoring the study". |
| Methods | Allocation: randomised. Funding: National Institutes for Health, Feinstein Institute for Medical Research | |
| Participants | Diagnosis: Structured Clinical Interview for DSM (SCID‐I) diagnosis of current schizophrenia, schizophreniform disorder, or schizoaffective disorder and a lifetime or current 3 months' history of cannabis abuse or dependence. Age: range 16 to 40 years; mean ˜21.7 years (risperidone), mean ˜21.7 years (olanzapine). Ethnicity: not stated. Inclusion criteria: Less than 12 weeks of lifetime antipsychotic medication treatment. Current positive symptoms evidenced by a rating of 4 or more on the severity of delusions, hallucinations, or thought disorder items of the Schedule for Affective Disorders and Schizophrenia Change Version with psychosis and disorganization items (SADS‐C+PD) or current negative symptoms demonstrated by a rating of 4 or more on the affective flattening, alogia, avolition, or anhedonia global items of the Hillside Clinical Trials version of the Scale for Assessment of Negative Symptoms (SANS). For women, a negative pregnancy test and agreement to use a medically accepted method of birth control. Competent and willing to sign informed consent. Exclusion criteria: 1) meeting DSM‐IV criteria for a current substance‐induced psychotic disorder, psychotic disorder due to a general medical condition, or mental retardation; 2) medical condition/treatment known to affect the brain; 3) any medical condition requiring treatment with a medication with psychotropic effects; 4) medical contraindications to treatment with olanzapine or risperidone; or 5) significant risk of suicidal or homicidal behaviour. | |
| Interventions | Risperidone: mean modal daily dose 4 mg. N = 21 Olanzapine: mean modal daily dose 15 mg. N = 28 All participants received psychoeducation about schizophrenia, were seen on a regular basis by allocated social workers and also had access to the ancillary treatment service available from 2 large departments of psychiatry. | |
| Outcomes | Mental state: positive psychotic symptoms ‒ average endpoint scores (SADS‐C‐PD scale, lower = better), negative symptoms (SADS‐C‐PD scale, lower = better). Substance use: stopped using cannabis (Urine testing and Substance Use Questionnaire). Substance use: stopped using alcohol (Substance Use Questionnaire). Leaving the study early. | |
| Notes | Authors e‐mailed for additional data and information: no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer pre‐generated block randomization list provided by the department of biostatistics and only accessible to the biostatisticians and dedicated research coordinators. |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether "research coordinators" were involved in patient recruitment. |
| Blinding of participants and personnel (performance bias) | High risk | Open label, both patients and staff were aware of treatments received. |
| Blinding of outcome assessment (detection bias) | Low risk | Diagnosis and psychopathology assessments were performed by masked ("blind") assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Although attrition was equal across groups (approximately 25%) no method of accounting for missing outcomes was present, i.e. ITT analysis with imputation. |
| Selective reporting (reporting bias) | Unclear risk | Secondary data analysis directed by analysis of primary study. Not clear if this could have influenced selection of outcomes. |
| Other bias | Unclear risk | Several authors have ties to drug companies; however unclear whether this could have an impact on the results as the parent study was supported by NIH grants K23 DA015541 (SS), MH60004 (DR), MH41960, and RR018535. It is stated that the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophreniform, schizophrenia or schizoaffective disorder, illicit drug or alcohol use 30 days prior to study entry as measured by the quantity/frequency sub‐scale of the Addiction Severity Index. N = 664 (236 with analysable data were substance users). Age: > 18 years; mean age ˜43 years. Ethnicity (parent study): Caucasian 361 (54%), African American 224 (34%), Other 79 (12%) Inclusion: psychotic symptom threshold of 18 or more on the Brief Psychiatric Rating Scale (BPRS). Individuals recently experiencing an adverse event attributable to current antipsychotic treatment (unless olanzapine or risperidone) were also eligible, although the vast majority met symptom criteria. Exclusion criteria: patients with very serious, unstable physical illnesses and other medical conditions or histories contraindicating use of any study medication. | |
| Interventions | Risperidone: suggested initiating dose 1 mg twice daily with flexible dosing and titration by study clinicians. N = 76 Olanzapine: suggested initiating dose 10 mg daily with flexible dosing and titration by study clinicians. N = 85 Conventional antipsychotics: 2 conventional agents (from: perphenazine, loxapine, haloperidol, fluphenazine, thiothixene) for minimum of 8 weeks consecutively as decided by study physicians based on prior history. N = 75 It is unclear what psychosocial interventions participants received. | |
| Outcomes | Unable to use: Time to discontinuation (skewed data) Numbers discontinuing treatment (no data) | |
| Notes | Authors e‐mailed for additional data and information: no response received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Treatment was described as open‐label. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Primary outcome and only outcome reported is time to all‐cause medication discontinuation, including leaving study for any reason. |
| Selective reporting (reporting bias) | Unclear risk | Secondary data analysis of existing trial. Analysis for primary trial not fully reported so unclear if this informed the aims and hypothesis of the secondary data analysis. |
| Other bias | High risk | Several authors have relationships with the pharmaceutical industry. Parent study funded by Eli Lilly. |
| Methods | Allocation: randomised | |
| Participants | Diagnosis: Structured Clinical Interview for DSM (SCID‐I) diagnosis of schizophrenia, with past history of more than one episode. Alcohol and illicit drug use was determined by a combination of self‐reported use, SCID‐I interviews, urine and hair samples, ratings on Clinician Alcohol and Drug Use Scale. N = 1432 cases available from the parent study for analysis; 643 were substance users. Age: 18 to 65 years; substance user group mean age ˜38.1 years, non‐substance user group mean age ˜42.6 years. Ethnicity: White 722 (50.4%), Non‐white 710 (49.5%). Inclusion: multi‐episode schizophrenia with or without illicit substance use disorder. Exclusion criteria: People with schizoaffective disorder, mental retardation or other cognitive disorders. A history of serious adverse reactions to the proposed treatments. Patients with only 1 schizophrenic episode or a history of treatment resistance, including non‐response to one of the proposed treatments or prior treatment with clozapine. Pregnant, breast‐feeding or presence of an unstable medical condition. | |
| Interventions | Risperidone: flexible dosing, allowable daily dose 1.5 mg to 6 mg, mean dose 3.8 mg/day. N = 157 Olanzapine: flexible dosing, allowable daily dose 7.5 mg to 30 mg, mean dose 20.0 mg/day. N = 142 Perphenazine: flexible dosing, allowable daily dose 8 mg to 32 mg, mean dose 20.4 mg/day. N = 124 Quetiapine: flexible dosing, allowable daily dose 200 mg to 800 mg, mean dose 515.1 mg/day. N = 137 Ziprasidone: flexible dosing, allowable daily dose 40 mg to 160 mg, mean dose 113.3 mg/day. N = 83 The investigators did not account for substance abuse treatments received, but they noted that very few were actively engaged in such treatments. | |
| Outcomes | Leaving the study early (any reason) Unable to use: Mental state: psychotic symptoms, positive psychotic symptoms, negative symptoms and general psychopathology (PANSS total and sub‐scales) (N and SD not available) Clinical Global Impression of Severtiy of illness (CGI‐severity) (N and SD not available) Readmission rate (no data) Adherence to antipsychotic medication (no SD) Adverse events: weight gain (no data), neurological side‐effects (no data) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no details given as to how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description of how sequence was kept concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as double blind with identically appearing capsules. Different medications had different side‐effect profiles and some overlap in side‐effect profiles for some medications (i.e. weight gain and sedation). Unclear if this could have favoured one or more medications over others. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No clear description of how blinding was maintained. Different side‐effect profiles may have unblinded medication and symptom severity ratings could have been influenced by this. Nevertheless, outcomes such as discontinuation would unlikely have been affected by blinding. |
| Incomplete outcome data (attrition bias) | High risk | High attrition rates with up to 69% of risperidone patients discontinuing treatment and high numbers (56% to 81%) in other treatment groups. Simple imputation with LOCF was used which could have biased results given such a large attrition rate. This would however not have affected the outcome of time to medication all‐cause discontinuation (as all patients were counted for medication discontinuation outcome), but could have impacted on measurement of mental state (i.e. LOCF imputations). |
| Selective reporting (reporting bias) | Unclear risk | The results from earlier analysis directed the current hypothesis in this study. Nevertheless the outcomes (time to all‐cause discontinuation), were similar to the original study protocol. It is unclear how earlier analyses could have directed results. |
| Other bias | Unclear risk | Several pharmaceutical companies provided medication for the study. A number of authors had ties to the pharmaceutical industry. The NIMH was responsible for the study was design, data collection, analysis, writing up and decision to publish the study. |
| Methods | Allocation: randomised | |
| Participants | Diagnosis: Structured Clinical Interview for DSM (SCID‐I) diagnosis of schizophreniform disorder, schizophrenia or schizoaffective disorder, cannabis self‐report and urine testing for cannabis. N = 138 (subgroup of 41 (29.7%) used cannabis). Age: 18 to 30 years, mean age ˜25 years Ethnicity: not reported Exclusion criteria: pregnant or lactating, no adequate contraception, known hypersensitivity to any ingredient of olanzapine or risperidone. Concomitant use of any other antipsychotic drug than olanzapine or risperidone. Use of depot anti‐psychotics for a period of at least three months prior to the study or the use of other psychotropic medication other than oxazepam or biperiden. Narrow‐angle glaucoma, neurological or endocrine disease. | |
| Interventions | Risperidone: flexible dosing, 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, titrated to a fixed dose within the first week. N = 21 Olanzapine: flexible dosing, 5 mg, 10 mg, 15 mg, 20 mg, titrated to fixed dose within first week. N = 20 | |
| Outcomes | Substance use: cannabis use self‐report scores ‒ change data (joints per week) Craving for substances: Obsessive Compulsive Drug Use Scale (OCDUS), Desires for Drug Questionairre (DDQ) ‒ endpoint data. Leaving the study early Unable to use: Subjective Well‐being: Subjective Well‐being Under Neuroleptics (SWN) score (no subgroup mean, SD or N) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description of where sequence was kept and who allocated participants. Nevertheless tablets were described as identical‐looking. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as "double blind" with identically appearing capsules, although no description is given as to how blinding was achieved. Different side‐effect profiles of the two medications could have lead to unblinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double blind" with identically appearing capsules, although no description is given as to how outcome assessors were kept masked from treatment. Different side‐effect profiles of the two medications could have lead to unblinding. |
| Incomplete outcome data (attrition bias) | High risk | ITT analysis with single imputation method (LOCF). Although attrition was comparable across groups it is unclear if groups differed with regards to other factors such as symptoms severity and other baseline measures. |
| Selective reporting (reporting bias) | Unclear risk | Only some outcomes stated in protocol are reported. Unclear how some factors, such as symptom severity that was not reported, could have impacted on reported outcomes of SWN and craving. |
| Other bias | High risk | Study funded by Eli‐Lilly |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: randomised Participants: people with schizophrenia who do not have co‐occurring substance misuse. | |
| Allocation: randomised Participants: people with schizophrenia, schizoaffective disorder; people with schizophrenia who do not have co‐occurring substance misuse. | |
| Allocation: randomised. Participants: patients with both schizophrenia and a cannabis use disorder. Intervention: risperidone vs. clozapine. Outcomes: cannabis use, negative symptoms, psychotic symptoms, neuropsychological function and quality of life. No data available: only published as study protocol, authors contacted for unpublished data: no response. | |
| Allocation: randomised. Participants: people with bipolar I disorder who do not have co‐occurring substance misuse. | |
| Allocation: randomised Participants: people with schizophrenia, schizophreniform, or psychosis not otherwise specified who do not have co‐occurring substance misuse. | |
| Allocation: randomised. No data available comparing risperidone with other medications. Study examined the impact of substance use on prognosis. | |
| Allocation: randomised. Participants: people with schizophrenia who do not have co‐occurring substance misuse. | |
| Allocation: randomised. | |
| Allocation: trial suspended, reported as non‐randomised, retrospective observational study. | |
| Allocation: randomised | |
| Allocation: randomised No data available. study protocol of terminated study. | |
| Allocation: randomised Study protocol only, authors contacted but no data provided. | |
| Allocation: randomised Participants: bipolar I and II disorder, recent manic or mixed episode with or without psychosis and with co‐occurring cocaine‐ or methamphetamine‐use disorder. Only 8.3% of total sample had psychotic features and 15.9% had bipolar type II disorder. | |
| Allocation: randomised | |
| Allocation: randomised | |
| Allocation: quasi‐randomisation (participants allocated "alternately"). | |
| Allocation: quasi‐randomisation (participants allocated "alternately"). | |
| Allocation: randomised | |
| Allocation: randomised No subgroups with substance use reported, authors contacted for unpublished data, no response. | |
| Allocation: randomised Participants: bipolar I disorder who do not have recent drug or alcohol use. | |
| Allocation: randomised Participants: schizophrenia, schizophreniform, schizoaffective disorder. No co‐occurring substance use disorders. Intervention: haloperidol, risperidone, placebo Outcomes: Obsessions and compulsions (Y‐BOCS), PANSS scores, CDSS scores. Authors contacted to determine if there were participants with co‐occurring substance use disorders. Authors clarified that there were no participants with co‐occurring substance use disorders. | |
| Allocation: randomised Participants: bipolar I and II. Excludes participants with drug or alcohol use in past 3 months. | |
| Allocation: quasi‐randomisation (allocation based on admission order) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: described as "double‐blind" * |
| Participants | Patients with schizophrenia and co‐occurring alcohol, cocaine, amphetamine, marijuana, opiate use disorder. N = 111 with substance use disorders |
| Interventions | Risperidone; (dose and delivery method unclear) N = 51. Quetiapine; (dose and delivery method unclear ) N = 40. Placebo. N = 20** |
| Outcomes | Mental state: psychotic symptoms, PANSS scale |
| Notes | * Randomisation could not be confirmed from authors, no response to e‐mails sent. ** Data from placebo group not used for this review. |
| Methods | Allocation: randomised, rater blinded, prospective head‐to‐head trial |
| Participants | Participants: schizophrenia, schizoaffective disorder, delusional disorder, affective psychosis (supplementary data with sample characteristics indicate that 3.8% of risperidone group had alcohol use disorder at baseline and 21.2% of risperidone group had drug misuse at baseline). |
| Interventions | risperidone, clinician determined dose, N = 53 (2 alcohol misuse in past 6 months, 11 drug misuse in past 6 months) olanzapine, clinician determined dose, N = 52 (5 alcohol misuse in past 6 months, 9 drug misuse in past 6 months) quetiapine, clinician determined dose, N = 50 (10 alcohol misuse in past 6 months, 7 drug misuse in past 6 months) ziprasidone, clinician determined dose, N = 58 (5 alcohol misuse in past 6 months, 11 drug misuse in past 6 months) |
| Outcomes | Outcomes: time to antipsychotic discontinuation, discharge and readmission. Improvement in PANSS, Calgary Depression Scale for Schizophrenia, CGI‐S, GAF,adverse effects, UKU Side Effect Rating Scale (UKU‐SERS). Baseline, 6 weeks, 3‐, 6‐, 12‐ and 24‐month measures. |
| Notes | Authors contacted for any subgroup data or analysis, no response to e‐mails sent. |
| Methods | Open (no masking), randomised, parallel assignment, superiority trial |
| Participants | Adults age 19 to 65 years with a diagnosis of schizophrenia or schizoaffective disorder and co‐occurring cocaine or methamphetamine abuse or dependence as diagnosed by Structured Clinical Interview for DSM‐IV. |
| Interventions | quetiapine or risperidone oral formulation |
| Outcomes | Primary: 50% or greater decrease in the drug use determined by the Time Line Follow Back (TLFB) method versus baseline Secondary: psychiatric symptoms assessed with the CGI, PANSS, BPRS, HAM‐D, and HAM‐A. Safety and tolerability assessed by patient‐ and physician‐reported adverse events and AIMS. Quality of life assessed with QoLI. |
| Notes | Authors were contacted via e‐mail but no response received. |
| Methods | Allocation: randomised |
| Participants | Participants: schizophrenia, schizophreniform, schizoaffective, bipolar, psychotic disorder NOS. Substantial subgroup used substances (cannabis: N = 64, 56.1%; alcohol: N = 87, 76.3%; cocaine: N = 24, 21.1%). |
| Interventions | Open‐label flexible‐doses of antipsychotic treatment with the following dose ranges: olanzapine 7.5 mg to 40 mg, N = 25 (cannabis = 15, alcohol = 19, cocaine = 4) risperidone 1.5 mg to 7.0 mg, N = 25 (cannabis = 14, alcohol = 17, cocaine = 5) quetiapine 100 mg to 1500 mg, N = 23 (cannabis = 11, alcohol = 17, cocaine = 2) |
| Outcomes | Time to medication discontinuation, PANSS scores, CDSS scores, Adverse effects |
| Notes | No data provided for substance misuse subgroup ‒ authors contacted and responded, no data provided. |
| Methods | Allocation: multicentre, randomised, placebo‐controlled trial Blindness: described as "double blind" Duration: 52 weeks Setting: Canadain and Brazilian academic centres |
| Participants | Bipolar I disorder in remission from recent manic or mixed episode on treatment with mood stabiliser (valproate or lithium) and either risperidone or olanzapine (N = 159, not clear how many had psychotic features). Total of 39% (62/159) of total sample had co‐occurring alcohol or substance use disorder. |
| Interventions | Discontinuation of risperidone or olanzapine at either 0 weeks, 24 weeks or 52 weeks and substitution with placebo. |
| Outcomes | Time to any mood episode, YMRS, HAMD‐21, MADRS, CGI‐BP, CGI‐S, Side‐effects UKU scale, ESRS, weight, metabolic measures (glucose, lipid profile). |
| Notes | Authors contacted. Responded that no data or analyses available at present for subgroups. No information provided on how many participants had bipolar type I with psychotic features. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Clozapine for Cannabis Use in Schizophrenia (CLOCS) |
| Methods | Double blind (subject, caregiver, investigator, outcomes assessor), randomised, parallel assignment, superiority trial, comparing the efficacy of clozapine with risperidone, Estimated recruitment target N = 132 |
| Participants | Adults 18 to 55 years, males and females, clinical diagnosis of schizophrenia and a co‐occurring cannabis use disorders (abuse or dependence) |
| Interventions | clozapine with target dose of 400 mg/day and maximum of 550 mg/day; risperidone with target dose of 4 mg/day and maximum of 6 mg/day |
| Outcomes | Primary: intensity (amount of cannabis used); frequency (number of days in past week) Secondary: symptoms of schizophrenia as measured by the BPRS, SANS, CGI; neuropsychological function by means of MATRICS Consensus Cognitive Battery; and reward responsiveness by means of a computerised Probablistic Reward Task. |
| Starting date | April 2013 |
| Contact information | |
| Notes | Estimated completion in Oct 2016 (recruitment); Oct 2017 (results) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: 1. General: average endpoint scores (PANSS subscale, lower=better) Show forest plot | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.14, 7.54] |
| Analysis 1.1  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 1 Mental state: 1. General: average endpoint scores (PANSS subscale, lower=better). | ||||
| 2 Mental state: 2. General: any change in general symptoms: Show forest plot | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.34] |
| Analysis 1.2  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 2 Mental state: 2. General: any change in general symptoms:. | ||||
| 3 Mental state: 3. Specific: positive, negative symptoms ‐ average endpoint scores (PANSS subscales, lower = better): Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 3 Mental state: 3. Specific: positive, negative symptoms ‐ average endpoint scores (PANSS subscales, lower = better):. | ||||
| 3.1 Mental state: Positive symptoms ‐ average endpoint score (PANSS positive subscale, lower=better) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐2.21, 4.01] |
| 3.2 Mental state: Negative symptoms ‐ average endpoint score (PANSS negative subscale, lower=better) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 4.0 [0.79, 7.21] |
| 4 Mental state: 4. Specific: anxiety symptoms Show forest plot | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| Analysis 1.4 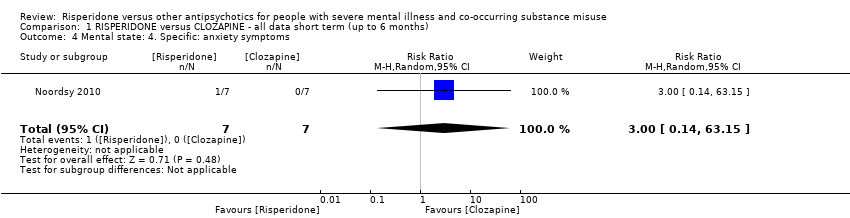 Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 4 Mental state: 4. Specific: anxiety symptoms. | ||||
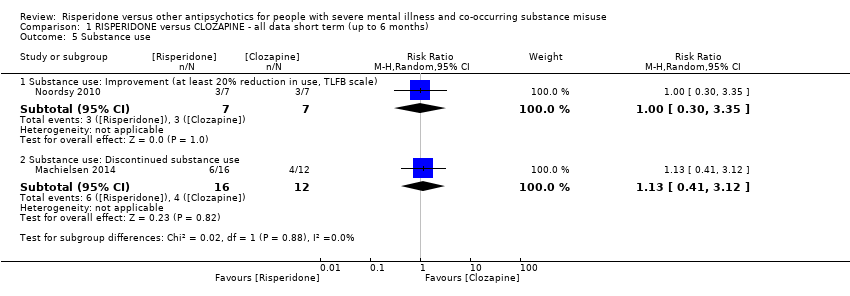
| 5 Substance use Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5 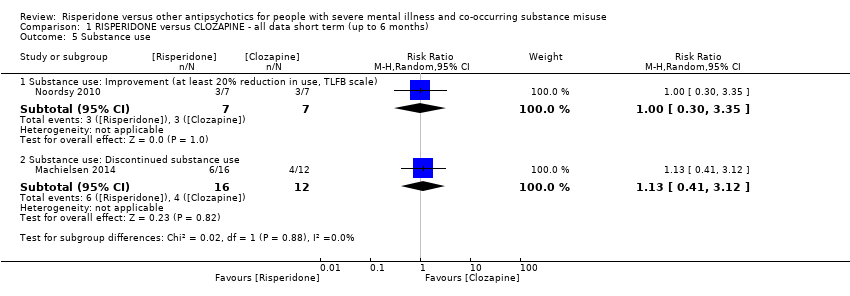 Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 5 Substance use. | ||||
| 5.1 Substance use: Improvement (at least 20% reduction in use, TLFB scale) | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.30, 3.35] |
| 5.2 Substance use: Discontinued substance use | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.41, 3.12] |
| 6 Subjective Well‐being: average endpoint scores (Subjective Well‐being under Neuroleptics scale, SWN scale, higher=better) Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐14.82, 2.82] |
| Analysis 1.6  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 6 Subjective Well‐being: average endpoint scores (Subjective Well‐being under Neuroleptics scale, SWN scale, higher=better). | ||||
| 7 Craving for substances Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7 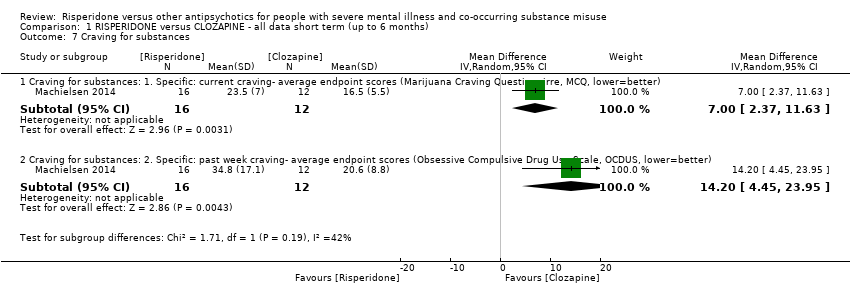 Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 7 Craving for substances. | ||||
| 7.1 Craving for substances: 1. Specific: current craving‐ average endpoint scores (Marijuana Craving Questionairre, MCQ, lower=better) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 7.00 [2.37, 11.63] |
| 7.2 Craving for substances: 2. Specific: past week craving‐ average endpoint scores (Obsessive Compulsive Drug Use Scale, OCDUS, lower=better) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 14.20 [4.45, 23.95] |
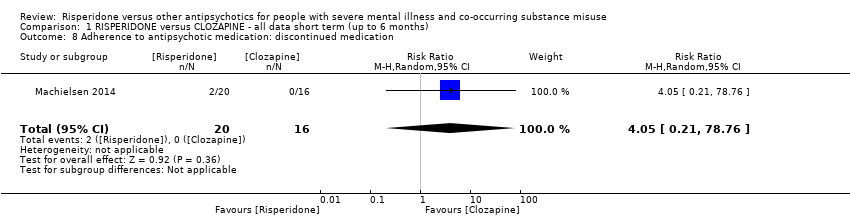
| 8 Adherence to antipsychotic medication: discontinued medication Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 4.05 [0.21, 78.76] |
| Analysis 1.8 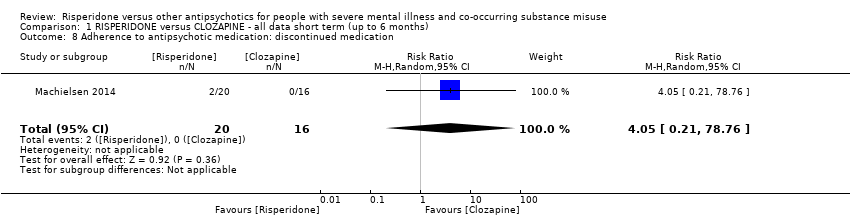 Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 8 Adherence to antipsychotic medication: discontinued medication. | ||||
| 9 Adverse effects. 1. Movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 9 Adverse effects. 1. Movement disorders. | ||||
| 9.1 any extrapyramidal side‐effects | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.30, 24.08] |
| 9.2 akathisia | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.34] |
| 10 Adverse effects: 2. Non‐movement disorder related side‐effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 10 Adverse effects: 2. Non‐movement disorder related side‐effects. | ||||
| 10.1 Cardiovascular: palpitations | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.2 Cardiovascular: hypotension | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.3 Central nervous system: headache | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.54] |
| 10.4 Central Nervous System: somnolence | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.30] |
| 10.5 Dermatological: acne | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.6 Endocrinological: decreased libido | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.7 Ear and labarynthine: ear canal blockage | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.8 Gastrointestinal: abdominal pain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.9 Gasstrointesinal: elevated liver function tests | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.10 Gastrointestinal: hypersalivation | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.74] |
| 10.11 General adverse effects: fatigue | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.12 Injuries: sprain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.13 Metabolic: increased appetite | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.14 Metabolic: weight gain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.19, 5.24] |
| 10.15 Musculosceletal: ankle pain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.16 Musculosceletal: knee and foot pain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.17 Musculosceletal: muscle twitch | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.18 Renal: urinary retention | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.19 Renal: urinary urgency | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
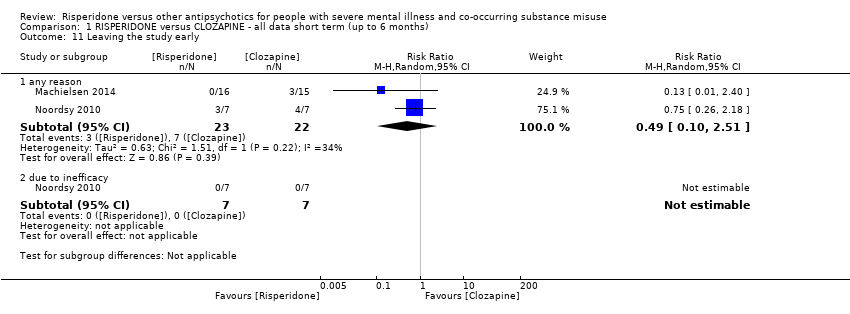
| 11 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 11 Leaving the study early. | ||||
| 11.1 any reason | 2 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.10, 2.51] |
| 11.2 due to inefficacy | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||
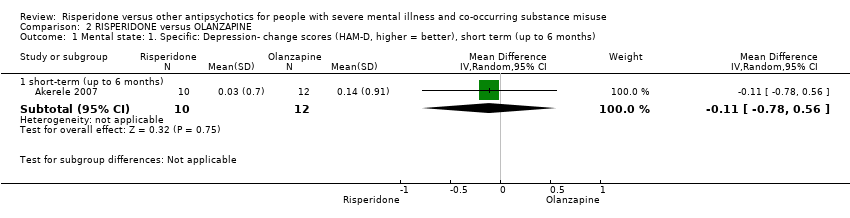
| 1 Mental state: 1. Specific: Depression‐ change scores (HAM‐D, higher = better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1 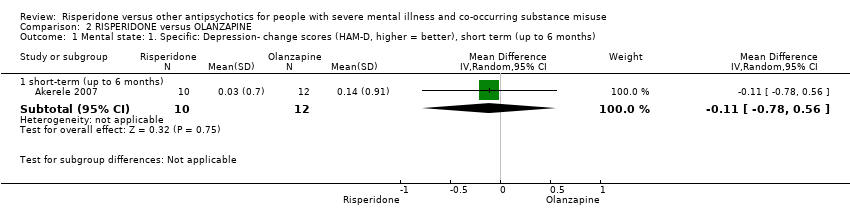 Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 1 Mental state: 1. Specific: Depression‐ change scores (HAM‐D, higher = better), short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 short‐term (up to 6 months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.78, 0.56] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Mental state: 2. Specific: Positive symptoms, total score‐ average endpoint scores (SADS‐C‐PD scale, lower=better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 2 Mental state: 2. Specific: Positive symptoms, total score‐ average endpoint scores (SADS‐C‐PD scale, lower=better), short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 short term, up to 6 months) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐3.82, 0.82] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Mental state: 3. Specific: Positive symptom subscales‐ average endpoint scores (SADS‐C‐PD subscores, lower=better), short term (up to 6 months)‐ skewed data Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 3 Mental state: 3. Specific: Positive symptom subscales‐ average endpoint scores (SADS‐C‐PD subscores, lower=better), short term (up to 6 months)‐ skewed data. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Mental state: 4. Specific: Negative symptoms, subscales‐ average endpoint scores (SANS subscales, lower=better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4 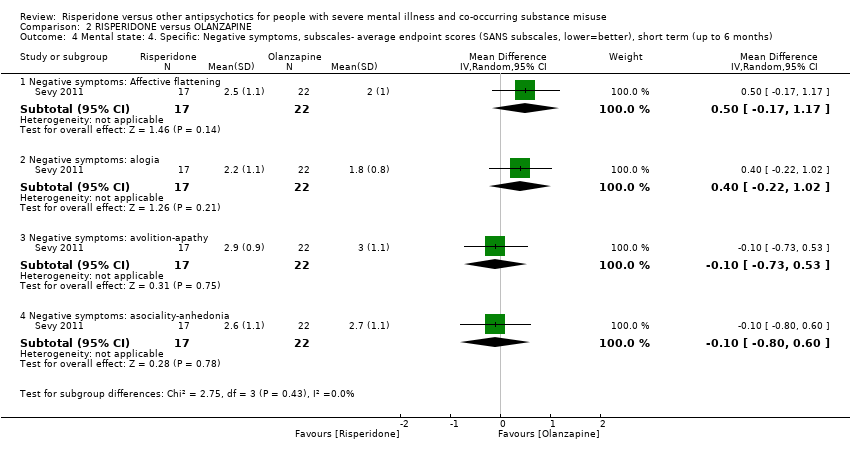 Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 4 Mental state: 4. Specific: Negative symptoms, subscales‐ average endpoint scores (SANS subscales, lower=better), short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Negative symptoms: Affective flattening | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.17, 1.17] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Negative symptoms: alogia | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.22, 1.02] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 Negative symptoms: avolition‐apathy | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.73, 0.53] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4.4 Negative symptoms: asociality‐anhedonia | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.80, 0.60] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Substance use: 1. Reduction of cannabis use‐change data (number of joints smoked/week, LOCF data, higher =better)‐ short term data (up to 6 months) Show forest plot | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐4.72, 5.52] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.5  Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 5 Substance use: 1. Reduction of cannabis use‐change data (number of joints smoked/week, LOCF data, higher =better)‐ short term data (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Substance use: 2. Discontinued substance use, short term (up to 6 months) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.6 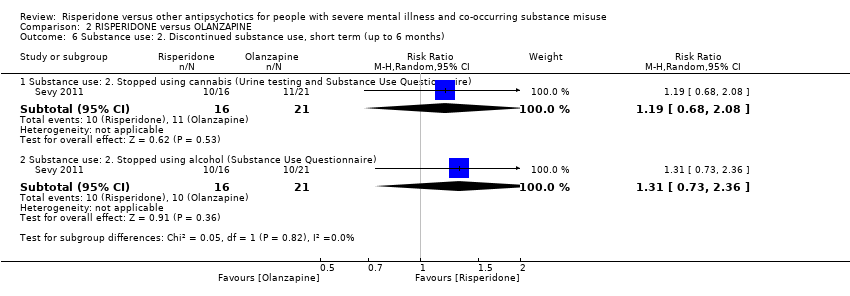 Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 6 Substance use: 2. Discontinued substance use, short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 Substance use: 2. Stopped using cannabis (Urine testing and Substance Use Questionnaire) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.68, 2.08] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 6.2 Substance use: 2. Stopped using alcohol (Substance Use Questionnaire) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.73, 2.36] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Craving for substances: 1. Obsessive Compulsive Drug Use Scale‐ average endpoint score (OCDUS, lower=better)‐short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.7  Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 7 Craving for substances: 1. Obsessive Compulsive Drug Use Scale‐ average endpoint score (OCDUS, lower=better)‐short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 short‐term (up to 6 months) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐3.51, 6.11] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Craving for substances: 2. Desires for Drug Questionnaire‐ average endpoint scores (DDQ, LOCF data, lower=better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.8 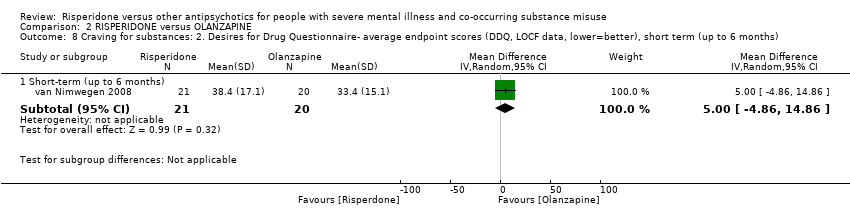 Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 8 Craving for substances: 2. Desires for Drug Questionnaire‐ average endpoint scores (DDQ, LOCF data, lower=better), short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 Short‐term (up to 6 months) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐4.86, 14.86] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Adverse effects Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.9  Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 9 Adverse effects. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Movement disorders: Parkinsonism‐ average endpoint score (SAS, high = worse)‐ short‐term (up to 6 months) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.21, 1.05] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.2 Non‐movement disorder related side‐effects: Weight gain‐ average endpoint score (BMI, lower=better)‐ short term (up to 6 months) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.99, 1.99] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Leaving study early: 1. Various reasons Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.10  Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 10 Leaving study early: 1. Various reasons. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 any reason, short term (up to 6 months) | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.35] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 10.2 any reason, long term (> 12 months) | 1 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.94, 1.21] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 10.3 readmission, short term (up to 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.45] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 10.4 intolerable adverse effects, short term (up to 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 10.5 participant loss of interest, short term (up to 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.33] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Leaving study early: 2. Weeks in the study‐ average endpoint data (high=good), short term (up to 6 months) Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.35, 3.35] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.11  Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 11 Leaving study early: 2. Weeks in the study‐ average endpoint data (high=good), short term (up to 6 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Leaving study early: 3. Weeks in study‐ average endpoint data (high=good), short term (up to 6 months)‐ skewed data Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.12
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 12 Leaving study early: 3. Weeks in study‐ average endpoint data (high=good), short term (up to 6 months)‐ skewed data. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early: all cause discontinuation, long term (>12 months) Show forest plot | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.92, 1.20] |
| Analysis 3.1  Comparison 3 RISPERIDONE versus PERPHENAZINE, Outcome 1 Leaving the study early: all cause discontinuation, long term (>12 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early: all cause discontinuation, long term (>12 months) Show forest plot | 1 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.07] |
| Analysis 4.1  Comparison 4 RISPERIDONE versus QUETIAPINE, Outcome 1 Leaving the study early: all cause discontinuation, long term (>12 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
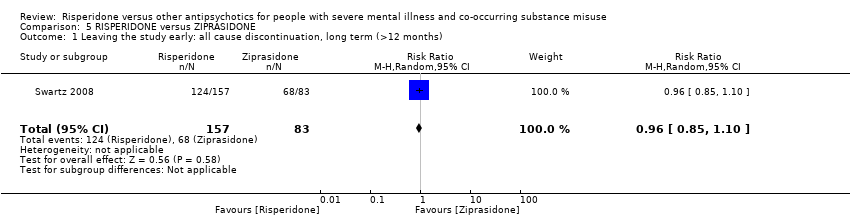
| 1 Leaving the study early: all cause discontinuation, long term (>12 months) Show forest plot | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.10] |
| Analysis 5.1  Comparison 5 RISPERIDONE versus ZIPRASIDONE, Outcome 1 Leaving the study early: all cause discontinuation, long term (>12 months). | ||||

Logic framework model with potential causal pathways: risperidone treatment in persons with dual diagnosis.

PRISMA flow diagram of study selection from 2016 and 2017 searches

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
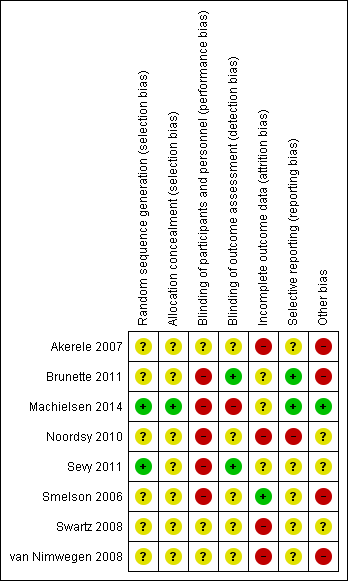
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 1 Mental state: 1. General: average endpoint scores (PANSS subscale, lower=better).

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 2 Mental state: 2. General: any change in general symptoms:.

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 3 Mental state: 3. Specific: positive, negative symptoms ‐ average endpoint scores (PANSS subscales, lower = better):.

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 4 Mental state: 4. Specific: anxiety symptoms.
Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 5 Substance use.

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 6 Subjective Well‐being: average endpoint scores (Subjective Well‐being under Neuroleptics scale, SWN scale, higher=better).

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 7 Craving for substances.
Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 8 Adherence to antipsychotic medication: discontinued medication.

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 9 Adverse effects. 1. Movement disorders.

Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 10 Adverse effects: 2. Non‐movement disorder related side‐effects.
Comparison 1 RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months), Outcome 11 Leaving the study early.
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 1 Mental state: 1. Specific: Depression‐ change scores (HAM‐D, higher = better), short term (up to 6 months).
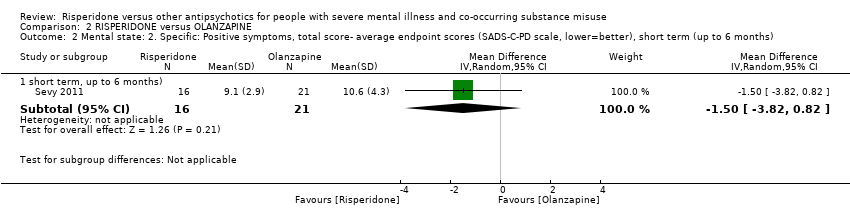
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 2 Mental state: 2. Specific: Positive symptoms, total score‐ average endpoint scores (SADS‐C‐PD scale, lower=better), short term (up to 6 months).
| Study | Intervention | Outcome (symptom subscore) | Mean | SD | N |
| Sevy 2011 | Risperidone | Delusions | 2.6 | 1.7 | 16 |
| Sevy 2011 | Olanzapine | 2.7 | 1.6 | 21 | |
| Sevy 2011 | Risperidone | Hallucinations | 1.8 | 1.2 | 16 |
| Sevy 2011 | Olanzapine | 2 | 1.6 | 21 | |
| Sevy 2011 | Risperidone | Thought disorder | 3.6 | 0.8 | 16 |
| Sevy 2011 | Olanzapine | 4.5 | 2.6 | 21 | |
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 3 Mental state: 3. Specific: Positive symptom subscales‐ average endpoint scores (SADS‐C‐PD subscores, lower=better), short term (up to 6 months)‐ skewed data.

Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 4 Mental state: 4. Specific: Negative symptoms, subscales‐ average endpoint scores (SANS subscales, lower=better), short term (up to 6 months).

Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 5 Substance use: 1. Reduction of cannabis use‐change data (number of joints smoked/week, LOCF data, higher =better)‐ short term data (up to 6 months).
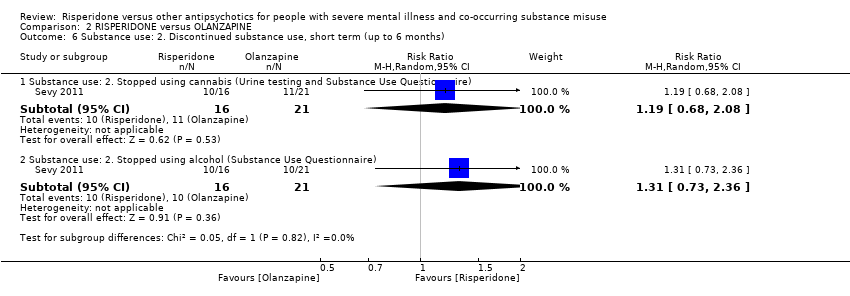
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 6 Substance use: 2. Discontinued substance use, short term (up to 6 months).

Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 7 Craving for substances: 1. Obsessive Compulsive Drug Use Scale‐ average endpoint score (OCDUS, lower=better)‐short term (up to 6 months).

Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 8 Craving for substances: 2. Desires for Drug Questionnaire‐ average endpoint scores (DDQ, LOCF data, lower=better), short term (up to 6 months).

Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 9 Adverse effects.
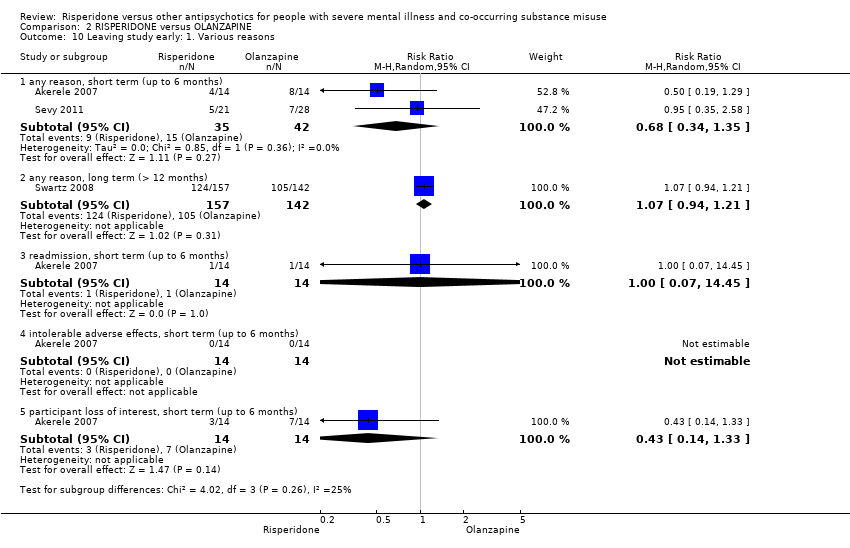
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 10 Leaving study early: 1. Various reasons.

Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 11 Leaving study early: 2. Weeks in the study‐ average endpoint data (high=good), short term (up to 6 months).
| Study | Intervention | Mean (number of weeks) | SD | N |
| Smelson 2006 | Risperidone | 207 | 142.9 | 76 |
| Smelson 2006 | Olanzapine | 267.9 | 127.4 | 85 |
Comparison 2 RISPERIDONE versus OLANZAPINE, Outcome 12 Leaving study early: 3. Weeks in study‐ average endpoint data (high=good), short term (up to 6 months)‐ skewed data.
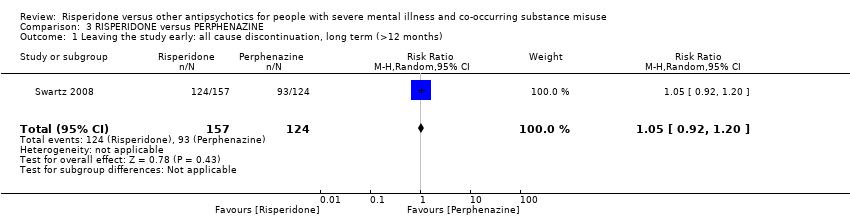
Comparison 3 RISPERIDONE versus PERPHENAZINE, Outcome 1 Leaving the study early: all cause discontinuation, long term (>12 months).

Comparison 4 RISPERIDONE versus QUETIAPINE, Outcome 1 Leaving the study early: all cause discontinuation, long term (>12 months).
Comparison 5 RISPERIDONE versus ZIPRASIDONE, Outcome 1 Leaving the study early: all cause discontinuation, long term (>12 months).
| Methods | Allocation: centralised sequence generation with table of random numbers or computer‐generated code, stratified by severity of illness, sequence concealed till interventions assigned. |
| Participants | Diagnosis: schizophrenia and co‐occurring ongoing substance misuse (clinical criteria). |
| Interventions | 1. Risperidone: clinically indicated dose. N = 150. 2. Olanzapine: clinically indicated dose. N = 150. |
| Outcomes | Global state: CGI‐I and CGI‐S. Substance use: pragmatic binary/continuous measure. Well‐being: pragmatic binary/continuous measure. Craving: pragmatic binary/continuous measure. Service outcomes: re‐hospitalisation, days in hospital, time attending psychiatric outpatient clinic. Quality of life: important change. Leaving the study early. Other routine data, such as incidents with the police, |
| Notes | * size of study to detect a 10% difference in improvement with 80% certainty. For all outcomes there should be binary cut‐off points of clinically important improvement, defined before the study starts. |
| RISPERIDONE versus CLOZAPINE ‐ all data short term (up to 6 months) for people with severe mental illness and co‐occurring substance misuse | ||||||
| Patient or population: for people with serious mental illness and co‐occurring substance misuse | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Clozapine | Risk with Risperidone | |||||
| Mental state: positive symptoms ‒average endpoint score (PANSS positive subscale, lower = better) | The mean positive symptoms (PANSS positive subscale, lower = better) in the intervention group was 0.9 higher (2.21 lower to 4.01 higher) | ‐ | 36 | ⊕⊝⊝⊝ | No trial reported "improvement in symptoms of severe mental illness" ‒ this continuous measure is the nearest proxy for this. | |
| Substance use: improvement ‒ (at least 20% reduction in use, TLFB scale) | Study population | RR 1.00 | 14 | ⊕⊝⊝⊝ | ||
| 429 per 1000 | 429 per 1000 | |||||
| Moderate | ||||||
| 429 per 1000 | 429 per 1000 | |||||
| Subjective well‐being: Subjective well‐being under neuroleptics scale ‒ average endpoint scores (SWN scale, higher = better) | The mean subjective well‐being under neuroleptics scale score (SWN scale, higher = better) in the intervention group was 6 lower (14.82 lower to 2.82 higher) | ‐ | 36 | ⊕⊝⊝⊝ | ||
| Craving for substances: Marijuana Craving Questionnaire ‒ average endpoint scores (MCQ, lower = better) | The mean craving for substances score on the Marijuana Craving Questionairre (MCQ, lower = better) in the intervention group was 7 higher (2.37 higher to 11.63 higher) | ‐ | 28 | ⊕⊝⊝⊝ | ||
| Adherence to antipsychotic medication: discontinued medication | Study population | RR 4.05 | 36 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1,000 | |||||
| Moderate | ||||||
| 0 per 1,000 | 0 per 1,000 | |||||
| Adverse effects. 1. Movement disorders ‐ any extrapyramidal | Study population | RR 2.71 | 50 | ⊕⊝⊝⊝ | Many adverse effects reported ‒ none designated 'clinically important' (extrapyramidal used as proxy). | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Leaving the study early ‒ any reason | Study population | RR 0.49 | 45 | ⊕⊝⊝⊝ | ||
| 318 per 1000 | 156 per 1000 | |||||
| Moderate | ||||||
| 386 per 1000 | 189 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High risk of performance bias and detection bias 2 Sample size is very small, optimal information size (OIS) not met to detect 25% difference 3 Performance bias, attrition bias, selective outcome reporting 4 Sample size is very small (n = 14) 5 High risk of performance bias, detection bias, attrition bias and selective outcomes reporting 6 Total sample size is very small (n<300), total event rate is very low and optimum information size (OIS) is not met | ||||||
| RISPERIDONE versus OLANZAPINE‐ all data short term (up to 6 months) for people with severe mental illness and co‐occurring substance misuse | ||||||
| Patient or population: people with serious mental illness and co‐occurring substance misuse | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Olanzapine | Risk with Risperidone | |||||
| Mental state: 2. Specific‐ Positive symptoms, total score‐ average endpoint scores (SADS‐C‐PD scale, lower = better) | The mean positive symptoms total score at endpoint (SADS‐C‐PD scale, lower = better) in the intervention group was 1.5 lower (3.82 lower to 0.82 higher) | ‐ | 37 | ⊕⊝⊝⊝ | ||
| Substance use: 1. Reduction of cannabis use‐change data (number of joints smoked/week) | The reduction of cannabis joints smoked (number of joints smoked/week‐short term data, up to 6 months) in the intervention group was 0.4 higher (4.72 lower to 5.52 higher) | ‐ | 41 | ⊕⊝⊝⊝ | ||
| Subjective well‐being | ‐ | ‐ | ‐ | No trial reported on this important outcome for participants with a co‐occurring substance use disorder | ||
| Craving for substances: 2. Drug Desires Questionnaire‐ average endpoint scores (DDQ, lower = better) | The mean endpoint. Drug Desires Questionnaire‐ endpoint scores (DDQ, lower = better), short term, up to 6 months‐in the intervention group was5 higher (4.86 lower to 14.86 higher) | ‐ | 41 | ⊕⊝⊝⊝ | ||
| Adherence to antipsychotic medication: number of missed doses, average endpoint data, short term (up to 6 months) | ‐ | ‐ | ‐ | ‐ | no useable data available for this outcome | |
| Adverse effects: Parkinsonism ‐ average endpoint score (SAS, high = worse) | The mean adverse effects: ‐ Parkinsonism‐ average endpoint score (SAS, high = worse)‐ short‐term‐ up to 6 months in the intervention group was 0.08 lower (1.21 lower to 1.05 higher) | ‐ | 16 | ⊕⊝⊝⊝ | ||
| Leaving study early: any reason | Study population | RR 0.68 | 77 | ⊕⊝⊝⊝ | ||
| 357 per 1000 | 243 per 1000 | |||||
| Moderate | ||||||
| 411 per 1000 | 279 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High risk for performance bias, allocation concealment, unknown risk for attrition and slective reporting 2 Very low sample size, optimal information size (OIS) not met 3 High risk of attrition bias, study sponsored by pharmaceutical industry 4 Very low sample size, optimal information criterion not met, CI crosses both appreciable harm and benefit 5 High attrition risk, high other risk of funding by pharmaceutical industry, all other risk items unclear risk of bias 6 High risk of performance, attrition and funding bias. Several domains with unclear risk of bias | ||||||
| RISPERIDONE versus PERPHENAZINE‐long term data (>12 months) for people with severe mental illness and co‐occurring substance misuse | ||||||
| Patient or population: people with severe mental illness and co‐occurring substance misuse | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PERPHENAZINE | Risk with RISPERIDONE | |||||
| Leaving the study early: any reason | Study population | RR 1.05 | 281 | ⊕⊕⊝⊝ | ||
| 750 per 1000 | 788 per 1000 | |||||
| Moderate | ||||||
| 750 per 1000 | 788 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High risk of attrition bias, but this does not affect this particular outcomes 2 Optimal information size criterion is met but the estimate includes no effect with both appreciable harm and benefit | ||||||
| RISPERIDONE versus QUETIAPINE‐ short and long term data (up to 6months and > 12 months) for people with severe mental illness and co‐occurring substance misuse | ||||||
| Patient or population: people with severe mental illness and co‐occurring substance misuse | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with QUETIAPINE | Risk with RISPERIDONE | |||||
| Leaving the study early: 1. any reason, long term (>12 months) | Study population | RR 0.96 | 294 | ⊕⊕⊝⊝ | ||
| 825 per 1000 | 792 per 1000 | |||||
| Moderate | ||||||
| 825 per 1000 | 792 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias unclear across all groups and with high risk of funding bias 2 Sample size meets optimal information threshold/ required sample size to detect 25% difference from control group in in PANSS score; at alpha of 0.05 and power of 80%. 3 Outcome not affected by risk of attrition bias 4 Optimal information criterion not met, estimate includes both appreciable harm and benefit | ||||||
| RISPERIDONE versus ZIPRASIDONE‐ all data long term data (>12 months) for people with severe mental illness and co‐occurring substance misuse | ||||||
| Patient or population: people with severe mental illness and co‐occurring substance misuse | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with ZIPRASIDONE | Risk with RISPERIDONE | |||||
| Leaving the study early: any reason | Study population | RR 0.96 | 240 | ⊕⊕⊝⊝ | ||
| 819 per 1000 | 787 per 1000 | |||||
| Moderate | ||||||
| 819 per 1000 | 787 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of attrition bias high but this does not affect this outcome 2 Optimal information size criterion met but estimate includes both appreciable harm and benefit. Total sample size small | ||||||
| Sequence generation |
|
| Allocation concealment |
|
| Blinding of participants and personnel |
|
| Blinding of outcome assessment |
|
| Incomplete outcome data |
|
| Selective reporting |
|
| Other forms of bias |
|
| Diagnostic tools | Abbreviation | Source of scale/ instrument | Study using instrument | Results reported or usable data for re‐analysis/ quantitative synthesis or qualitative results/data only |
| Structured Clinical Interview for DSM Disorders | SCID‐I | Not an outcome measure | ||
| Mental state scales | ||||
| Brief Psychiatric Rating Scale | BPRS | No results or usable data reported or obtained | ||
| Clinical Global Impression scale | CGI | No results or usable data reported or obtained | ||
| Hamilton Depression Rating Scale | HAM‐D | Results reported; usable data for quantitative synthesis | ||
| Positive and Negative Syndrome Scale | PANSS | Results reported; usable data for quantitative synthesis | ||
| Schedule for Affective Disorders and Schizophrenia ‒ Change Version with Psychosis and Disorganization items | SADS‐C‐PD | Results reported; usable data for quantitative synthesis | ||
| Schedule for the Assessment of Negative Symptoms | SANS | No results or usable data reported or obtained | ||
| Substance use scales | ||||
| Addiction Severity Index | ASI | No results or usable data reported or obtained | ||
| Composite International Diagnostic Interview | CIDI | Not an outcome measure | ||
| Substance Use Questionnaire | SUQ | Sevy 2011, Locally derived instrument/ non‐validated | Results reported, used together with urine testing; usable data for quantitative synthesis | |
| Time‐Line Follow‐Back | TLFB | Results reported in dichotomised format for quantitative synthesis | ||
| Quantitative Substance Use Inventory | Locally derived instrument/ non‐validated | Non‐validated scale | ||
| Subjective‐Wellbeing Scales | ||||
| Subjective Well‐being Under Neuroleptics Scale | SWN | Results reported; usable data for quantitative synthesis | ||
| Craving for substances measures | ||||
| Cocaine Craving Report | No usable data for quantitative synthesis | |||
| Desires for Drug Questionnaire | DDQ | Results reported; usable data for quantitative synthesis | ||
| Marijuana Craving Report | No usable data for quantitative synthesis | |||
| Marijuana Craving Questionnaire | MCQ | Results reported; usable data for quantitative synthesis | ||
| Obsessive Compulsive Drug Use Scale | OCDUS | Results reported; usable data for quantitative synthesis | ||
| Adverse effect scales | ||||
| Abnormal Involuntary Movement Scale | AIMS | No results or usable data reported or obtained | ||
| Barnes Akathisia Rating Scale | BARS | No results or usable data reported or obtained | ||
| Simpson Angus Scale | SAS | Results reported; usable data for quantitative synthesis | ||
| Other measures (categorical or time to event) | ||||
| Urine assay for cannabis and cocaine use (proportion of treatment group positive per week) | No usable data for quantitative synthesis | |||
| Number of participants with improvement in substance use (categorised as improved or not‐improved versus unchanged) | Results reported; usable data for quantitative synthesis (dichotomised) | |||
| Days of self‐reported drug use in past week | No usable data for quantitative synthesis | |||
| Weeks in treatment | Results reported; usable data for quantitative synthesis | |||
| Number of participants not completing the study | Akerele 2007; Machielsen 2014; Noordsy 2010; Sevy 2011; Swartz 2008 | Results reported, usable data for quantitative synthesis | ||
| Compliance with medication (missed doses) | No usable data for quantitative synthesis | |||
| Study tag | Participants | Comparison | Relevant review | ||
| Primary problem | Co‐morbidity | Note (reasons for exclusion) | |||
| schizophrenia | Excludes participants with alcohol or drug abuse in past year | Excludes comorbidity | risperidone, haloperidol, methotrimeprazine | ‐ | |
| schizophrenia, schizoaffective disorder | Excludes participants with alcohol or drug abuse in past year | Excludes comorbidity | risperidone, olanzapine, conventional antipsychotics vs. risperidone long‐acting injectable (RLAI),or quetiapine | ‐ | |
| schizophrenia | cannabis use disorder | Excluded as this was a study protocol, authors contacted for unpublished data, no response | risperidone vs. clozapine | ‐ | |
| bipolar type I disorder | No measure of substance use | Excludes comorbidity | risperidone, quetiapine | ‐ | |
| schizophrenia, schizophreniform, psychosis not otherwise specified | No measure of substance use | Excludes comorbidity | risperidone, aripirazole | ‐ | |
| schizophrenia | Co‐occurring substance use in subgroup of 44.9% | No comparison of study medication, mainly a prognostic study of the impact of substance use | risperidone, quetiapine, perphenazine, olanzapine, ziprasidone | ‐ | |
| schizophrenia | No measure of substance use | Excludes comorbidity | risperidone, aripiprazole | ‐ | |
| mental disorders due to alcohol use | substance induced mental disorders | Exclusion criterion | risperidone, olanzapine | Suggested review: risperidone versus other antipsychotics for substance induced psychosis | |
| schizophrenia | Co‐occurring cannabis use disorder | Study protocol, authors contacted for unpublished data no response | risperidone, clozapine | ||
| schizophrenia, schizoaffective disorder | Co‐occurring alcohol use disorder (abuse or dependence) | Comparison of same medication (risperidone) in different preparations (oral vs depot) | oral risperidone, depot risperidone | ‐ | |
| schizophrenia, schizoaffective disorder | Co‐occurring alcohol and substance use disorder | Study protocol, study terminated, authors contacted for unpublished data no response | clozapine, conventional antipsychotics, atypical antipsychotics | ‐ | |
| schizophrenia, schizoaffective disorder | Co‐occurring cannabis use disorder | Study protocol, authors contacted no data provided | clozapine, conventional antipsychotics, atypical antipsychotics | ‐ | |
| bipolar I and II disorder, recent manic or mixed episode with or without psychosis | co‐occurring cocaine or methamphetamine use disorder | Only 8.3% of total sample had psychotic features and 15.9% had bipolar type II disorder. | risperidone, quetiapine | Suggested review: Risperidone versus other antipsychotics in bipolar disorder with co‐occurring substance use disorders | |
| bipolar I disorder with mania or mixed states | Excludes participants with recent substance use | Excludes comorbidity. Excludes bipolar disorder with psychotic features. | risperidone or olanzapine | ‐ | |
| bipolar disorder, acute mania | Excludes participants with drug or alcohol use in past 3 months | Excludes comorbidity | aripiprazole, risperidone | ‐ | |
| schizophrenia | Co‐occurring substance use disorders | Quasi‐randomisation | risperidone long‐acting depot, zuclopenthixol long‐acting depot | ‐ | |
| schizophrenia | Co‐occurring substance use disorders | Quasi‐randomisation | risperidone (oral), zuclopenthixol (oral), zuclopenthixol long acting depot | ‐ | |
| bipolar with current manic or mixed episode | Study excludes participants with drug or alcohol in past 1 months | Excludes comorbidity | mood stabiliser augmentation with risperidone, haloperidol or placebo | ‐ | |
| psychotic disorders: schizoaffective disorder, bipolar I disorder, major depressive disorder, delusional disorder, Alzheimer's dementia, schizophreniform disorder, vascular dementia, and substance abuse dementia | No subgroups with substance use reported | Excludes comorbidity. Authors contacted for unpublished data, no response. | risperidone, quetiapine | ‐ | |
| bipolar I disorder | Excludes participants with recent substance use | Excludes comorbidity | haloperidol, risperidone, placebo | ‐ | |
| schizophrenia, schizophreniform, schizoaffective disorder | No measure of substance use | Excludes comorbidity | haloperidol, risperidone, placebo | ‐ | |
| bipolar I and II | Excludes participants with drug or alcohol use in past 3 months. | Excludes comorbidity | continuation of oral risperidone, olanzapine, quetiapine or switch to long‐acting injectable LAI risperidone | ‐ | |
| schizophrenia or schizophrenia‐like illnesses | Excludes participants with alcohol or substance dependence | Quasi‐randomisation.Excludes comorbidity. | aripiprazole versus risperidone | ‐ | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: 1. General: average endpoint scores (PANSS subscale, lower=better) Show forest plot | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.14, 7.54] |
| 2 Mental state: 2. General: any change in general symptoms: Show forest plot | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.34] |
| 3 Mental state: 3. Specific: positive, negative symptoms ‐ average endpoint scores (PANSS subscales, lower = better): Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Mental state: Positive symptoms ‐ average endpoint score (PANSS positive subscale, lower=better) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐2.21, 4.01] |
| 3.2 Mental state: Negative symptoms ‐ average endpoint score (PANSS negative subscale, lower=better) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 4.0 [0.79, 7.21] |
| 4 Mental state: 4. Specific: anxiety symptoms Show forest plot | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 5 Substance use Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Substance use: Improvement (at least 20% reduction in use, TLFB scale) | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.30, 3.35] |
| 5.2 Substance use: Discontinued substance use | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.41, 3.12] |
| 6 Subjective Well‐being: average endpoint scores (Subjective Well‐being under Neuroleptics scale, SWN scale, higher=better) Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐14.82, 2.82] |
| 7 Craving for substances Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Craving for substances: 1. Specific: current craving‐ average endpoint scores (Marijuana Craving Questionairre, MCQ, lower=better) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 7.00 [2.37, 11.63] |
| 7.2 Craving for substances: 2. Specific: past week craving‐ average endpoint scores (Obsessive Compulsive Drug Use Scale, OCDUS, lower=better) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 14.20 [4.45, 23.95] |
| 8 Adherence to antipsychotic medication: discontinued medication Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 4.05 [0.21, 78.76] |
| 9 Adverse effects. 1. Movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 any extrapyramidal side‐effects | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.30, 24.08] |
| 9.2 akathisia | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.34] |
| 10 Adverse effects: 2. Non‐movement disorder related side‐effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Cardiovascular: palpitations | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.2 Cardiovascular: hypotension | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.3 Central nervous system: headache | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.54] |
| 10.4 Central Nervous System: somnolence | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.30] |
| 10.5 Dermatological: acne | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.6 Endocrinological: decreased libido | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.7 Ear and labarynthine: ear canal blockage | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.8 Gastrointestinal: abdominal pain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.9 Gasstrointesinal: elevated liver function tests | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.10 Gastrointestinal: hypersalivation | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.74] |
| 10.11 General adverse effects: fatigue | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.12 Injuries: sprain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.13 Metabolic: increased appetite | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.14 Metabolic: weight gain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.19, 5.24] |
| 10.15 Musculosceletal: ankle pain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.16 Musculosceletal: knee and foot pain | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.17 Musculosceletal: muscle twitch | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 63.15] |
| 10.18 Renal: urinary retention | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 10.19 Renal: urinary urgency | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.02] |
| 11 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 any reason | 2 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.10, 2.51] |
| 11.2 due to inefficacy | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: 1. Specific: Depression‐ change scores (HAM‐D, higher = better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 short‐term (up to 6 months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.78, 0.56] |
| 2 Mental state: 2. Specific: Positive symptoms, total score‐ average endpoint scores (SADS‐C‐PD scale, lower=better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 short term, up to 6 months) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐3.82, 0.82] |
| 3 Mental state: 3. Specific: Positive symptom subscales‐ average endpoint scores (SADS‐C‐PD subscores, lower=better), short term (up to 6 months)‐ skewed data Show forest plot | Other data | No numeric data | ||
| 4 Mental state: 4. Specific: Negative symptoms, subscales‐ average endpoint scores (SANS subscales, lower=better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Negative symptoms: Affective flattening | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.17, 1.17] |
| 4.2 Negative symptoms: alogia | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.22, 1.02] |
| 4.3 Negative symptoms: avolition‐apathy | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.73, 0.53] |
| 4.4 Negative symptoms: asociality‐anhedonia | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 5 Substance use: 1. Reduction of cannabis use‐change data (number of joints smoked/week, LOCF data, higher =better)‐ short term data (up to 6 months) Show forest plot | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐4.72, 5.52] |
| 6 Substance use: 2. Discontinued substance use, short term (up to 6 months) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Substance use: 2. Stopped using cannabis (Urine testing and Substance Use Questionnaire) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.68, 2.08] |
| 6.2 Substance use: 2. Stopped using alcohol (Substance Use Questionnaire) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.73, 2.36] |
| 7 Craving for substances: 1. Obsessive Compulsive Drug Use Scale‐ average endpoint score (OCDUS, lower=better)‐short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 short‐term (up to 6 months) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐3.51, 6.11] |
| 8 Craving for substances: 2. Desires for Drug Questionnaire‐ average endpoint scores (DDQ, LOCF data, lower=better), short term (up to 6 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Short‐term (up to 6 months) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐4.86, 14.86] |
| 9 Adverse effects Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Movement disorders: Parkinsonism‐ average endpoint score (SAS, high = worse)‐ short‐term (up to 6 months) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.21, 1.05] |
| 9.2 Non‐movement disorder related side‐effects: Weight gain‐ average endpoint score (BMI, lower=better)‐ short term (up to 6 months) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.99, 1.99] |
| 10 Leaving study early: 1. Various reasons Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 any reason, short term (up to 6 months) | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.35] |
| 10.2 any reason, long term (> 12 months) | 1 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.94, 1.21] |
| 10.3 readmission, short term (up to 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.45] |
| 10.4 intolerable adverse effects, short term (up to 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 participant loss of interest, short term (up to 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.33] |
| 11 Leaving study early: 2. Weeks in the study‐ average endpoint data (high=good), short term (up to 6 months) Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.35, 3.35] |
| 12 Leaving study early: 3. Weeks in study‐ average endpoint data (high=good), short term (up to 6 months)‐ skewed data Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early: all cause discontinuation, long term (>12 months) Show forest plot | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.92, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early: all cause discontinuation, long term (>12 months) Show forest plot | 1 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early: all cause discontinuation, long term (>12 months) Show forest plot | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.10] |


