نقش تصویربرداری در رد تشخیص آمبولی ریوی در بارداری
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the prevalence of PE and other lung diseases among pregnant women with suspected PE and to calculate radiation exposure. Type of study: retrospective cohort. Enrolled/eligible: 127/188. Inclusion period: 2009–2013. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE. Exclusion criteria: none. Setting: Sweden, tertiary care, 83% inpatients, 17% outpatients. Age: 30 years, 18–48 (mean, range). Gestational age: 24% 1st trimester; 46% 2nd trimester; 30% 3rd trimester. Presenting symptoms: 54% dyspnoea, 41% chest pain, 12% cough, 6% calf/thigh swelling, 5% haemoptysis, 2% collapse. Suspicion of DVT: 5.5%. Prior testing: 15/127 had chest X‐ray, 6 of which were abnormal. 5/127 had compression ultrasonography, 1 of which showed DVT. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: unclear. Second presentations included: unclear. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: SPECT. Original assessment or re‐assessment for study: original. Assessed by: not specified. Diagnostic criteria: not specified. Single or multiple protocols used during study: multiple according to trimester. Ventilation scanning performed: in proportion of patients. Technical specifications: 1‐ or 2‐day protocol. Perfusion 99mTc‐MAA 50 or 120 MBq. Venilation 99mTc‐Technegas 30 MBq. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: Patient files from all hospitals in the region were checked for potential later PE or DVT. | ||
| Flow and timing | Duration of follow‐up: during the same pregnancy and puerperal period. Loss to follow‐up: none. Criteria for choosing between index tests: unclear, in part according to after hours availability. | ||
| Comparative | |||
| Notes | The 61 CTPA scans performed in this study were excluded because they did not meet our inclusion criteria regarding the reference standard. Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate VTE outcomes in patients with negative MDCT‐PA during pregnancy. Type of study: retrospective cohort. Enrolled/eligible: 343/343. Inclusion period: April 2004‐June 2008. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE who underwent MDCT. Exclusion criteria: none. Setting: USA, tertiary care, inpatients and outpatients. Age: 29 ± 6.7 years (mean ± SD). Gestational age: unclear. Presenting symptoms: dyspnoea 75.6%, pleuritic pain 19.5%, non‐pleuritic chest pain 45.6%, cough 18.3%, calf swelling 14.3%, wheezing 5.2%, haemoptysis 0.9%. Suspicion of DVT: in at least 14.3%. Prior testing: unclear. Comorbidity: lung disease n = 84 (asthma 79.5%, prior VTE 14.5%, pulmonary hypertension 0.02%, sarcoidosis 0.01%, lung metastases 0.01%, pneumothorax 0.01%), heart disease n = 25, obstructive sleep apnoea n = 3. Anticoagulant therapy within 24 hours before testing: at least 2.1%. Second presentations included:yes, 6/349 scans. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: CTPA. Original assessment or re‐assessment for study: original. Assessed by: board‐certified radiologist. Diagnostic criteria: Study was labelled as ‘‘non‐diagnostic’’ subjectively if vessel opacification was poor and PE could not be excluded at the segmental level or more proximally. Single or multiple protocols used during study: interpretation on film or on a Picture Archiving and Communication System (PACS). Otherwise, the same. Technical specifications: 4‐MDCT. Collimation 4 × 2.5 mm. Rotation time 0.8 second. Pitch 1.5–1. 120 kV. Auto mAs. Further details: Data used for current review reflect scans as unit of analysis. Data with individual patients as unit of analysis could not be extracted. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: medical record review of own institution and imaging study query at 2 other institutions for evidence of VTE. If no data were available, the patient was considered loss to follow‐up. | ||
| Flow and timing | Duration of follow‐up: 3 months or 6 weeks postpartum (whichever came later). Loss to follow‐up: 16. Criteria for choosing between index tests: NA. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to quantitatively and qualitatively evaluate pulmonary 64‐MDCT angiography image quality during pregnancy and puerperium. Type of study: retrospective cohort. Enrolled/eligible: 70/70. Inclusion period: 3‐year period. | ||
| Patient characteristics and setting | Inclusion criteria: women with suspicion of PE during pregnancy and puerperium and matched controls. Exclusion criteria: none. Setting: Ireland. Age: 31.1 ± 6.1 years (mean ± SD). Gestational age: 1.4% 1st trimester; 44.3% 2nd trimester; 54.3% 3rd trimester. Presenting symptoms: unclear. Suspicion of DVT: unclear. Prior testing: 5 of the pregnant or puerperium patients had 2 scans, and 1 had 3 scans. Other prior testing was unclear. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: unclear. Second presentations included: unclear. Re‐scan after inconclusive scan included in results: yes. | ||
| Index tests | Index test: CTPA. Original assessment or re‐assessment for study: re‐assessment. Assessed by: radiologists with 7 and 15 years of experience. Diagnostic criteria: not specified. Single or multiple protocols used during study: single. Technical specifications: 64‐MDCT. Pitch 0.9. Rotation time 0.5 second. Collimation 32 × 0.6 mm. 100–120 kVp. Current was adjusted to patient weight. | ||
| Target condition and reference standard(s) | Target condition: PE at different levels. Segmental or more proximal PE considered as target condition for current review. Reference standard: checking hospital radiology system for subsequent imaging. | ||
| Flow and timing | Duration of follow‐up: 6 months. Loss to follow‐up: none. Criteria for choosing between index tests: NA. | ||
| Comparative | |||
| Notes | Attempted to contact study authors in July 2015. We received no reply. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to study the distribution of lung scan results and the safety of V/Q scanning, as well as the safety of withholding anticoagulation therapy following a normal or non‐diagnostic scan in pregnant women. Type of study: retrospective cohort. Enrolled/eligible: 105/105. Inclusion period: January 1990‐April 2000. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE undergoing perfusion scintigraphy. Exclusion criteria: full‐dose anticoagulation therapy. Setting: Canada, 53% secondary and 47% tertiary care. Age: 32 (17‐41) years. Gestational age: 9.9% 1st trimester; 42.5% 2nd trimester; 47.9% 3rd trimester. Presenting symptoms: 62% dyspnoea, 46% pleuritic pain, 19% non‐pleuritic chest pain. Suspicion of DVT: unclear. Prior testing: time relation of testing unclear. 50% had chest radiograph, 40% of which were abnormal. 61% had leg ultrasonography or impedance plethysmography. No patient had a DVT. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: none. Second presentations included: no. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: lung scintigraphy. Original assessment or re‐assessment for study: re‐assessment. Assessed by: 2 experts. Diagnostic criteria: high probability: > 75% subsegmental or greater perfusion defects with normal ventilation. Single or multiple protocols used during study: multiple. Ventilation scintigraphy performed: yes. Technical specifications: perfusion 99mTc‐MAA. Venilation 99mTc methylene diphosphonate aerosol or 99mTc sulphur colloid. | ||
| Target condition and reference standard(s) | Target condition: pulmonary embolism. Reference standard: clinical follow‐up. In part by telephone, otherwise through family physician and review of medical records. Further details: 2 patients had pulmonary angiography, 1 of which was positive. This patient had a non‐diagnostic index test but was excluded because of therapeutic anticoagulation. | ||
| Flow and timing | Duration of follow‐up: for telephone contact 20.6 months, 0.5‐108.0 (mean, range). Unclear duration for GP contact and record review. Loss to follow‐up: none. Criteria for choosing between index tests: NA. | ||
| Comparative | |||
| Notes | Study authors were contacted but could not provide additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine if clinical prediction models have potential diagnostic validity. Type of study: retrospective cohort. Enrolled/eligible: 183/216. Inclusion period: 2007‐2010. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women who underwent imaging for PE. Exclusion criteria: unclear. Setting: 2 tertiary centres in Australia and the United Kingdom. Age: 30 years; 18‐44 (median; range). Gestational age: 12% 1st trimester; 33% 2nd trimester; 55% 3rd trimester. Presenting symptoms: unclear. Suspicion of DVT: unclear. Prior testing: modified Wells determined retrospectively: 58% likely and 42% unlikely. D‐dimer was performed in 51 women: 48 were positive. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: unclear. Second presentations included: NA. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: lung scintigraphy. Original assessment or re‐assessment for study: re‐assessment. Assessed by: 2 experienced radiologists. Diagnostic criteria: not specified. Single or multiple protocols used during study: not specified. Ventilation scintigraphy performed: not specified. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: review of medical record. | ||
| Flow and timing | Duration of follow‐up: unclear, but through postpartum period. Loss to follow‐up: none. Criteria for choosing between index tests: NA. | ||
| Comparative | |||
| Notes | Study authors provided additional data. Portion of cohort used for earlier abstract publication, "The utility of the wells clinical prediction model and ventilation‐perfusion scanning for pulmonary embolism in pregnancy”, published in Journal of Thrombosis and Haemostasis in 2011. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the incidence of PE in pregnant patients investigated for this condition. Type of study: retrospective cohort. Enrolled/eligible: 49/49. Inclusion period: January 2005‐January 2010. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE. Retrospectively identified by positive bHCG collected at the time of the investigation. Exclusion criteria: none. Setting: Australia, tertiary care hospital. Age: 32.6 ± 5.5 years (mean ± SD). Gestational age: 4% 1st trimester; 53% 2nd trimester; 39% 3rd trimester; 4% unknown. Presenting symptoms: unclear. Suspicion of DVT: unclear. Prior testing: 14 positive D‐dimer and 1 negative in 49 patients. 27 of 44 patients had a chest X‐ray. 18 of 42 patients had compression ultrasonography, which revealed no cases of DVT. 1 patient in the scintigraphy group had a prior CTPA. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: At least 7/49 received clexane. Second presentations included: NA. Re‐scan after inconclusive scan included in results: yes, unclear number of re‐scans. | ||
| Index tests | Index test: lung scintigraphy. Original assessment or re‐assessment for study: not specified. Assessed by: not specified. Diagnostic criteria: not specified. Single or multiple protocols used during study: not specified. Ventilation scintigraphy performed: in all. Technical specifications: perfusion 99mTc‐MAA 100 MBq. Ventilation Technegas 20‐25 MBq. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: medical record review for patients with initial positive scan and for patients who re‐presented to the hospital. | ||
| Flow and timing | Duration of follow‐up: until delivery. Loss to follow‐up: none. Criteria for choosing between index tests: clinician and patient preference. | ||
| Comparative | |||
| Notes | We excluded the 21 CTPA scans performed in this study for not meeting our inclusion criteria of a minimum sample size of 25. The publication was in abstract form. Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the effect of this reduced‐dose pulmonary CTA protocol on radiation dose and image quality in pregnant patients. Type of study: retrospective cohort. Enrolled/eligible: 26/unclear. Inclusion period: July 2006 to July 2007. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with a suspicion of PE undergoing CTA. Exclusion criteria: known allergy to contrast material, renal insufficiency, suspected hyperparathyroidism. Setting: USA. Emergency department. Age: 29 ± 5 years (mean ± SD) Gestational age: 19 ± 10 (mean ± SD) Presenting symptoms: unclear. Suspicion of DVT: unclear. Prior testing: D‐dimer in proportion of women, results unclear. Compression ultrasonography in 18, negative in 18. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: unclear. Second presentations included: NA. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: CTPA. Original assessment or re‐assessment for study: re‐assessment. Assessed by: radiologists with 7 and 5 years of experience. Diagnostic criteria: not specified. Single or multiple protocols used during study: single. Technical specifications: 64‐row multi‐detector CT. Collimation 0.625 × 64 mm. Pitch 0.984. Rotation time 0.35 second. 100 kVp. 100 mAs. | ||
| Target condition and reference standard(s) | Target condition: segmental PE. Reference standard: hospital information system checked for rehospitalisation and PE or DVT diagnosis after index test. | ||
| Flow and timing | Duration of follow‐up: 18 ± 5 months (mean ± SD). Loss to follow‐up: none. Criteria for choosing between index tests: NA. | ||
| Comparative | |||
| Notes | We attempted to contact study authors by email in July 2015 and received no reply. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to investigate ruling out PE by CTPA in pregnant patients with clinical suspicion of PE. Type of study: retrospective cohort. Enrolled/eligible: 134/141. Inclusion period: February 2004‐December 2012. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE. Exclusion criteria: treatment with therapeutic dose of heparins, younger than 18 years, allergy to contrast. Setting: the Netherlands, secondary and tertiary care, inpatients and outpatients. Age: 31 years, 27‐35 (median, IQR). Gestational age: 8.2% 1st trimester; 40.3% 2nd trimester; 50.0% 3rd trimester; 1.5% unclear. Presenting symptoms: unclear. Suspicion of DVT: none. Prior testing: unclear. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: none at therapeutic dose. Prophylactic dose unclear. Second presentations included: unclear. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: CTPA. Original assessment or re‐assessment for study: original assessment. Performed by: local attending radiologist. Diagnostic criteria: if contrast material outlined an intraluminal defect or if a vessel was totally occluded by low‐attenuation material on at least 2 adjacent slices. Single or multiple protocols used during study: multiple. Technical specifications: single‐detector or multi‐detector‐row systems. No further specifications. | ||
| Target condition and reference standard(s) | Target condition: pulmonary embolism. Reference standard: outpatient visit or telephone interview. Further details: only with negative or inconclusive CTPA. Follow‐up consisted of a scheduled outpatient visit or telephone interview at 3 months. Patients were additionally instructed to contact the study centre or their general practitioner in the event of symptoms suggestive of DVT or PE. | ||
| Flow and timing | Duration of follow‐up: 3 months. Loss to follow‐up: none. Criteria for choosing between index tests: NA. Further details: The aim was to assess negative predictive value; therefore, follow‐up was performed only for patients without a confirmed PE on initial CTPA. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare diagnostic adequacy of lung scintigraphy vs pulmonary CTPA in pregnant patients with suspected PE and to identify causes of diagnostic inadequacy. Type of study: retrospective cohort. Enrolled/eligible: 50/50. Inclusion period: July 2006‐April 2008. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE, aged 15‐45. Exclusion criteria: none. Setting: Ireland, maternity hospital emergency department. Age: CTPA 32.6 ± 5.6 years, lung scintigraphy 31.8 ± 5.4 years (mean ± SD). Gestational age: unclear. Presenting symptoms: unclear. Suspicion of DVT: none. Prior testing: D‐dimer was performed in an unknown proportion of patients. All patients in scintigraphy group had normal chest X‐ray. 3/25 in CTPA group had abnormal chest X‐ray.. Two patients underwent lung scintigraphy after the initial non‐diagnostic CTPA, and 1 had lung scintigraphy after 2 non‐diagnostic CTPA scans. Comorbidity: unknown. Anticoagulant therapy within 24 hours before testing: unclear. Second presentations included: NA. Re‐scan after inconclusive scan included in results: yes, 3 re‐scans in CTPA group. | ||
| Index tests | Index test: CTPA (n = 28), lung scintigraphy (n = 25). Original assessment or re‐assessment for study: original. Assessed by: unclear. Diagnostic criteria: not specified. For CTPA ‐ Single or multiple protocols used during study: single. ‐ Technical specifications: 64‐MDCT scanner. Collimation 0.6 mm. Rotation speed 0.33 second. Pitch 0.9. 120 kV. 200 mAs. For lung scintigraphy ‐ Ventilation scintigraphy performed: minority of patients. ‐ Single or multiple protocols used during study: multiple. ‐ Technical specifications: perfusion 99mTc‐labeled MAA 90 MBq. Venilation 99mTc‐carbon particles 485 ± 72 MBq. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: chart review and imaging review on re‐presentation. | ||
| Flow and timing | Duration of follow‐up: 3 months. Loss to follow‐up: none. Criteria for choosing between index tests: physician preference; if abnormality on initial chest X‐ray, CTPA. | ||
| Comparative | |||
| Notes | Study authors provided additional data. Portion of cohort used for the publication, "Pulmonary CT angiography protocol adapted to the haemodynamic effects of pregnancy", published in American Journal of Roentgenology in 2011. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine if the diagnostic utility of perfusion scintigraphy in pregnant patients with suspected PE could be optimised by careful patient selection. Type of study: retrospective cohort. Enrolled/eligible: 96/96 for lung scintigraphy. Another 9 patients had CTPA. Inclusion period: January 2001‐December 2005. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant women with suspicion of PE who underwent imaging. Exclusion criteria: abnormal chest X‐ray, asthma or chronic lung disease. Setting: United Kingdom. Age: 29.8 years (range 16–48). Gestational age: 8% 1st trimester; 23% 2nd trimester; 69% 3rd trimester. Presenting symptoms: unclear. Suspicion of DVT: unclear. Prior testing: All had negative chest X‐ray. Comorbidity: no asthma or chronic lung disease, no other comorbidity reported. Anticoagulant therapy within 24 hours before testing: unclear. Second presentations included: one. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: lung scintigraphy. Original assessment or re‐assessment for study: re‐assessment but original assessments were extractable from the article. Assessed by: not specified. Diagnostic criteria: not specified. Ventilation scintigraphy performed: no. Single or multiple protocols used during study: multiple. Technical specifications: perfusion 99mTc‐MAA 40 or 80 MBq. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: review of institutional obstetrical records for pregnancy outcome and assessment of any imaging during follow‐up period. | ||
| Flow and timing | Duration of follow‐up: reported only for the group with non‐diagnostic scan: 744 days; 87–1421 (mean; range). Loss to follow‐up: none. Criteria for choosing between index tests: standardised protocol. Patients with normal chest X‐ray and no pulmonary comorbidity underwent scintigraphy; other patients underwent CTPA. Protocol was not followed in 100% of cases. | ||
| Comparative | |||
| Notes | Study authors were contacted but could not provide additional data. We excluded the 9 patients who had CTPA because this group met the exclusion criterion of a sample size smaller than 25. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine whether CTPA or perfusion scanning has better image quality and to determine the likelihood of subsequent PE after a negative interpretation. Type of study: retrospective cohort. Enrolled/eligible: 199/unclear. Inclusion period: 2000–2007. | ||
| Patient characteristics and setting | Inclusion criteria: pregnant patients who underwent CTPA or perfusion scanning for suspected PE. Exclusion criteria: DVT diagnosed on ultrasonography. Setting: USA, 47% at private hospital, 53% at university hospital. Age: 31 years, 18‐39 (mean, range). Gestational age: 27% 1st trimester; 31% 2nd trimester; 42% 3rd trimester. Presenting symptoms: 90% dyspnoea, 65% chest pain, 15% calf/thigh pain, 2% collapse, 1% haemoptysis. Suspicion of DVT: unclear. Prior testing: abnormal chest X‐ray: 8% in CTPA group and 5% in perfusion scan group. The rest had normal chest X‐ray. 58% underwent bilateral compression ultrasonography, all negative for DVT. In both CTA and perfusion scan groups, 3 patients underwent the other index test after non‐diagnostic scan. Comorbidity: unclear. Anticoagulant therapy within 24 hours before testing: 8%. Second presentations included: unclear. Re‐scan after inconclusive scan included in results: no re‐scans. | ||
| Index tests | Index test: CTPA (n = 106), lung scintigraphy (n = 99). Original assessment or re‐assessment for study: All re‐assessments were in agreement with the original ones. For CTPA ‐ Assessed by: 2 radiologists with 4 and 25 years of experience. ‐ Diagnostic criteria: filling defect in or failure of opacification of a pulmonary artery. ‐ Single or multiple protocols used during study: multiple. ‐ Technical specifications: 8‐, 16‐, or 64‐MDCT scanner. Pitch 1. 120–140 kV. 150–300 mAs. For lung scintigraphy ‐ Assessed by: a fellow in nuclear medicine and in some cases a nuclear medicine specialist (30 years of experience). ‐ Diagnostic criteria: threshold of more than 1.5 segmental perfusion defects for high probability. ‐ Ventilation scintigraphy performed: no. ‐ Technical specifications: 1–1.5 mCi (37–55 MBq) of 99mTc‐labeled MAA. | ||
| Target condition and reference standard(s) | Target condition: PE. Reference standard: clinical follow‐up. Information from hospital charts on suspicion, detection or treatment of PE. | ||
| Flow and timing | Duration of follow‐up: 3 months. Loss to follow‐up: 2%. Criteria for choosing between index tests: unclear. | ||
| Comparative | |||
| Notes | Attempted to contact study authors in July 2015. We received no reply. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
99mTc: technetium 99m.
bHCG: beta human chorionic gonadotropin.
CT: computed tomography.
CTA: computed tomography angiography.
CTPA: computed tomography pulmonary angiography.
DVT: deep vein thrombosis.
GP: general practitioner.
IQR: interquartile range.
MAA: macro aggregated albumin.
MDCT: multi‐detector computed tomography.
MDCT‐PA: multi‐detector computed tomography with pulmonary angiography.
NA: not applicable.
PACS: Picture Archiving and Communication System.
PE: pulmonary embolism.
SD: standard deviation.
SPECT: single‐photon emission computed tomography.
V/Q: ventilation/perfusion.
VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Duration of follow‐up not described. | |
| Discrepancies identified between additional data provided by study authors on the pregnant patient subgroup and data reported in the article, which could not be resolved by study authors. | |
| Fewer than 25 pregnant women. Study on MRA (not a reason for exclusion). | |
| Discrepancies identified between additional data provided by study authors and data reported in the article, which could not be resolved by study authors. | |
| Portion of cohort overlaps with included study Ridge 2009. The remaining portion of the cohort, with separate CTPA protocol, consisted of fewer than 25 pregnant women. | |
| Required data could not be extracted from the published abstract and was not provided by study authors. |
CTPA: computed tomography pulmonary angiography.
MRA: magnetic resonance angiography.
Characteristics of studies awaiting classification [ordered by study ID]
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | Full text could not be obtained. | ||
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
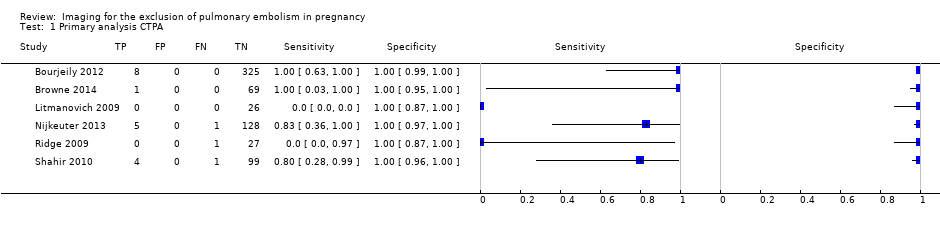
| 1 Primary analysis CTPA Show forest plot | 6 | 695 |
| Test 1 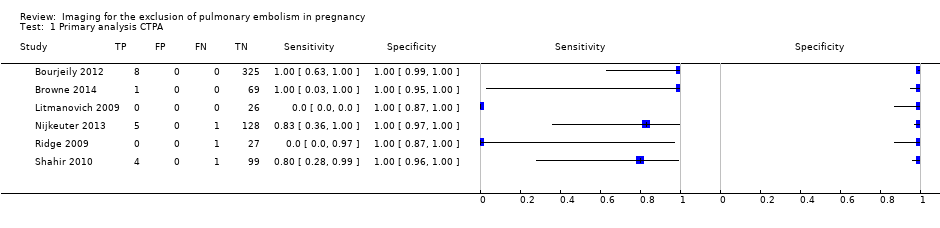 Primary analysis CTPA. | ||
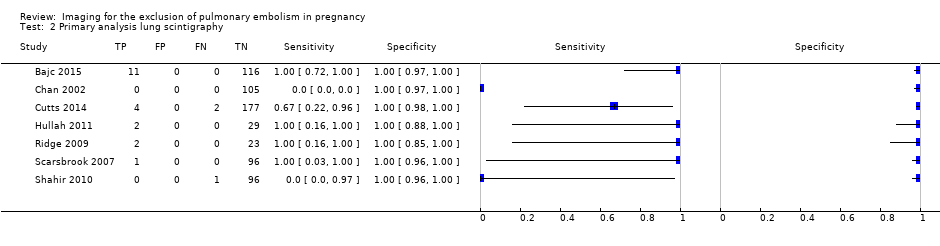
| 2 Primary analysis lung scintigraphy Show forest plot | 7 | 665 |
| Test 2 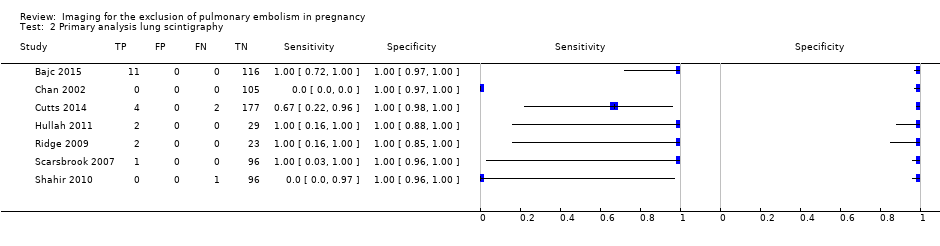 Primary analysis lung scintigraphy. | ||
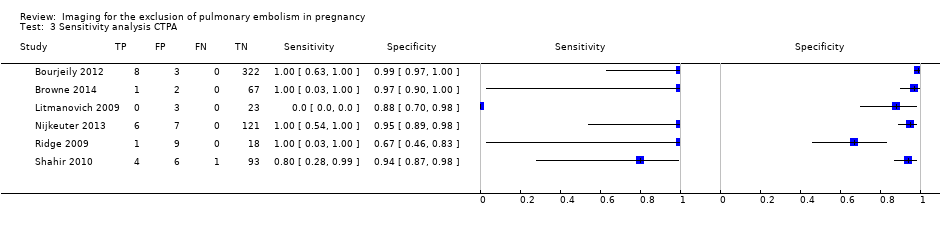
| 3 Sensitivity analysis CTPA Show forest plot | 6 | 695 |
| Test 3  Sensitivity analysis CTPA. | ||
| 4 Sensitivity analysis lung scintigraphy Show forest plot | 7 | 665 |
| Test 4 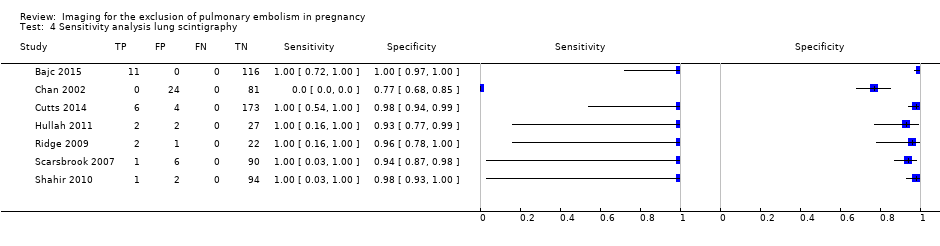 Sensitivity analysis lung scintigraphy. | ||

Study flow diagram.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Primary analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as negative.

Primary analysis. Forest plot of CTPA with inconclusives regarded as negative.

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as positive.

Sensitivity analysis. Forest plot of CTPA with inconclusives regarded as positive.
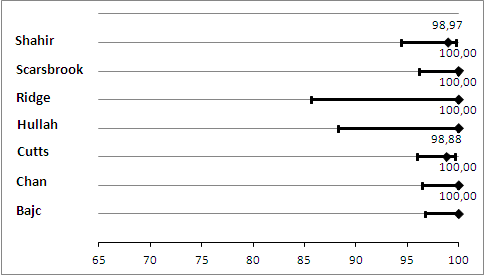
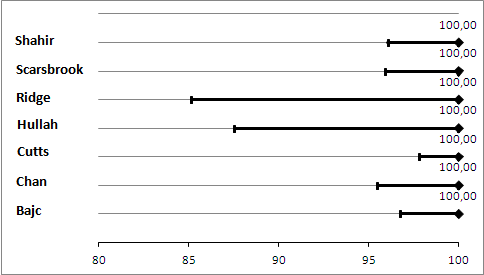
Primary analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as negative.
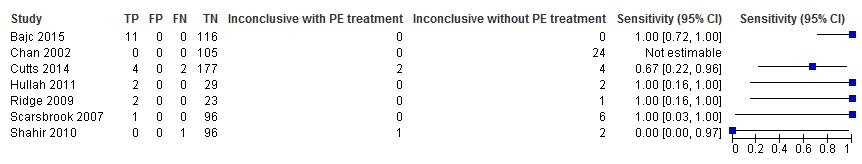
Primary analysis. Forest plot of lung scintigraphy with inconclusives regarded as negative.
Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as positive.
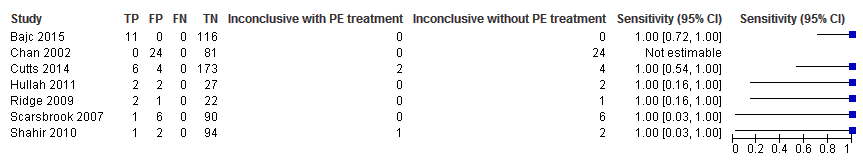
Sensitivity analysis. Forest plot of lung scintigraphy with inconclusives regarded as positive.
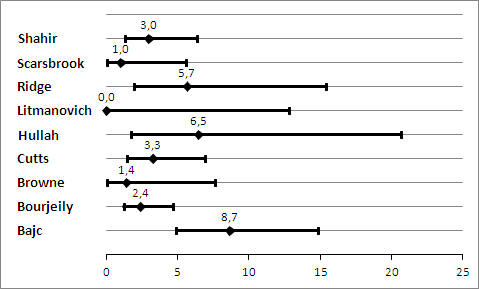
Prevelance of pulmonary embolism (%) with 95% confidence interval.
Primary analysis CTPA.
Primary analysis lung scintigraphy.
Sensitivity analysis CTPA.

Sensitivity analysis lung scintigraphy.
| What is the diagnostic accuracy of imaging tests for the diagnosis of pulmonary embolism (PE) in pregnancy? | ||||||
| Patients | Pregnant women with clinical suspicion of PE. | |||||
| Prior testing and prevalence | Varied. Most often performed were chest X‐ray and imaging for deep venous thrombosis. The median prevalence of PE was 3.3% (range 0.0% to 8.7%), as assessed by the applied reference standard, which has limitations. | |||||
| Settings | Secondary and tertiary care, both inpatients and outpatients. | |||||
| Index test | Computed tomography pulmonary angiography (CTPA), lung scintigraphy and magnetic resonance angiography (MRA). No studies on MRA were included. Inconclusive test results were regarded as negative in the primary analysis. | |||||
| Importance | Pregnant women are often suspected of PE because of increased risk and physiological signs that mimic symptoms of PE. Pregnant women are often excluded from diagnostic imaging studies. These imaging tests might perform differently during pregnancy, and radiation and other risks are weighed differently. | |||||
| Reference standard | Clinical follow‐up of at least 6 weeks. In almost all studies, follow‐up was performed to identify PE, not to exclude it. Pulmonary angiography was preferred but was applied by none of the studies. | |||||
| Studies | Cross‐sectional cohort studies were included. Case‐control studies were excluded. All studies were retrospective. | |||||
| Test | Number of studies (number of index test results) | Median negative predictive value (range) | Median sensitivity (range) | Median inconclusive test results (range) | Overall risk of bias (QUADAS‐2) | Overall applicability (QUADAS‐2) |
| CTPA | 6 (695) | 100% (96%‐100%) | 83% (0%‐100%) | 5.9% (0.9%‐36%) | High risk | High concern |
| Lung scintigraphy | 7 (665) | 100% (99%‐100%) | 100% (0%‐100%) | 4% (0%‐23%) | High risk | High concern |
| CAUTION: The results in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | ||||||
| CTPA: computed tomography pulmonary angiography. | ||||||
|
| Item plus signalling questions | Criteria for scoring 'yes', 'no' and 'unclear' |
| 1 | PATIENT SELECTION Was a consecutive or random sample of patients enrolled? Was a case‐control design avoided? Did the study avoid inappropriate exclusions? | We will score this item 'yes' when patients were consecutively or randomly selected; 90% or more were evaluated at the hospital; 5% or less of had received anticoagulant therapy within 24 hours before testing; 30% or less were given a diagnosis of comorbidity such as chronic obstructive pulmonary disease or other pulmonary disease, malignancy or pregnancy complications (preeclampsia, syndrome of haemolysis, elevated liver enzymes and low platelets or eclampsia); and 10% or less had undergone prior testing for this episode of suspected PE. We will score 'no' if one of these criteria was not met. |
| 2 | INDEX TEST Were index test results interpreted without knowledge of results of the reference standard? | We will score this item 'yes' in the following cases: if study authors state that the index test interpreter was unaware of the result of the reference test; or if the order of testing was index test before reference test for every patient. Even if clinical follow‐up was the reference test, the order of testing has to be stated for the item to be scored 'yes'. We will score the item ‘no’ for studies in which it is stated that the interpreter of the index test was aware of the result of the reference test. In other cases, we will score this as 'unclear'. In cases of studies directly comparing the diagnostic accuracy of 2 index tests against the reference standard, these test results had to be interpreted without knowledge of the results of the comparator index test, and we will score this item similarly to the approach described above. |
| 3 | REFERENCE STANDARD Is the reference standard likely to correctly classify the target condition? Were reference standard results interpreted without knowledge of results of the index test? | We considered both PA and clinical follow‐up of at least 6 weeks as useful for correct classification of the target condition, the latter only if objective diagnostic tests are used in cases of suspected venous thromboembolism. We will score this item 'yes' if study authors state that reference tests were interpreted without knowledge of results of the index test. Furthermore, in cases of clinical follow‐up as a reference standard, any clinical suspicion of venous thrombosis during follow‐up needs to be followed by objective diagnostic testing (i.e. CUS or venography for suspicion of DVT, and scintigraphy, CTPA or pulmonary angiography for clinical suspicion of PE). If a patient died during follow‐up, we classified death as caused by PE in cases of confirmation by autopsy, in cases of an objective test positive for PE before death or if PE could not be confidently excluded as the cause of death. |
| 4 | FLOW AND TIMING Was an appropriate interval between index test(s) and reference standard provided? Did all patients receive a reference standard? Did all patients receive the same reference standard? Were all patients included in the analysis? | With PA, we will consider a time period of less than 24 hours between index and reference tests as short enough to ensure that the target condition did not change between tests, either because of natural progression of the disease or because of therapeutic intervention. For studies using pulmonary angiography as the reference test, we will score this item 'yes' if the time between index and reference tests was less than 24 hours. Similarly, for studies directly comparing diagnostic accuracy of index tests, we will consider a time period of less than 24 hours between index tests and the reference test as short enough. During clinical follow‐up, the disease may diminish through natural progression or through intervention. Or the condition may arise during follow‐up if it was not present at the time of the index test. Therefore, we will score studies using clinical follow‐up 'no' for this item. We will score this item 'no' if less than 90% or a non‐random selection of patients underwent the reference test. We will score this item 'no' if less than 90% of patients who had an index test result underwent pulmonary angiography or had clinical follow‐up as the reference test. |
| CTPA: computed tomography pulmonary angiography. | ||
| Test | No. of studies | No. of participants |
| 1 Primary analysis CTPA Show forest plot | 6 | 695 |
| 2 Primary analysis lung scintigraphy Show forest plot | 7 | 665 |
| 3 Sensitivity analysis CTPA Show forest plot | 6 | 695 |
| 4 Sensitivity analysis lung scintigraphy Show forest plot | 7 | 665 |

