نقش تصویربرداری در رد تشخیص آمبولی ریوی در بارداری
چکیده
پیشینه
آمبولی ریه (pulmonary embolism) یکی از علل اصلی مرگومیر ناشی از بارداری است. تشخیص دقیق در بیماران باردار برای پیشگیری از آمبولی ریوی درمان نشده و همچنین درمان آنتیکوآگولانت غیر‐ضروری و اقدامات پیشگیرانه در آینده ضروری است. تکنیکهای تصویربرداری کاربردی ممکن است بهطور متفاوتی در این بیماران جوانتر با کوموربیدیتی کمتر و تغییر فیزیولوژی انجام شود، که عمدتا از مطالعات تشخیصی حذف شدهاند.
اهداف
تعیین دقت تشخیصی توموگرافی کامپیوتری آنژیوگرافی ریه (computed tomography pulmonary angiography; CTPA)، سینتیگرافی ریه (lung scintigraphy) و آنژیوگرافی رزونانس مغناطیسی (magnetic resonance angiography; MRA) برای تشخیص آمبولی ریه در دوران بارداری.
روشهای جستوجو
ما MEDLINE و Embase را تا جولای 2015 جستوجو کردیم. از مطالعات وارد شده به عنوان بذر در جستوجوی استنادها و از دکمه «پیدا کردن مشابه» استفاده کردیم و فهرست منابع را جستوجو کردیم. به کارشناسان در این زمینه نزدیک شدیم تا به ما در شناسایی مطالعات غیر‐ایندکس شده کمک کنند.
معیارهای انتخاب
مجموعههای متوالی از بیماران باردار مشکوک به آمبولی ریه را وارد کردیم که تحت یکی از تست شاخص (آنژیوگرافی ریوی توموگرافی کامپیوتری (CT)، سینتیگرافی ریه، MRA) و پیگیری بالینی یا آنژیوگرافی ریه به عنوان یک تست مرجع تست شدند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور استخراج دادهها و ارزیابی کیفیت را انجام دادند. با محققان مطالعات بالقوه واجد شرایط تماس گرفتیم تا اطلاعات ازدسترفته را دریافت کنیم. در تجزیهوتحلیل اولیه، نتایج تست شاخص نامنسجم را به عنوان یک تست مرجع منفی و درمان آمبولی ریه پس از یک تست نامنسجم شاخص به عنوان یک تست مرجع مثبت مورد بررسی قرار دادیم.
نتایج اصلی
11 مطالعه (چهار CTPA؛ پنج سینتیگرافی ریه؛ هر دو) را با مجموع نتایج 695 CTPA و 665 سینتیگرافی ریه وارد کردیم. سینتیگرافی ریه با روشهای مختلفی انجام شدند. هیچ مطالعه MRA با معیارهای ورود ما همخوانی نداشت.
در مجموع، خطر سوگیری (bias) و نگرانی در مورد قابلیت کاربرد در تمام مطالعات، به واسطه سوال مرور، بالا بودند، زیرا در روشهای مطالعه ناهمگونی وجود داشت. متاآنالیز انجام ندادیم. تمام مطالعات از پیگیری بالینی به عنوان یک استاندارد مرجع استفاده کردند، اما نه به روشی که امکان شناسایی قابل اعتماد را از مثبت کاذب فراهم کند. حساسیت و ارزش اخباری منفی تنها معیار معتبر برای دقت تست بود.
میانه ارزش اخباری منفی برای CTPA برابر با 100% (محدوده 96% تا 100%) بود. میانه حساسیت برابر با 83% (محدوده 0% تا 100%) بود.
میانه ارزش اخباری منفی برای سینتیگرافی ریه برابر با 100% (محدوده 99% تا 100%) بود. میانه حساسیت برابر با 100% (محدوده 0% تا 100%) بود.
میانه فراوانی نتایج غیر‐قاطع برابر با 5.9% (محدوده 0.9% تا 36%) برای CTPA و 4.0% (محدوده 0% تا 23%) برای سینتیگرافی ریه بود. میانه کلی شیوع آمبولی ریوی 3.3% (محدوده 0.0% تا 8.7%) گزارش شد.
نتیجهگیریهای نویسندگان
هر دو CTPA و سینتیگرافی ریه به نظر میرسد برای رد تشخیص آمبولی ریوی در دوران بارداری مناسب است. با این حال، کیفیت شواهد مستلزم پذیرش محتاطانه این نتیجهگیری است. محدودیتهای مهم شامل استانداردهای مرجع ضعیف، فرضیههای لازم در تجزیهوتحلیل نتایج تست حاصل غیر‐قابل اعتماد و عدم توانایی ذاتی مطالعات وارد شده برای شناسایی مثبت کاذب بوده است. مشخص نیست که کدام تست دارای بالاترین دقت است. نیاز به مقایسه مستقیم بین روشهای تشخیصی، از جمله MR، در مطالعات تشخیصی تصادفیسازی شده آیندهنگر وجود دارد.
خلاصه به زبان ساده
تکنیکهای تصویربرداری برای رد تشخیص آمبولی ریه در دوران بارداری
آمبولی ریه (pulmonary embolism) یک لخته خونی است که جریان خون را به بخشی از ریهها مسدود میکند. زنان باردار در معرض خطر بالای آمبولی ریه قرار دارند و علت اصلی مرگومیر در دوران بارداری است. زنان در معرض خطر با داروهای رقیق کننده خون درمان میشوند. مهم است که هیچ بیماری از دست نرود و از درمان بیمورد در زنان بدون بیماری پیشگیری شود. این بیماری را میتوان از طریق تکنیکهای مختلف اسکن تشخیص داد. در مورد عملکرد این تستها در دوران بارداری اطلاعات کمی در دست است، که ممکن است متفاوت از عملکرد آنها در بارداری باشد. ما این مرور را انجام دادیم تا دقت تستهای تصویربرداری زیر را برای تشخیص آمبولی ریه در دوران بارداری تعیین کنیم: آنژیوگرافی توموگرافی کامپیوتری ریوی (computed tomography pulmonary angiography)، سینتیگرافی ریه (lung scintigraphy) و آنژیوگرافی رزونانس مغناطیسی (magnetic resonance angiography).
11 مطالعه (موجود تا جولای 2015) را پیدا کردیم که نتایج 695 آنژیوگرافی توموگرافی کامپیوتری ریوی، نتایج 665 سینتیگرافی ریوی و هیچ موردی را از آنژیوگرافی رزونانس مغناطیسی توصیف کردهاند. مطالعات در مورد سینتیگرافی ریوی تکنیکهای مختلفی داشتند. به طور کلی، این مطالعات کیفیت پائین داشتند؛ بنابراین، نمیتوانیم نتایج را با هم مقایسه کنیم تا تخمین دقیقی از دقت آنها را به دست آوریم. مطالعات شناسایی شده از نظر بالینی بیماران را پیگیری کردند تا تایید عدم وجود آمبولی ریوی که در اسکن اولیه نشان داده شده، مسجل شود، ازاینرو میتوان از اطلاعات فقط برای نتیجهگیری در مورد توانایی این تستهای تصویربرداری برای رد تشخیص آمبولی ریوی، نه بر توانایی آنها در ایجاد تشخیص، استفاده کرد.
هر دو تست آنژیوگرافی توموگرافی کامپیوتری ریوی و سینتیگرافی ریوی برای رد تشخیص آمبولی ریوی در دوران بارداری مناسب است. تقریبا هیچ موردی از دست نرفته بود، به خصوص زمانی که تست تصویربرداری عدم بیماری را بدون شک و تردید نشان داده بود. با این حال، این نتیجه باید با توجه به کیفیت پائین و تفاوت بین مطالعات شناسایی شده تفسیر شود. حدود 5% از اسکنها نامشخص بود، اما این میزان در یک مطالعه 36% بود. حدود 3% از همه زنان که در این مطالعه بودند، آمبولی ریه داشتند. ما نمیدانیم که کدام تست بهتر است، زیرا تستها بهطور مستقیم در همان بیماران مقایسه نمیشود و جنبههای دیگر نیز علاوه بر دقت تست باید مورد توجه قرار گیرند. محدودیتهای عمده این مرور عبارتند از استفاده از پیگیری بالینی درون مطالعات برای تایید عدم وجود بیماری، نتایج غیر‐قابل تشخیص و عدم توانایی مطالعات برای ارائه اطلاعات در مورد دقت این تستها در ایجاد و نه رد تشخیص. پژوهشهای با کیفیت بالا برای بررسی استفاده از آنژیوگرافی ریوی، سینتیگرافی ریوی و آنژیوگرافی ریوی مغناطیسی در گروههای مشابه انجام میشود.
Authors' conclusions
Summary of findings
| What is the diagnostic accuracy of imaging tests for the diagnosis of pulmonary embolism (PE) in pregnancy? | ||||||
| Patients | Pregnant women with clinical suspicion of PE. | |||||
| Prior testing and prevalence | Varied. Most often performed were chest X‐ray and imaging for deep venous thrombosis. The median prevalence of PE was 3.3% (range 0.0% to 8.7%), as assessed by the applied reference standard, which has limitations. | |||||
| Settings | Secondary and tertiary care, both inpatients and outpatients. | |||||
| Index test | Computed tomography pulmonary angiography (CTPA), lung scintigraphy and magnetic resonance angiography (MRA). No studies on MRA were included. Inconclusive test results were regarded as negative in the primary analysis. | |||||
| Importance | Pregnant women are often suspected of PE because of increased risk and physiological signs that mimic symptoms of PE. Pregnant women are often excluded from diagnostic imaging studies. These imaging tests might perform differently during pregnancy, and radiation and other risks are weighed differently. | |||||
| Reference standard | Clinical follow‐up of at least 6 weeks. In almost all studies, follow‐up was performed to identify PE, not to exclude it. Pulmonary angiography was preferred but was applied by none of the studies. | |||||
| Studies | Cross‐sectional cohort studies were included. Case‐control studies were excluded. All studies were retrospective. | |||||
| Test | Number of studies (number of index test results) | Median negative predictive value (range) | Median sensitivity (range) | Median inconclusive test results (range) | Overall risk of bias (QUADAS‐2) | Overall applicability (QUADAS‐2) |
| CTPA | 6 (695) | 100% (96%‐100%) | 83% (0%‐100%) | 5.9% (0.9%‐36%) | High risk | High concern |
| Lung scintigraphy | 7 (665) | 100% (99%‐100%) | 100% (0%‐100%) | 4% (0%‐23%) | High risk | High concern |
| CAUTION: The results in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | ||||||
| CTPA: computed tomography pulmonary angiography. | ||||||
Background
Target condition being diagnosed
Pulmonary embolism (PE) is a leading cause of maternal mortality (Chang 2003; Kobayashi 2008; Lewis 2007). Approximately 30% of maternal deaths directly related to pregnancy between 2003 and 2005 in the United Kingdom were caused by thromboembolism (Lewis 2007). The incidence approximates 3 per 10,000 pregnancies, including the postpartum period, around 30% of which occur before the time of delivery (Meng 2015). Mortality in untreated patients with PE is reported to be as high as 18% to 35%, although these figures are based on older studies (Calder 2005). Treatment of women with pregnancy‐related PE consists of subcutaneous low‐molecular‐weight heparin (LMWH) (Bates 2012). Drawbacks of LMWH treatment include the need for daily injections, the occurrence of skin reactions and bruising in 2% to 29% of women (Bank 2003; Greer 2005), restricted epidural analgesics during labor and theoretical risks of heparin‐induced thrombocytopenia and major bleeding.
Pulmonary embolism together with deep venous thrombosis (DVT) is considered to be one disease entity, and this combination is referred to as venous thromboembolism (VTE). These conditions often co‐occur and share the same risk factors and pathophysiology. Pregnant women have a five‐fold increased risk of VTE compared with age‐matched controls (Pomp 2008). However, absolute numbers of pregnant women with clinical suspicion of PE are still relatively low. Most physicians working in the field of thrombosis infrequently encounter pregnant women with PE, thus experience is greatly lacking. A major challenge is that many symptoms of PE are similar to physiological manifestations of pregnancy, such as swollen legs, dyspnoea and tachycardia. Among pregnant women with suspected PE, a VTE diagnosis is confirmed in 4%, as compared with 12% among non‐pregnant patients (Kline 2014). Pulmonary angiography, which traditionally has been considered the gold standard for PE, is an X‐ray‐based imaging technique that uses contrast agent to visualise thrombi. However, this approach is rarely used anymore because of associated complications, high radiation doses, high costs and declining availability and expertise (Rosenberg 2007; Somarouthu 2010).
Index test(s)
This review focuses on computed tomography pulmonary angiography (CTPA), lung scintigraphy and magnetic resonance angiography (MRA), performed as first‐line tests or after other diagnostic testing in pregnant patients suspected of PE. CTPA is an X‐ray‐based imaging modality that uses intravenous contrast along with computer processing to create tomographic images. CTPA has a clinical validity comparable with that of pulmonary angiography (Quiroz 2005). It is widely available and has the potential of providing an alternative diagnosis. Outside of pregnancy, it is the imaging test most often performed to identify PE. An intraluminal filling defect is diagnostic of PE, and absence of a filling defect rules out the diagnosis. An inconclusive test result is reported for only a small percentage of CTPAs performed outside of pregnancy (0.9% to 4.6% (Mos 2009)). Disadvantages of CTPA include allergic reactions to the contrast agent, risk of contrast‐related nephropathy and exposure of both mother and foetus to radiation. The level of foetal exposure seems acceptable provided appropriate methods of dose reduction are applied (Nijkeuter 2006). Concerns have been raised that breast tissue, which is especially susceptible to carcinogenic effects, receives particularly high doses of radiation (Einstein 2007). Iodinated contrast agents cross the placenta and carry the theoretical risk of neonatal thyroid function depression, although this risk is not supported by findings of clinical studies (Bourjeily 2010; Webb 2005).
Lung scintigraphy is another well‐established diagnostic modality for PE. Radioisotopes are infused and inhaled to evaluate perfusion and ventilation of the lungs, respectively. This procedure uses gamma cameras to make two‐dimensional projection images. Results of ventilation and perfusion scanning are combined to identify a mismatch indicative of PE. Ventilation/perfusion single‐photon emission computed tomography, or V/Q SPECT, is based on the same technique but provides three‐dimensional imaging. Therefore, V/Q SPECT is in effect a distinct diagnostic modality. It is recommended as a first‐line diagnostic procedure by the European Association of Nuclear Medicine guidelines (Bajc 2009). Scan results can be divided into three categories: 'PE present' (high probability), 'PE absent' (very low probability or normal) and 'inconclusive'. Outside pregnancy, the sensitivity of a high‐probability lung scintigraphy scan was reported to be 77% and the specificity of a very low probability or normal scan was 98% after non‐diagnostic readings were excluded (Sostman 2008). The high percentage (around 39%) of non‐diagnostic readings is the main drawback of the V/Q scan and necessitates further imaging (PIOPED 1990). This proportion can be reduced by performing V/Q scan only in patients with a normal chest X‐ray. A normal chest X‐ray can also justify leaving out the ventilation scan, thereby limiting radiation exposure (Torbicki 2014).
The mother is generally exposed to less radiation with lung scintigraphy than with CTPA (Hurwitz 2006). Radiation exposure of the foetus during lung scintigraphy is equal to or greater than that reported in CTPA, depending on the CT scanner model and imaging protocol used, as well as the trimester and method of estimated exposure (Doshi 2008; Hurwitz 2006a; Winer‐Muram 2002). The main risk of in utero exposure to radiation is induction of malignancy. The number of cases of excess malignancy up to age 15 years following irradiation in utero is considered to be 1 in 16,000 per mSv (Streffer 2003). CTPA exposes the foetus to 0.013 mSv of radiation, and a perfusion scan to 0.11 to 0.20 mSv (Nijkeuter 2004). Given the difficulty of calculating radiation and variation in the protocols used, these numbers should be interpreted as equally low. The small amounts justify the use of radiation to prevent a potentially life‐threatening PE. Of course, radiation exposure should be kept as low as possible, in line with the As Low As Reasonably Achievable principle (Hendee 1986). At the same time, clinicians must be aware that risks of radiation should be weighed against risks of untreated PE or complications of unnecessary anticoagulant therapy.
Magnetic resonance angiography (MRA) applies magnetic fields and radiofrequency pulses to visualise blood vessels using flow effects or contrast material. This approach offers the advantage that it is radiation‐free. However, MRA is not applied in daily practice because of long acquisition times, and few studies have reported on this technique (Kanal 1992; Oudkerk 2002). Another concern is the safety of gadolinium‐containing contrast agents during pregnancy (Chen 2008). No deleterious effects have been documented during human pregnancy, but supraclinical doses show deleterious effects on both pregnancy and foetal development in animal studies (Tremblay 2012). Progress has been made with contrast‐free magnetic resonance imaging (Kluge 2004). Although magnetism and radiofrequency pulses carry theoretical risks for the foetus, limited research has produced no evidence of harmful effects on the (unborn) child or on pregnancy (Tremblay 2012).
Clinical pathway
In practice, the diagnostic pathway in the case of suspicion of PE in pregnant women may differ among different physicians and different patients. Depending on the clinical presentation, a chest X‐ray or an electrocardiogram can be used to rule out other diseases. As PE and DVT are manifestations of the same disease entity (VTE), physicians sometimes opt for compression ultrasonography (CUS) of the legs in cases of suspected PE, when symptoms of DVT are also present. When the diagnosis of DVT is confirmed, no further imaging of the lungs is necessary, as treatment for patients with PE is the same as treatment for patients with DVT.
For the diagnosis of PE outside of pregnancy, different tests and clinical decision rules can be combined to provide the safest diagnostic strategies. Clinical decision rules are used to determine a patient’s pretest probability of PE. In non‐pregnant patients, a low to moderate pretest probability combined with a normal D‐dimer test is safe to rule out PE without the need for further diagnostic imaging. In pregnancy, however, several items of these rules constitute normal physiological manifestations of pregnancy, for example, swollen legs or heart rate above 100 beats/min. Other items are seldom seen in pregnancy, such as the presence of cancer. Clinical decision rules for PE have not been validated in pregnant women. In addition, during normal pregnancy, D‐dimer levels gradually rise. A normal D‐dimer level is infrequently encountered in the second and third trimesters of pregnancy (Chabloz 2001; Quiroz 2005); therefore, current practice seldom includes D‐dimer levels or clinical decision rules in the diagnostic strategy for ruling out PE during pregnancy. We have not included these tests in this review.
Rationale
It is important to regard pregnant women as a distinct subgroup for which tests might perform differently and additional risks need to be considered. Other research groups have reviewed the available literature on PE diagnostics specifically for pregnant women (Bourjeily 2010a; Brown 2010; Duran‐Mendicuti 2011; Nijkeuter 2006; Rodger 2010; Rosenberg 2007; Tan 2011). These narrative reviews are useful as guides in the clinical dilemma of suspected PE in pregnancy. However, they lack the rigorous and explicit methods of a Cochrane review with transparent guidelines, minimisation of bias and periodic updates.
An objectively confirmed diagnosis of PE is imperative in pregnancy. Anticoagulant treatment is potentially harmful and has implications for delivery, and a PE diagnosis determines future decisions on thromboprophylaxis and oral contraceptive use. On the other hand, untreated PE is a potentially fatal disease. Physiological changes during pregnancy may mimic the symptoms of PE; therefore, PE can be suspected more often in pregnant women. D‐dimer testing and clinical decision rules available for non‐pregnant patients are not useful for pregnant women.
Diagnostic imaging tests for PE have been extensively studied in non‐pregnant patients (Bounameaux 2010), but pregnant women usually are excluded from these diagnostic and management studies. A strong evidence base for the management of pregnancy‐related PE is therefore missing (Middeldorp 2011). Extrapolating results from studies and guidelines on non‐pregnant patients is not advisable because pregnant women form a clinically distinct subgroup. The average age of pregnant women is lower than the age of patients in the studies from which diagnostic accuracy data are derived. Therefore, pregnant women are less likely to have cardiorespiratory comorbidity, which can result in abnormal V/Q scans (Matthews 2006). In pregnancy, a raised diaphragm might result in abnormal perfusion scan results.
Another issue complicating the diagnosis of PE specifically in pregnancy is that imaging tests expose the foetus to ionising radiation. Although the level of foetal radiation exposure is far below the threshold for inducing carcinogenic and teratogenic effects, physicians may be hesitant to perform imaging tests. Given the high index of suspicion and the dangers of misdiagnosis encouraging diagnostic efforts, along with radiation exposure and other test complications discouraging testing, optimal diagnostic strategies are even more crucial in pregnant women than in non‐pregnant patients.
Objectives
To determine the diagnostic accuracy of CTPA, lung scintigraphy and MRA for the diagnosis of PE during pregnancy. and to calculate the diagnostic accuracy of these tests in relation to the reference standard of pulmonary angiography or clinical follow‐up for at least six weeks for occurrence of symptomatic PE.
Secondary objectives
To determine the number of inconclusive test results for each diagnostic test or diagnostic strategy, defined as neither showing nor excluding PE.
We aimed to investigate the effects of following clinical factors on the negative predictive value of index tests.
1. Prior testing (e.g. using clinical decision rule results and D‐dimer testing).
2. Gestational age.
3. Type of reference standard.
4. Technological advances of CTPA (multi‐slice vs single‐slice).
Methods
Criteria for considering studies for this review
Types of studies
We considered studies with a prospective or retrospective consecutive patient series design eligible for inclusion. The minimum sample size was set at 25 per index test, in light of the a priori chance of PE of 4%; we deemed smaller studies uninformative. Studies could still be included if patients received one of multiple index tests and the choice between tests was not random and was not based on a clear protocol, as long as recruitment was consecutive. We considered inclusion consecutive if study authors described it using this or similar terms. The quality item on consecutive inclusion could still be scored 'no' on the basis of criteria provided in Table 1 (see below). In studies in which the index test could be performed multiple times for one patient, study authors reported the index test result on a patient or scan basis. In both cases, data on index test results in relation to reference results had to be extractable from the article or provided on request by study authors. We excluded case‐control studies.
|
| Item plus signalling questions | Criteria for scoring 'yes', 'no' and 'unclear' |
| 1 | PATIENT SELECTION Was a consecutive or random sample of patients enrolled? Was a case‐control design avoided? Did the study avoid inappropriate exclusions? | We will score this item 'yes' when patients were consecutively or randomly selected; 90% or more were evaluated at the hospital; 5% or less of had received anticoagulant therapy within 24 hours before testing; 30% or less were given a diagnosis of comorbidity such as chronic obstructive pulmonary disease or other pulmonary disease, malignancy or pregnancy complications (preeclampsia, syndrome of haemolysis, elevated liver enzymes and low platelets or eclampsia); and 10% or less had undergone prior testing for this episode of suspected PE. We will score 'no' if one of these criteria was not met. |
| 2 | INDEX TEST Were index test results interpreted without knowledge of results of the reference standard? | We will score this item 'yes' in the following cases: if study authors state that the index test interpreter was unaware of the result of the reference test; or if the order of testing was index test before reference test for every patient. Even if clinical follow‐up was the reference test, the order of testing has to be stated for the item to be scored 'yes'. We will score the item ‘no’ for studies in which it is stated that the interpreter of the index test was aware of the result of the reference test. In other cases, we will score this as 'unclear'. In cases of studies directly comparing the diagnostic accuracy of 2 index tests against the reference standard, these test results had to be interpreted without knowledge of the results of the comparator index test, and we will score this item similarly to the approach described above. |
| 3 | REFERENCE STANDARD Is the reference standard likely to correctly classify the target condition? Were reference standard results interpreted without knowledge of results of the index test? | We considered both PA and clinical follow‐up of at least 6 weeks as useful for correct classification of the target condition, the latter only if objective diagnostic tests are used in cases of suspected venous thromboembolism. We will score this item 'yes' if study authors state that reference tests were interpreted without knowledge of results of the index test. Furthermore, in cases of clinical follow‐up as a reference standard, any clinical suspicion of venous thrombosis during follow‐up needs to be followed by objective diagnostic testing (i.e. CUS or venography for suspicion of DVT, and scintigraphy, CTPA or pulmonary angiography for clinical suspicion of PE). If a patient died during follow‐up, we classified death as caused by PE in cases of confirmation by autopsy, in cases of an objective test positive for PE before death or if PE could not be confidently excluded as the cause of death. |
| 4 | FLOW AND TIMING Was an appropriate interval between index test(s) and reference standard provided? Did all patients receive a reference standard? Did all patients receive the same reference standard? Were all patients included in the analysis? | With PA, we will consider a time period of less than 24 hours between index and reference tests as short enough to ensure that the target condition did not change between tests, either because of natural progression of the disease or because of therapeutic intervention. For studies using pulmonary angiography as the reference test, we will score this item 'yes' if the time between index and reference tests was less than 24 hours. Similarly, for studies directly comparing diagnostic accuracy of index tests, we will consider a time period of less than 24 hours between index tests and the reference test as short enough. During clinical follow‐up, the disease may diminish through natural progression or through intervention. Or the condition may arise during follow‐up if it was not present at the time of the index test. Therefore, we will score studies using clinical follow‐up 'no' for this item. We will score this item 'no' if less than 90% or a non‐random selection of patients underwent the reference test. We will score this item 'no' if less than 90% of patients who had an index test result underwent pulmonary angiography or had clinical follow‐up as the reference test. |
CTPA: computed tomography pulmonary angiography.
CUS: compression ultrasonography.
DVT: deep vein thrombosis.
PA: pulmonary angiography.
PE: pulmonary embolism.
Participants
Participants were pregnant women with clinical suspicion of PE. Conditions for suspicion of PE and confirmation of pregnancy were left to the discretion of the authors of included studies. We did not include data on postpartum or asymptomatic patients.
If not all women in the study met the inclusion criteria of the present review, data had to be extractable for relevant participants. If relevant data were not extractable and could not be supplied by the study author, we excluded the study. Study populations from secondary and tertiary care settings, comprising inpatients and outpatients, were eligible for inclusion. We excluded primary care patients because test accuracy can vary with care setting.
Index tests
Studies investigating CTPA, lung scintigraphy, either planar or V/Q SPECT and either with or without ventilation scanning, and MRA as an index test were eligible for inclusion. If the patient had undergone the same index test more than once, we included only data on the first index test performed. If the first test was followed by a different index test, we included this test result. Data on original assessments of the index test result in the clinical setting were preferred over re‐assessments for study purposes.
Target conditions
The target condition of this review is symptomatic PE. For studies that provided multiple definitions of non‐diagnostic scans according to the level of the pulmonary vasculature, we considered scans as non‐diagnostic when they were of insufficient quality to allow visualisation of PE at segmental or more proximal levels.
Reference standards
We accepted as reference standards both pulmonary angiography and clinical follow‐up for the occurrence of symptomatic PE for at least six weeks. We anticipated that few or no studies would use pulmonary angiography as the reference standard. We considered a PE confirmed within three months of the index test as a positive reference test.
The diagnosis of PE was confirmed on pulmonary angiography in the case of a new intraluminal filling defect, with cut‐off of contrast material in a vessel with diameter greater than 2.5 mm.
If clinical follow‐up was applied as the reference standard, objective diagnostic testing had to be performed in the case of symptoms of PE. Objective criteria for the diagnosis of PE included a (new) intraluminal filling defect on CTPA; cut‐off of contrast material in a vessel greater than 2.5 mm in diameter; a new perfusion defect involving at least 75% of a segment, with corresponding normal ventilation (i.e. a high‐probability lung scan); a new non‐diagnostic lung scan accompanied by documentation of DVT on ultrasonography or venography; and confirmation of a new PE at autopsy. For clinical follow‐up, PE was also diagnosed when symptoms of PE were combined with a diagnosis of DVT. The objective criterion for the diagnosis of DVT was a (new) non‐compressible venous segment or a substantial increase (4 mm or more) in the diameter of the thrombus during full compression in a previously abnormal segment on ultrasonography, or a new intraluminal filling defect on venography. In case of an inconclusive index test, we considered the reference test positive if the patient was treated on clinical grounds without objective evidence of VTE on follow‐up. This provides a conservative estimate of both negative predictive value and sensitivity in the primary analysis.
When both pulmonary angiography and clinical follow‐up were performed, we considered pulmonary angiography the reference standard.
Search methods for identification of studies
We applied no language restrictions.
Electronic searches
We searched MEDLINE and Embase (OvidSP) systematically for potentially eligible studies until July 2015. We specified the search strategies used in Appendix 1 and Appendix 2. We used three sets of terms reflecting the research question of the review: the tests (index tests), the target condition (PE) and the patients (pregnant women). We executed searches with venous thrombosis or thromboembolism as the target condition to increase sensitivity. We did not use a methodological search filter or a search term such as 'diagnostic' because doing this has been shown to decrease the sensitivity of the search without significantly benefiting the specificity (Leeflang 2006; Ritchie 2007).
Searching other resources
We used studies identified by the electronic search as eligible for inclusion in the review as 'seeds' for the 'related article' feature in PubMed and the 'find similar' feature in Embase to identify other potentially relevant articles. We performed a citations search for these studies in Web of Science to identify articles that cited them. We handsearched the reference lists of included studies and of previous reviews on this topic for potentially relevant studies. Finally, we contacted experts in the field (MV Huisman, M Righini, WS Chan) to enquire about unpublished studies and studies not included in Embase or MEDLINE.
Data collection and analysis
Selection of studies
Review authors retrieved articles identified by the search and reviewed all abstracts of potentially relevant studies, judging them first by title with a very low threshold. We then conducted a full‐text review of publications considered potentially relevant on the basis of the abstract review to determine whether they met the inclusion criteria defined above. Two review authors independently completed this process. We resolved disagreements by discussion or, when necessary, through involvement of a third review author, whose judgement was then decisive.
Data extraction and management
Two review authors used standardised, piloted data extraction forms to independently extract data from all selected studies. We resolved disagreements by discussion or, when necessary, through involvement of a third review author, whose judgement was then decisive. Review authors collected the following data from each study for each test: general information (title, study authors, year of publication, status of publication, period of recruitment, study design); descriptives of the relevant patient population (age, sex, ethnicity, country of recruitment, inpatients or outpatients, healthcare setting, PE symptoms); information on the test under study; reference test used (clinical follow‐up or pulmonary angiography, or both); items for study quality assessment (see below); data on intraobserver and interobserver variability; numbers of inconclusive test results in diseased and non‐diseased groups; and numbers of true and false positives and true and false negatives for relevant women. We contacted the authors of the original studies in cases of missing data.
Assessment of methodological quality
Two review authors independently assessed the methodological quality of selected studies using the QUADAS‐2 instrument (Table 1) ‐ an evidence‐based tool for assessment of the methodological quality of diagnostic studies included in systematic reviews (Whiting 2011). Review authors scored each item 'yes', 'no' or 'unclear' according to the criteria outlined in Table 1 and provided additional judgements when quality issues fell outside the scope of the criteria. We resolved disagreements by discussion or, when necessary, with involvement of a third review author, whose judgement was then decisive.
Statistical analysis and data synthesis
From each study, we extracted available data on true and false negatives and positives for relevant participants to calculate specificity, sensitivity and post‐test probabilities. We planned to pool the data in a meta‐analysis for each diagnostic test if a sufficient number of studies of sufficient quality and methodological uniformity were available. For PE prevalence, we divided true positives plus false negatives by total sample size.
We intended to use a bivariate random‐effects model in calculating summary estimates of sensitivity and specificity (Reitsma 2005) and to translate summary estimates into likelihood ratios. To illustrate the findings, we intended to present post‐test probabilities for a range of plausible values of pretest probabilities of PE.
Besides the possibility of being positive or negative, a test might be inconclusive. In practice, patients with an inconclusive test result often undergo further testing. The conventional approach to calculating sensitivity and specificity does not provide a way of incorporating this third outcome category. Ignoring the inconclusive category would lead to overestimations of sensitivity and specificity. Calculating the sensitivity by dividing true positives by the total number of participants with the disease suggests that 100% minus that percentage is the proportion of diseased participants missed by the test. However, this is not the case, because this proportion also comprises participants who did not have a conclusive test result. Even though they did not have a conclusive test result, these participants are not necessarily falsely classified. Calculating specificity for a test with an inconclusive result category is similarly thwarted. For index tests with inconclusive results, we explicitly stated the proportions of participants with an inconclusive test result in the diseased group and in the healthy group, respectively. In our primary analyses, we calculated test accuracy measures while considering intermediate results as 'negatives', with the aim of providing conservative estimates rather than overestimations. In sensitivity analyses, we re‐analysed the data while treating inconclusive results as 'positives'.
Clinicians may be especially interested in the proportion of patients with PE that will be missed after a negative index test, or after an inconclusive index test. Therefore, we also estimated summary post‐test probabilities of PE for the three index test result categories if meta‐analysis provided summary estimates for sensitivity and specificity. This could be done by extending the bivariate model for multiple index test categories, provided sufficient data were available, and provided the models converged (Bipat 2007; Leeflang 2012).
Investigations of heterogeneity
To explore anticipated sources of heterogeneity, we intended to add predefined covariates to the meta‐regression. We would undertake this only for index tests or strategies that were investigated in more than three studies reporting on the particular covariate (Higgins 2009; Lambert 2002; Morton 2004; Thompson 2002).
Clinical factors included the following.
-
Prior testing and clinical decision rule results. Patients with suspected PE are often stratified into high‐ and low‐risk groups on the basis of clinical decision rules or empirical clinical likelihood. The prevalence of PE differs between these groups. Results of prior diagnostic tests also influence the likelihood that the target condition is present. Therefore, we would record and introduce into the analysis of heterogeneity any clinical decision rule or other prior diagnostic test that was not the index test of the particular study.
-
Stage of pregnancy. For various reasons, pregnant women should be considered a distinct subgroup with regard to the diagnosis of PE. The importance of these reasons can vary during the course of pregnancy, for example, the progressive rise in D‐dimer levels or signs of pregnancy that mimic symptoms of PE.
-
Reference standard. It is desirable to allow only one reference standard for all studies. Pulmonary angiography used to be the gold standard, but in more recent literature, it has been replaced by clinical follow‐up. Therefore, we decided to include both as acceptable reference standards. This might lead to differential verification and may cause heterogeneity.
-
Technological advances of index tests: multi‐slice versus single‐slice CTPA.
We intended to also explore heterogeneity more informally by visually inspecting forest plots stratified by potential factors of heterogeneity.
Sensitivity analyses
We planned to perform sensitivity analysis to explore effects on overall results of historical versus current inclusion of participants. Diagnostic accuracy studies are by definition cross‐sectional because two tests are compared on the basis of their ability to provide a diagnosis at the same moment in the course of a disease. However, inclusion of patients can be historical, that is, retrospective. This design is more prone to selection bias and is therefore an anticipated source of heterogeneity.
We also investigated the effect of considering inconclusive index test results as 'positive' and compared these results with when they were considered to be 'negative'.
Assessment of reporting bias
We did not explore publication bias because adequate methods of detecting publication bias have not yet been developed for use in diagnostic accuracy studies (Deeks 2005).
Results
Results of the search
See Figure 1.

Study flow diagram.
Our search yielded 5841 hits in Embase and 2303 hits in MEDLINE. We identified another 17 articles through handsearching of references. Other elements of the search strategy revealed no additional potentially eligible studies. After duplicates were removed, 6772 publications remained for title and abstract screening. We deemed a total of 195 as potentially eligible and marked them for full‐text review. Of these, we excluded 183 for reasons listed in Figure 1. We could not obtain the full text of one study (Vin 2009). We included the remaining 11 studies in the present review (Bajc 2015; Bourjeily 2012; Browne 2014; Chan 2002; Cutts 2014; Hullah 2011; Litmanovich 2009; Nijkeuter 2013; Ridge 2009; Scarsbrook 2007; Shahir 2010). The original authors of eight studies provided additional data or clarification on their study methods, as did study authors for three studies that we eventually excluded. We identified 11 studies that presented data on a study population that was also reported in another publication.
Characteristics of studies
We provided characteristics and quality assessments of individual included studies in the Characteristics of included studies table. We listed selected excluded studies that readers might consider in light of the research question, or that appeared on first inspection to be eligible, in the Characteristics of excluded studies table, along with reasons for exclusion. We listed in Figure 1 reasons for exclusion of all 183 studies that we excluded after full‐text review. We identified only one potentially eligible study on MRA (Herédia 2012) but excluded this study because of its small sample size (n < 25).
Of the included studies, five reported on lung scintigraphy (Bajc 2015; Chan 2002; Cutts 2014; Hullah 2011; Scarsbrook 2007), four on CTPA (Bourjeily 2012; Browne 2014; Litmanovich 2009; Nijkeuter 2013) and two on both lung scintigraphy and CTPA (Ridge 2009; Shahir 2010). CTPA was also reported in Hullah 2011 and Scarsbrook 2007 but their sample size was too small. The total numbers of index test results that we could include in the current review were 695 for CTPA and 665 for lung scintigraphy.
All studies were retrospective consecutive patient series that used clinical follow‐up as a reference standard. Not all were designed with the aim of determining the diagnostic accuracy of index tests. Other aims included evaluating image quality (Browne 2014), testing diagnostic validity of clinical prediction models (Cutts 2014) and assessing radiation dose (Litmanovich 2009).
Of the CTPA studies, one used single‐detector or multi‐detector scanners (Nijkeuter 2013), and the others used only multi‐detector scanners (Bourjeily 2012; Browne 2014; Litmanovich 2009; Ridge 2009; Shahir 2010). Of the lung scintigraphy studies, one used SPECT and performed ventilation scanning in a proportion of patients (Bajc 2015). The other studies used planar scintigraphy. Two studies performed V/Q scanning for all patients (Chan 2002; Hullah 2011), and two performed only perfusion scanning (Scarsbrook 2007; Shahir 2010). All patients in these studies had undergone a prior chest X‐ray. One study did not specifically state whether ventilation scanning was performed (Cutts 2014). One lung scintigraphy study performed ventilation scanning in a minority of patients (Ridge 2009).
For studies reporting the duration of follow‐up, median duration was six months (Browne 2014; Chan 2002; Litmanovich 2009; Nijkeuter 2013; Ridge 2009; Scarsbrook 2007; Shahir 2010). Two studies provided follow‐up throughout pregnancy and the postpartum period (Bajc 2015; Cutts 2014); one over the postpartum period or three months, whichever was longer (Bourjeily 2012) and one until delivery (Hullah 2011).
Recruitment starting year ranged from 1990 to 2009. The recruitment end year ranged from 2000 to 2013. Countries of inclusion were USA (n = 3), Australia (n = 2), Ireland (n = 2), United Kingdom (n = 2), Canada (n = 1), the Netherlands (n = 1) and Sweden (n = 1). Four studies recruited only at tertiary care centres (Bajc 2015; Bourjeily 2012; Cutts 2014; Hullah 2011), one at a secondary care centre (Ridge 2009) and three at both types of centres (Chan 2002; Nijkeuter 2013; Shahir 2010). For the remaining three studies, this information was unclear. Two studies recruited patients from the emergency department (Litmanovich 2009; Ridge 2009), and three recruited both inpatients and outpatients (Bajc 2015; Chan 2002; Nijkeuter 2013). For the remaining six studies, this information was unclear.
The median of reported mean or median age was 31 years (range 29 to 32.6 years). Two studies did not report gestational age (Bourjeily 2012; Ridge 2009). In one study, mean gestational age was 19 weeks (Litmanovich 2009). In the other studies, a median of 9% of patients presented in the first trimester, a median of 41% in the second trimester and a median of 49% in the third trimester.
Four studies described presenting symptoms (Bajc 2015; Bourjeily 2012; Chan 2002; Shahir 2010). In all four studies, the most common symptom was dyspnoea, and the second most common was chest pain.
Nine studies described some extent of prior or additional testing (Bajc 2015; Browne 2014; Chan 2002; Cutts 2014; Hullah 2011; Litmanovich 2009; Ridge 2009; Scarsbrook 2007; Shahir 2010). A total of 230 patients underwent imaging for DVT. One case of DVT was found. Other often performed tests included D‐dimer and chest X‐ray. Four studies reported that a small proportion of up to six patients underwent the index test multiple times or received a combination of lung scintigraphy and CTPA (Browne 2014; Hullah 2011; Ridge 2009; Shahir 2010).
Methodological quality of included studies
We summarised risk of bias and applicability concerns in Figure 2 and Figure 3. None of the studies had the main aim of estimating specificity of the index test, and only some were designed to estimate the sensitivity or negative predictive value (Bajc 2015; Bourjeily 2012; Nijkeuter 2013; Ridge 2009; Scarsbrook 2007). We assessed quality in terms of risk of bias and concerns regarding applicability in light of the research question of the current review, not the research questions of individual studies. From this perspective, the overall quality of the included studies was low. We deemed that no studies were low risk, and that none produced a low level of concern on more than three items of the QUADAS‐2 tool. The item that had lowest risk of bias overall was the index test. We scored a relatively high proportion of items as unclear, in part because of the divergence between the research question of the current review and the research questions of individual studies.
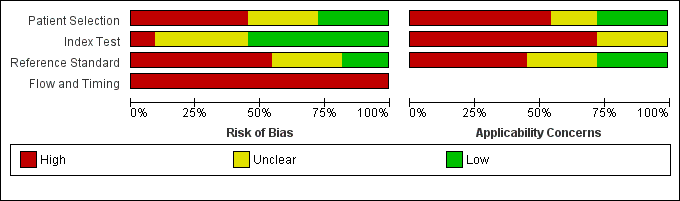
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
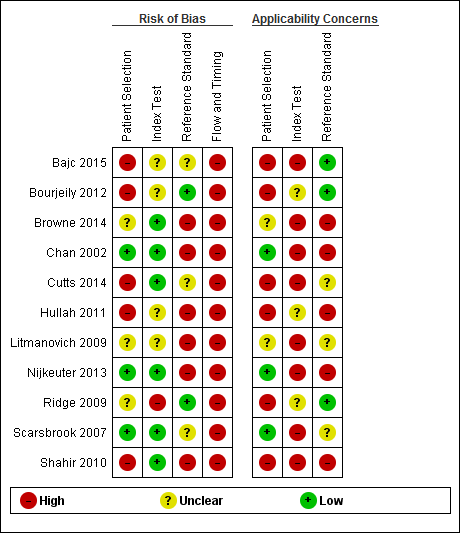
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Patient selection
We judged patient selection to carry low risk of bias in three studies (Chan 2002; Nijkeuter 2013; Scarsbrook 2007), unclear risk in three studies (Browne 2014; Litmanovich 2009; Ridge 2009) and high risk in five studies (Bajc 2015; Bourjeily 2012; Cutts 2014; Hullah 2011; Shahir 2010). Concern about applicability was low in three studies (Chan 2002; Nijkeuter 2013; Scarsbrook 2007), unclear in two studies (Browne 2014; Litmanovich 2009) and high in six studies (Bajc 2015; Bourjeily 2012; Cutts 2014; Hullah 2011; Ridge 2009; Shahir 2010). Causes for high concern and high risk of bias included non‐systematic selection between index tests (Bajc 2015; Hullah 2011; Ridge 2009; Shahir 2010), anticoagulant treatment that was started before testing was begun in over 5% of patients (Hullah 2011; Shahir 2010), a suboptimal patient selection procedure for a consecutive series of pregnant women (Cutts 2014; Hullah 2011) and high prevalence of comorbidity (Bourjeily 2012).
Index test
We judged the risk that conduct or interpretation of the index test had introduced bias as low in six studies (Browne 2014; Chan 2002; Cutts 2014; Nijkeuter 2013; Scarsbrook 2007; Shahir 2010), high in one (Ridge 2009) and unclear in the remaining four (Bajc 2015; Bourjeily 2012; Hullah 2011; Litmanovich 2009). Among low‐risk studies, interpretation of the index test was blinded, but in the other studies this was unclear. In none of the studies was concern regarding applicability low. Concern was high in eight studies (Bajc 2015; Browne 2014; Chan 2002; Cutts 2014; Litmanovich 2009; Nijkeuter 2013; Scarsbrook 2007; Shahir 2010)and unclear in three (Bourjeily 2012; Hullah 2011; Ridge 2009). Reasons for high concern included use of multiple index test procedures (Nijkeuter 2013; Ridge 2009; Scarsbrook 2007; Shahir 2010), re‐assessment of index test results by researchers for study purposes (Browne 2014; Chan 2002; Cutts 2014; Litmanovich 2009) and use of SPECT scintigraphy (Bajc 2015).
Reference standard
We judged the risk that conduct or interpretation of the reference test had introduced bias as low in two studies (Bourjeily 2012; Ridge 2009), high in six studies (Browne 2014; Chan 2002; Hullah 2011; Litmanovich 2009; Nijkeuter 2013; Shahir 2010) and unclear in the remaining three studies (Bajc 2015; Cutts 2014; Scarsbrook 2007). Concern regarding applicability was high in five studies (Browne 2014; Chan 2002; Hullah 2011; Nijkeuter 2013; Shahir 2010), low in three studies (Bajc 2015; Bourjeily 2012; Ridge 2009) and unclear in three studies (Cutts 2014; Litmanovich 2009; Scarsbrook 2007). Methods of clinical follow‐up varied from a query regarding the hospital radiology system used for any imaging for PE after the index test (Browne 2014) to extensive medical record and radiology system review in all hospitals in the region plus telephone interviews and contact with the general practitioner (Chan 2002). Seven studies used a reference standard that could not be used to identify false positives (Bajc 2015; Bourjeily 2012; Browne 2014; Chan 2002; Litmanovich 2009; Nijkeuter 2013; Shahir 2010), mainly because follow‐up was specifically targeted to find evidence of VTE. None of these studies aimed to investigate false positives. Three studies used a reference standard that had the potential to identify false positives (Cutts 2014; Hullah 2011; Ridge 2009), and in one study, this was unclear (Scarsbrook 2007). No studies stated that interpretation of the reference test was blinded.
Flow and timing
We deemed all studies to have high risk of bias in the domain of flow and timing. All studies used clinical follow‐up as the reference standard. During the follow‐up period there is the possibility that the PE diminishes through the natural course of the disease in case of a PE that was not identified by the index test, or that it occurs in a patient that did not have a PE at the time of the index test. Therefore, clinical follow‐up can introduce bias in the domain of flow and timing. In two studies, investigators stated that only cases with a negative or inconclusive index test underwent the reference standard (Chan 2002; Nijkeuter 2013), whereas others did not clearly describe this. Among studies in which patients could undergo different imaging tests for PE, the criteria used to choose between tests were unclear or non‐standardised in four (Bajc 2015; Hullah 2011; Ridge 2009; Shahir 2010) and were standardised in one (Scarsbrook 2007). In total, 20 patients were described as lost to follow‐up (Bourjeily 2012; Shahir 2010).
Findings
We deemed meta‐analysis of results of individual studies inappropriate because of heterogeneity and overall low methodological quality. We identified numerous sources of heterogeneity. Methods used for patient selection and clinical follow‐up varied considerably in thoroughness. Where two index tests were available, determining which one the patient should receive was not performed systematically, (i.e. randomly or on the basis of a clear protocol), except in one study (Scarsbrook 2007). Lung scintigraphy studies used different imaging modalities (perfusion scintigraphy, ventilation/perfusion scintigraphy or V/Q SPECT). If a proportion of patients underwent multiple scans, some studies reported on scans as the unit of analysis, whereas others reported on patients, (i.e. a patient was considered false negative, or a single scan was considered false negative). Studies used either the original clinical assessment of the index test or a re‐assessment for study purposes. Prior and additional testing performed was unclear in almost all studies. Comorbidity varied between studies, as did blinding to index test results. Almost no study reported cut‐offs or definitions for index test results. Follow‐up often was not designed to identify false positives and was not provided for all patients in some studies. We could not perform a sensitivity analysis of historical versus current inclusion because all studies were retrospective in design.
None of the studies was designed with the aim of establishing the accuracy of the index test, in terms of sensitivity and specificity, which was the aim of the current review. Clinical follow‐up is inherently poor in detecting false positives. If the index test indicates PE, anticoagulant treatment is started and symptoms decline. In the case of a false positive, with no or self‐limiting pathology instead of a PE, symptoms also subside. Furthermore, some studies did not follow patients with a positive index test. Most aimed only to identify the occurrence of VTE during follow‐up, usually by searching for medical notes or imaging data from re‐admissions after the index test. Clearly, false positives are not detected in this way. Therefore, the only reliable measures of test accuracy that can be established with the available data are sensitivity and negative predictive value (NPV). In the primary analysis, inconclusive index test results were regarded as negative.
CTPA
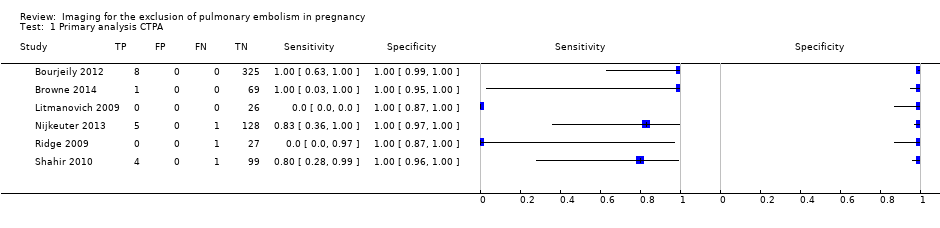
The median NPV of CTPA was 100%. The lowest reported NPV was 96%, and the highest was 100% (Figure 4). Median sensitivity in the CTPA studies was 83%. For one study, we could not estimate sensitivity because there were no cases of PE (Litmanovich 2009). Therefore the median sensitivity does not derive from exactly the same set of studies as the median NPV. Sensitivity ranged from 0% to 100%, as displayed in Figure 5. The study with a sensitivity of 0% had no positive results on 28 CTPA scans, but one of the patients with an inconclusive scan was treated for PE on clinical grounds, and thus was regarded as false negative (Ridge 2009). No studies identified any false‐positive cases.
Primary analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as negative.

Primary analysis. Forest plot of CTPA with inconclusives regarded as negative.
The median percentage of inconclusive results was 5.9%, and the percentage reported ranged from 0.9% to 36%. The absolute numbers are displayed in Figure 5. The total number of inconclusive scans was 32. Two women in total had an inconclusive CTPA and were considered to have a positive reference standard. For one woman, lung scintigraphy showed PE (Ridge 2009), whereas the other was treated on clinical grounds (Nijkeuter 2013).
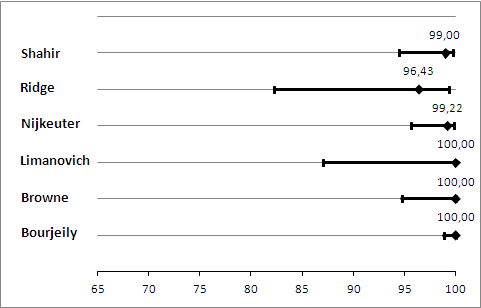
We performed a sensitivity analysis in which we regarded inconclusive index test results as positive. In this analysis, the median NPV was 100%, and NPV ranged from 99% to 100% (Figure 6). Median sensitivity in this analysis was 100%, and sensitivity ranged from 80% to 100% (Figure 7).

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as positive.

Sensitivity analysis. Forest plot of CTPA with inconclusives regarded as positive.
Lung scintigraphy
For lung scintigraphy, the median NPV was 100%. The lowest reported NPV was 99%, and the highest was 100% (Figure 8). Median sensitivity was 100%, and sensitivity ranged from 0% to 100%, as displayed in Figure 9. The study with a sensitivity of 0% had no positive results on 97 lung scintigraphy scans, but one of the three patients with an inconclusive scan was found to have PE on a subsequent CTPA, and thus was regarded as false negative (Shahir 2010). No studies identified any false‐positive cases.
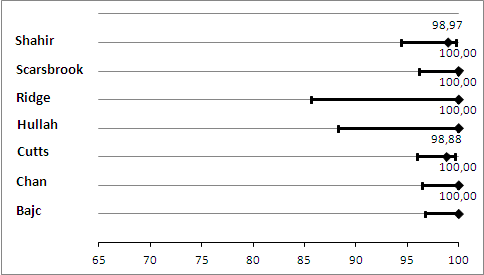
Primary analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as negative.
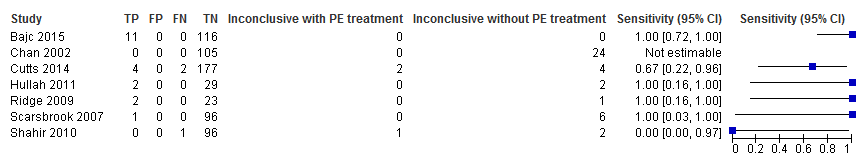
Primary analysis. Forest plot of lung scintigraphy with inconclusives regarded as negative.
The median percentage of inconclusive results was 4.0%, and the percentage reported by study authors ranged from 0% to 23%. The absolute numbers are displayed in Figure 9. The total number of inconclusive scans was 42. Three women in total had an inconclusive lung scintigraphy and were considered to have a positive reference standard. One was an incomplete study because the patient was unable to lie on the scanning table (Shahir 2010). Subsequent CTPA showed PE. The other two patients were treated for PE on clinical grounds (Cutts 2014).
We performed a sensitivity analysis in which we regarded inconclusive index test results as positive. In this analysis, the NPV was 100% in all studies, as shown in Figure 10, reflecting the absence of PE revealed by the reference test after a negative lung scintigraphy. The sensitivity was not estimable in one study because patients receiving PE treatment were excluded and no other PEs were identified by the reference test (Chan 2002). In all other studies, the sensitivity was 100% in this analysis (Figure 11).

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as positive.
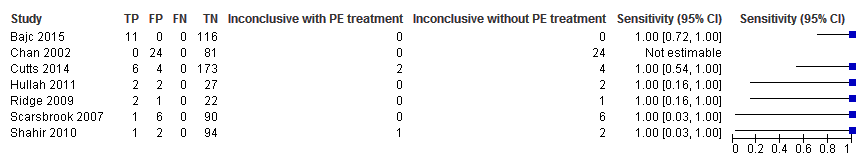
Sensitivity analysis. Forest plot of lung scintigraphy with inconclusives regarded as positive.
PE prevalence
When we considered all cases with a positive index or reference test as having PE, including treatment after an inconclusive test, we found that the median prevalence in all studies combined was 3.3%, and prevalence ranged from 0.0% to 8.7%, as displayed in Figure 12. We excluded two studies from this analysis because they excluded women receiving therapeutic doses of LMWH (Chan 2002; Nijkeuter 2013).

Prevelance of pulmonary embolism (%) with 95% confidence interval.
Discussion
Summary of main results
The aim of this review was to determine the diagnostic accuracy of imaging tests for the diagnosis of pulmonary embolism (PE) in pregnancy. We included a total of 11 studies reporting on computed tomography pulmonary angiography (CTPA), lung scintigraphy or both. Six studies investigated CTPA (Bourjeily 2012; Browne 2014; Litmanovich 2009; Nijkeuter 2013; Ridge 2009; Shahir 2010), one single‐photon emission computed tomography (SPECT) with scintigraphy (Bajc 2015), two planar ventilation/perfusion scintigraphy (Chan 2002; Hullah 2011), two perfusion scintigraphy (Scarsbrook 2007; Shahir 2010) and one perfusion with ventilation scintigraphy in a small proportion of patients (Ridge 2009); another study was unclear on the lung scintigraphy technique used (Cutts 2014). We identified no studies on magnetic resonance angiography (MRA) that met the inclusion criteria of this review. summary of findings Table displays a summary of our findings.
All studies were retrospective and used clinical follow‐up as the reference standard. Almost all applied clinical follow‐up to identify PE, not to exclude it, thus precluding conclusions on test specificity and positive predictive value. No studies identified any false‐positive cases. No studies had the aim of determining test specificity, and only some studies were designed to determine sensitivity. We assessed study quality in light of the research question presented in the current review, not in light of research questions posed by the individual studies. From this perspective overall study quality was low and concern regarding applicability was high. We considered meta‐analysis inappropriate for both CTPA and lung scintigraphy studies because of heterogeneity and overall low study quality. Sources of heterogeneity included scintigraphy techniques, thoroughness of clinical follow‐up, handling of multiple scans for one patient, use of original assessments or re‐assessments for research into index test results, robustness of patient selection, prior testing and blinding.
In six studies reporting a total of 695 CTPA index test results, the median negative predictive value (NPV) was 100%, and NPV ranged from 96% to 100%. CTPA had a median sensitivity of 83%, ranging from 0% to 100%. We found a median of 5.9% inconclusive CTPA test results, ranging from 0.9% to 36%.
In seven lung scintigraphy studies reporting a total of 665 index test results, the median NPV was also 100%, and NPV ranged from 99% to 100%. Median sensitivity was 100%, and sensitivity ranged from 0% to 100%. The median percentage of inconclusive results was 4.0%, with a range of 0% to 23%.
Overall, median PE prevalence was 3.3%, and PE prevalence ranged from 0.0% to 8.7%.
Strengths and weaknesses of the review
Given that the search used broad inclusion criteria without language restrictions and search filters and yielded many screened publications, the chance of missed relevant publications up to the date of the search is small. We attempted to contact all authors of included studies to ensure maximum completeness of study data. By excluding primary care patients and using original assessments of index test results, when available, we optimised the applicability of review findings.
We made methodological choices with the aim of providing conservative estimates rather than overestimations of test accuracy. There is no proper way to deal with inconclusive test results when determining diagnostic accuracy measures. Excluding them from the analysis leads to overestimation. Artificially regarding them as negative or positive increases specificity or sensitivity, respectively. We regarded inconclusive scans as negative index test results in the primary analysis. Large differences between forest plots in the primary analysis and the sensitivity analysis underline the inherent problem of conventional test accuracy measures when an inconclusive category is involved. Another dilemma is that patients with an inconclusive scan who are treated with anticoagulants on clinical grounds will likely not have a PE identified or excluded during follow‐up. This precludes false positives and false negatives in these cases. We regarded treatment after an inconclusive scan as a positive reference standard that provides conservative estimates of NPV and sensitivity.
We judged the overall risk of bias in both CTPA and lung scintigraphy studies as high for the research question of this review. This reflected in part divergence from research questions posed by individual studies, which were not all specifically designed to establish test accuracy. For the clinical research question at hand, one should consider the quality of evidence low and should interpret results cautiously. Major potential causes of bias included non‐systematic selection between index tests in four studies (Bajc 2015; Hullah 2011; Ridge 2009; Shahir 2010), unclear blinding to index test results in all studies and use of clinical follow‐up.
Clinical follow‐up has inherent limitations as a reference standard. First, follow‐up is not strictly cross‐sectional, so PE may occur or resolve between the time of the index test and the time of the reference test. We chose an upper cut‐off of three months between the index test and occurrence of a PE during follow‐up. Second, in studies with unscrupulous follow‐up, there is a risk of overestimating sensitivity and NPV because of missed PE cases. The meticulousness of follow‐up varied between studies. Last, it was not clear in all studies whether cases with an index test positive for PE were followed up, and two studies explicitly did not follow up these cases. In studies in which all cases did undergo the reference test, the aim most often was to identify PE during follow‐up, not to exclude it. We dealt with this partial verification by reporting only on sensitivity and NPV. These measures are useful in providing answers to the clinically most relevant question of whether it is safe to withhold treatment after a negative and inconclusive scan. However there is always a trade‐off between the sensitivity and specificity; therefore, important information is missing from the included studies.
Although a retrospective design is not considered to carry high risk of bias in the QUADAS‐2 assessment, retrospective studies are potentially at risk for selection bias and for incomplete or inaccurate data collection. For instance, if inconclusive scans are at risk of not being registered in the radiology information system, which serves as the reference standard, the percentage of inconclusive scans might be underestimated.
Applicability of findings to the review question
In general, one should apply the results of this review with caution because of the low quality and high degree of heterogeneity of the included studies. Owing to above described limitations of conventional test accuracy measures when a third diagnostic category is included, we regard inconclusives as negative in both NPV and sensitivity. This means that reported accuracy measures apply to negative and inconclusive results as a group, whereas clinicians might be particularly interested in the risk of PE after a negative index test. NPV and sensitivity should be regarded as a conservative best guess.
The above described limitations also translate into uncertainty in the estimate of PE prevalence. For this estimation, we regarded all patients with a positive index test as having had PE, which assumes a 100% positive predictive value. We regarded treatment for PE after an inconclusive test as a positive index test, which also might lead to an overestimation of prevalence.
Overall, study samples appeared representative of the target population. We included patients with pregnancies in all trimesters. When described, the presenting symptoms appeared representative of the population of pregnant patients with suspicion of PE. We included both secondary and tertiary care inpatients and outpatients.
Non‐systematic selection between available index tests might hamper the generalisability of results. This occurred in four studies (Bajc 2015; Hullah 2011; Ridge 2009; Shahir 2010). This study design confers the risk that a certain patient group might be selected for one index test, and another for the second index test.
In one of the CTPA studies, patients underwent single‐detector or multi‐detector computed tomography (CT) (Nijkeuter 2013). The other studies used multi‐detector CT scanners for all patients. Two of the scintigraphy studies used only perfusion scintigraphy after chest X‐ray (Scarsbrook 2007; Shahir 2010). This is a common diagnostic strategy in clinical practice. Investigators in the other scintigraphy studies performed ventilation scanning in all or a proportion of the study sample, although this was unclear in one study (Cutts 2014). One study used SPECT lung scintigraphy (Bajc 2015), which might be viewed as a separate technique. Four studies employed multiple scanning protocols (Nijkeuter 2013; Ridge 2009; Scarsbrook 2007; Shahir 2010). Results should be taken to as applying to lung scintigraphy or CTPA when the same technique is used.
When possible, we excluded from analysis any second imaging with the same index test. Review results thus apply to first‐time scanning. If a different index test was performed before, then the result could still be included (e.g. lung scintigraphy after an inconclusive CTPA). Review results therefore apply to the index test, regardless of prior testing, except when the same index test was used.When reported, we included the original assessment of the index test. Four studies reported only re‐assessments for study purposes (Browne 2014; Chan 2002; Cutts 2014; Litmanovich 2009). Results of these studies are less applicable to the clinical situation.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Primary analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as negative.

Primary analysis. Forest plot of CTPA with inconclusives regarded as negative.

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as positive.

Sensitivity analysis. Forest plot of CTPA with inconclusives regarded as positive.

Primary analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as negative.
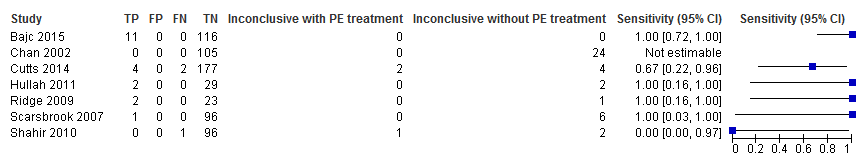
Primary analysis. Forest plot of lung scintigraphy with inconclusives regarded as negative.

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as positive.
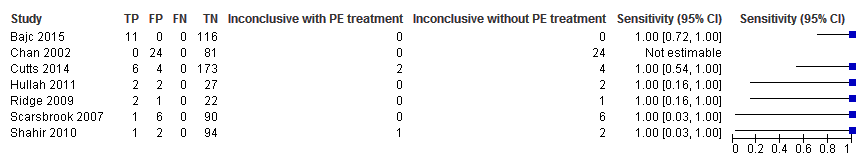
Sensitivity analysis. Forest plot of lung scintigraphy with inconclusives regarded as positive.

Prevelance of pulmonary embolism (%) with 95% confidence interval.
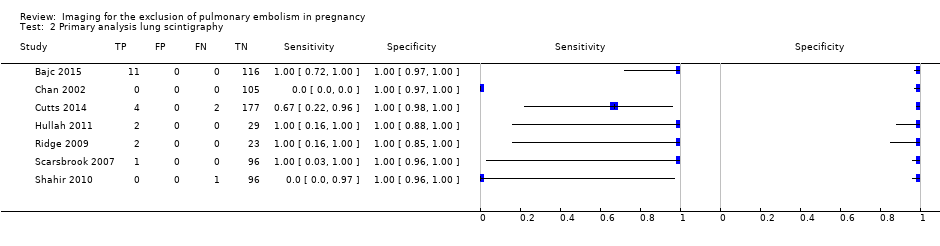
Primary analysis lung scintigraphy.
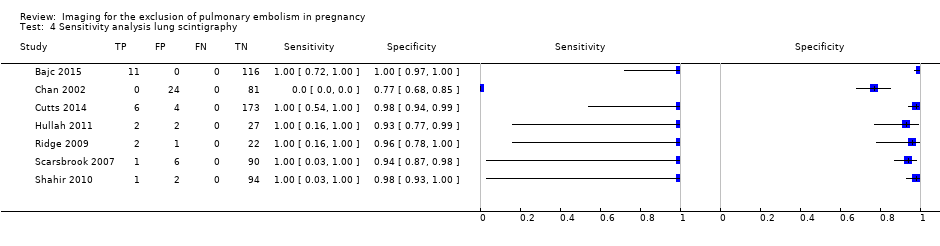
Sensitivity analysis lung scintigraphy.
| What is the diagnostic accuracy of imaging tests for the diagnosis of pulmonary embolism (PE) in pregnancy? | ||||||
| Patients | Pregnant women with clinical suspicion of PE. | |||||
| Prior testing and prevalence | Varied. Most often performed were chest X‐ray and imaging for deep venous thrombosis. The median prevalence of PE was 3.3% (range 0.0% to 8.7%), as assessed by the applied reference standard, which has limitations. | |||||
| Settings | Secondary and tertiary care, both inpatients and outpatients. | |||||
| Index test | Computed tomography pulmonary angiography (CTPA), lung scintigraphy and magnetic resonance angiography (MRA). No studies on MRA were included. Inconclusive test results were regarded as negative in the primary analysis. | |||||
| Importance | Pregnant women are often suspected of PE because of increased risk and physiological signs that mimic symptoms of PE. Pregnant women are often excluded from diagnostic imaging studies. These imaging tests might perform differently during pregnancy, and radiation and other risks are weighed differently. | |||||
| Reference standard | Clinical follow‐up of at least 6 weeks. In almost all studies, follow‐up was performed to identify PE, not to exclude it. Pulmonary angiography was preferred but was applied by none of the studies. | |||||
| Studies | Cross‐sectional cohort studies were included. Case‐control studies were excluded. All studies were retrospective. | |||||
| Test | Number of studies (number of index test results) | Median negative predictive value (range) | Median sensitivity (range) | Median inconclusive test results (range) | Overall risk of bias (QUADAS‐2) | Overall applicability (QUADAS‐2) |
| CTPA | 6 (695) | 100% (96%‐100%) | 83% (0%‐100%) | 5.9% (0.9%‐36%) | High risk | High concern |
| Lung scintigraphy | 7 (665) | 100% (99%‐100%) | 100% (0%‐100%) | 4% (0%‐23%) | High risk | High concern |
| CAUTION: The results in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | ||||||
| CTPA: computed tomography pulmonary angiography. | ||||||
|
| Item plus signalling questions | Criteria for scoring 'yes', 'no' and 'unclear' |
| 1 | PATIENT SELECTION Was a consecutive or random sample of patients enrolled? Was a case‐control design avoided? Did the study avoid inappropriate exclusions? | We will score this item 'yes' when patients were consecutively or randomly selected; 90% or more were evaluated at the hospital; 5% or less of had received anticoagulant therapy within 24 hours before testing; 30% or less were given a diagnosis of comorbidity such as chronic obstructive pulmonary disease or other pulmonary disease, malignancy or pregnancy complications (preeclampsia, syndrome of haemolysis, elevated liver enzymes and low platelets or eclampsia); and 10% or less had undergone prior testing for this episode of suspected PE. We will score 'no' if one of these criteria was not met. |
| 2 | INDEX TEST Were index test results interpreted without knowledge of results of the reference standard? | We will score this item 'yes' in the following cases: if study authors state that the index test interpreter was unaware of the result of the reference test; or if the order of testing was index test before reference test for every patient. Even if clinical follow‐up was the reference test, the order of testing has to be stated for the item to be scored 'yes'. We will score the item ‘no’ for studies in which it is stated that the interpreter of the index test was aware of the result of the reference test. In other cases, we will score this as 'unclear'. In cases of studies directly comparing the diagnostic accuracy of 2 index tests against the reference standard, these test results had to be interpreted without knowledge of the results of the comparator index test, and we will score this item similarly to the approach described above. |
| 3 | REFERENCE STANDARD Is the reference standard likely to correctly classify the target condition? Were reference standard results interpreted without knowledge of results of the index test? | We considered both PA and clinical follow‐up of at least 6 weeks as useful for correct classification of the target condition, the latter only if objective diagnostic tests are used in cases of suspected venous thromboembolism. We will score this item 'yes' if study authors state that reference tests were interpreted without knowledge of results of the index test. Furthermore, in cases of clinical follow‐up as a reference standard, any clinical suspicion of venous thrombosis during follow‐up needs to be followed by objective diagnostic testing (i.e. CUS or venography for suspicion of DVT, and scintigraphy, CTPA or pulmonary angiography for clinical suspicion of PE). If a patient died during follow‐up, we classified death as caused by PE in cases of confirmation by autopsy, in cases of an objective test positive for PE before death or if PE could not be confidently excluded as the cause of death. |
| 4 | FLOW AND TIMING Was an appropriate interval between index test(s) and reference standard provided? Did all patients receive a reference standard? Did all patients receive the same reference standard? Were all patients included in the analysis? | With PA, we will consider a time period of less than 24 hours between index and reference tests as short enough to ensure that the target condition did not change between tests, either because of natural progression of the disease or because of therapeutic intervention. For studies using pulmonary angiography as the reference test, we will score this item 'yes' if the time between index and reference tests was less than 24 hours. Similarly, for studies directly comparing diagnostic accuracy of index tests, we will consider a time period of less than 24 hours between index tests and the reference test as short enough. During clinical follow‐up, the disease may diminish through natural progression or through intervention. Or the condition may arise during follow‐up if it was not present at the time of the index test. Therefore, we will score studies using clinical follow‐up 'no' for this item. We will score this item 'no' if less than 90% or a non‐random selection of patients underwent the reference test. We will score this item 'no' if less than 90% of patients who had an index test result underwent pulmonary angiography or had clinical follow‐up as the reference test. |
| CTPA: computed tomography pulmonary angiography. | ||
| Test | No. of studies | No. of participants |
| 1 Primary analysis CTPA Show forest plot | 6 | 695 |
| 2 Primary analysis lung scintigraphy Show forest plot | 7 | 665 |
| 3 Sensitivity analysis CTPA Show forest plot | 6 | 695 |
| 4 Sensitivity analysis lung scintigraphy Show forest plot | 7 | 665 |