نقش عوامل ضد‐میکروبی موضعی در درمان زخمهای پا در افراد مبتلا به دیابت
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT Setting: Hospital, 1 centre Country: Pakistan Duration of follow‐up: 8 weeks Duration of treatment: Not noted Funding source: Not reported Unit of analysis: Participant | |
| Participants | 60 participants Inclusion criteria: Grade I or II foot ulcer in person with diabetes (grading assessed using Meggitt‐Wagner scale and corresponds to absence of necrosis and osteomyelitis) of more than 4 weeks' duration. Adequate controlled diabetes with fasting blood sugar of 110 to 130 mg/dL on 2 consecutive days prior to recruitment in the study. Exclusion criteria: Patients with a history of hepatic or renal disease, those on corticosteroid therapy, and those with impalpable dorsalis pedis or posterior tibial arteries. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported) Group 1: All Wagner grade II ulcers; ulcer area 1107.53 SD: 486.5 Group 2: All Wagner grade II ulcers: ulcer area 1310.10 SD: 489.2 Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = 30) Pyodine bath and saline and Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder (from capsules, no information on concentration) applied in a thin, uniform layer plus pyodine bath and saline/Vaseline gauze dressing as for Group 1. The amount of powder depended on ulcer area. Additional treatment information: Dressings were changed daily or on alternate days depending on need. | |
| Outcomes | Primary review outcomes: None reported Secondary review outcomes: None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study patients were divided in two equal groups randomly by lottery method" Comment: Whilst limited information is presented, we assumed that the a lottery approach refers to a random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "No information was provided on who conducted randomisation and if or how allocation was concealed" Comment: No information provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: Flow chart reports 0 lost to follow‐up in either group. Comment: Assumed all participants followed up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained, all outcomes stated in methods reported. However, key outcomes not presented; unclear if these were measured. |
| Other bias | Low risk | None noted. |
| Methods | RCT Setting: Hospital, 1 centre Country: Sweden Duration of follow‐up: 12 weeks Duration of treatment: Not reported Funding source: For‐profit organisation Unit of analysis: Participant | |
| Participants | 41 participants Inclusion criteria: Caucasian > 40 years of age with previously known diabetes, an exudating cavity ulcer below the ankle (Wagner grade I or II) with an ulcer area > 1 cm² and systolic toe pressure > 30 mmHg or a systolic ankle pressure > 80 mmHg. Exclusion criteria: Patients with ulcers > 25 cm², deep abscess, osteomyelitis, or gangrene (Wagner grade III to IV). Patients undergoing investigation of the thyroid gland or unlikely to adhere to study protocol. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = 19) Gentamicin solution (Garamycin, Schering‐Plough); streptodornase/streptokinase (Varidase, Lederle); dry saline gauze. Group 2: (n = 22) Cadexomer iodine ointment (Iodosorb) changed once daily during the first week and daily or every second or third day in subsequent weeks. Additional comments: All participants were offered the same basic treatment during the study. Prior to inclusion footwear was corrected or special footwear provided whenever required to relieve local pressure. Oral antibiotics used in signs of infection. If the ulcer was infected, gentamicin solution (80 mg/mL) was prescribed twice daily, streptodornase/streptokinase was used for necrotic lesions. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed. Secondary review outcomes: Surgical resection; adverse events. | |
| Notes | Stratifìcation was based on size and type of ulcer (Wagner grade I to II). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated list of randomly permuted blocks of patients, the size of the blocks was unknown to the investigator." Comment: Adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of allocation concealment process |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label" Comment: Assumed staff and participants not blinded to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "... with blinded photo evaluation ..." Comment: Blinded outcome assessment for healing |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "... with blinded photo evaluation ..." Comment: Blinded outcome assessment for healing |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information was provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Patients were withdrawn from the study in case [sic] of hospitalisation (n = 2), lack of compliance (n = 1), violation of inclusion criteria (n = 2)" Comment: Data presented for 35 participants, suggesting 6 dropped out (study started with 41 participants), for a loss of 15%. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
| Methods | Open‐label RCT Setting: Hospital, 4 centres Country: Gothenburg, Sweden Duration of follow‐up: Up to 24 weeks Duration of treatment: 12 weeks Funding source: Vinnova and RLS Global AB co funded the study Unit of analysis: Participant | |
| Participants | 41 participants Inclusion criteria: Type 1 and 2 diabetes, age 18 years or older (67.5 ± 11.8 years in chloramine group; 74.5 ± 12.3 years in control group) and an infected foot for more than 4 weeks. Exclusion criteria: Patients with end‐stage renal disease, impaired blood circulation, or in need of vascular intervention, or a vascular intervention performed less than 3 months before the study, a history of kidney or pancreas transplant, treatment with cortisone > 60 mg daily, chemotherapy or any immune‐modulating agents during the past year, identified conditions, in the ulcer area (e.g. cancer), or generally poor health of the participant and at risk of requirement of hospitalisation. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected | |
| Interventions | Group 1: (n = 19) Standard care alone. Ulcer was cleaned and debrided according to the guidelines of the International Working Group on the Diabetic Foot once weekly. The ulcer was dressed with foam, hydrocolloid, or alginate dressing. In a few cases an adjusted antiseptic agent, silver or polyhexamethylene biguanide, was used. Group 2: (n = 21) Chloramine plus standard care. Trialist applied a preparation containing sodium hypochlorite and amino acid, which are converted to chloramine by mixing the 2 components immediately prior to treatment. The gel was applied to the ulcer once a week. Debridement was done with the gel left on the ulcer surface to provide antibacterial protection for the exposed tissue. Additional comments: Nurses and podiatrist performed cleansing and debridement of the ulcer in both groups at least once weekly for 12 weeks. All participants were given standard care advice on the treatment of diabetes and risk factors. Oral antibiotic treatment was offered if signs of significant infection were observed, particularly affecting underlying tissues or bones. Appropriate off‐loading was considered in all participants. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time‐to‐event data (partially reported); resolution of signs of infection Secondary review outcomes: Surgical resection; adverse events | |
| Notes | The original study performed both ITT and PP analyses for efficacy outcomes, but did not state which analysis was used in the report, hence we assumed the numbers reported were completers only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised in blocks of 4 ..." Comment: Block randomisation is implied. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "in an explorative open randomised controlled multi‐centre study" Comment: No mention of allocation concealment process |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "open‐label" Comment: Assumed staff and participants not blinded to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a photo was taken every week after treatment ... the area of ulcer was subsequently measured by an independent observer" Comment: Adequate blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a photo was taken every week after treatment ... the area of ulcer was subsequently measured by an independent observer" Comment: Adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: It is unclear how the adverse events were assessed. |
| Incomplete outcome data (attrition bias) | Low risk | Chloramine group: Violation of protocol (n = 1 had percutaneous angioplasty; n = 1 was accidentally included in 2 centres; n = 1 lower toe blood pressure < 30 mmHg), 2 ulcer coalesced and unable to assess (n = 1). Control group: lost to follow‐up (n = 1), withdrew informed consent (n = 1) Comment: The dropout rate did not differ significantly between groups; although more people dropped out of the intervention group, the reasons for dropout were mostly not related to the treatment. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | |
| Methods | RCT; prospective, 2‐centre, randomised, controlled, double‐blind, pilot study Setting: Hospital and community, 2 centres Country: UK Duration of follow‐up: 4 weeks Duration of treatment: Weekly treatment for 4 weeks Funding source: For‐profit organisation Unit of analysis: Participant | |
| Participants | 20 participants Inclusion criteria: Chronic (4 weeks’ duration), non‐clinically infected foot ulcers (colonised) where necrotic tissue was present and mechanical debridement was indicated. A foot ulcer was defined as a full‐thickness break of the epithelium distal to the medial and lateral malleoli. Only 1 ulcer per participant was included. Exclusion criteria: Ulcers larger than 25 cm², ulcers defined as grade III in the University of Texas classification, osteomyelitis, peripheral arterial disease (absent pulses/ankle‐brachial index < 0.8), prescription use of anticoagulants, immunosuppressive drug treatment, or known allergies to chlorine (present in Dermacyn). Clinically infected wounds were excluded on the grounds of antibiotic use. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer duration (weeks) 13.7 SD: 12.0 Group 2: Ulcer duration (weeks) 9.7 SD: 8.1 Infection status at baseline: Not infected | |
| Interventions | Group 1 (n = 10): Saline solution Group 2 (n = 10): Super‐oxidised aqueous solution Additional comments: Both solutions used with the Versajet lavage system for removing necrotic tissue. Each solution was used with the Versajet lavage system in a clinic treatment room, followed by a wound rinse and a 10‐minute soak with the respective solution. After the soaking, all of the wounds were dressed with a hydrogel dressing that was changed at regular intervals of 3 to 4 days, as specified by the treating physician. Saline or super‐oxidised aqueous solution was applied at every dressing change. | |
| Outcomes | Primary review outcomes: Proportion of wounds healed (partially reported) Secondary review outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Ten subjects were randomised to each group using a computer‐generated block randomization scheme" Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Both medical centers were provided with sealed randomization envelopes for conducting the treatment assignment" Comment: It is unclear if the envelopes were opaque, but we assume it is a reporting issue. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This was a prospective, two‐center, randomised, controlled, double‐blind, pilot study." Comment: No further information was provided on who was blinded or how the blinding was achieved. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information was provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information was provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information was provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not provided |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
| Methods | RCT Setting: Hospital, 2 centres Country: Denmark Duration of follow‐up: 14 weeks Duration of treatment: Not reported Funding source: For‐profit Unit of analysis: Participant | |
| Participants | 39 participants Inclusion criteria: Diabetic patients aged 35 to 80 years with an ulcer of at least 30 days' duration. Ulcer defined as diabetic foot ulcer, Wagner grade II to III. No local or systemic signs of infection with normal leukocyte levels. Patient willing to return to centre for dressing changes and wound evaluation. Exclusion criteria: Known allergies to any of the contents of PROMOGRAN PRISMA (collagen, oxidised regenerated cellulose, or silver oxidised regenerated cellulose); clinical signs of infection; pregnancy or lactating; history of drug misuse or excessive alcohol consumption; currently undergoing chemotherapy; wound is considered to be malignant; peripheral arterial disease or toe pressure 45 mmHg, or both; patient is unable to walk; patient had haemolytic anaemia and/or iron deficiency anaemia and/or malnutrition, severe cardiac and/or hepatic and/or renal and/or pulmonary insufficiency or chronic administration of cortisone for chronic inflammatory disease and/or autoimmune disease. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer duration (months) 16.9 SD: 36.6; wound area (cm²) 4.4 SD: 6.3 Group 2: Ulcer duration (months) 12.9 SD: 13.0; wound area (cm²) 2.1 SD: 3.1 Infection status at baseline: Not infected | |
| Interventions | Group 1: (n = 15) Foam dressing (Biatain, Coloplast, Humlebæk, Denmark) for moderately exuding wounds and a more absorbent dressing (Mesorb, Mölnlycke Health Care, Gothenburg, Sweden) for highly secreting wounds. Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing (Promogran Prisma, Systagenix Wound Management Ltd., Gatwick, UK). Applied directly to wound. Where there was a low level of wound exudates, the dressing was pre‐wet before applying to the wound. The study protocol suggests that the control dressings were also used in the intervention group, but timing was unclear. Additional comments: The same type of dressings were used in the test and control group and consisted of a foam dressing (Biatain, Coloplast, Humlebæk, Denmark) for moderately exuding wounds and a more absorbent dressing (Mesorb, Mölnlycke Health Care, Gothenburg, Sweden) for highly secreting wounds. The dressings were changed at least twice a week according to the condition of the wound. Patients in both groups were treated with standard wound treatment protocol including debridement and off‐loading, based on specialist clinical evaluation. | |
| Outcomes | Primary review outcomes: Proportion of wounds healed; wound infection (defined by a clinical specialist evaluation based on the classical infection signs, with no microbiological assessment) Secondary review outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed independently of the research team using random number tables and group assignment was kept in sealed envelopes until the end of the study" Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed independently of the research team using random number tables and group assignment was kept in sealed envelopes until the end of the study" Comment: Adequate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Figure shows loss of 3 participants at 14 weeks' follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
| Methods | RCT Setting: Outpatients and inpatients admitted to the Department of Burn and Plastic Surgery of Dazhou Central Hospital Country: China Duration of follow‐up: 4 weeks Duration of treatment: 4 weeks Funding source: Not reported Unit of analysis: Not reported | |
| Participants | Inclusion criteria: Patients with foot ulcers with over 2 years' history of diabetes and glycated haemoglobin > 6.5%. Exclusion criteria: Patients with severe heart or lung disease, high blood pressure, severe mental illness, required immediate amputation, malnutrition, severe sinusitis, detachment of retina, diabetic ketosis in the last 2 weeks, diabetic ketoacidosis, severe infection, and other patients at high risk of being non‐compliant. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer area 12.34 SD: 3.42 (cm²) Group 2: Ulcer area 11.85 SD: 2.91 (cm²) Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improvement in microcirculation). Group 2: (n = 40) Silver ion dressing plus standard care (including blood glucose control, nutritional support, improve microcirculation). Additional information: Dressing was changed daily in Group 1; dressing was changed daily and silver ion was refreshed once a week in Group 2. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to healing (partially reported) Secondary review outcomes: None reported | |
| Notes | English abstract; text translated from Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers were generated with computer programme and managed by an assigned team member" Comment: Adequate method of generating random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "researchers, clinicians and patients do not know the allocation sequence before the trial" Comment: Although we do not know how the trialists concealed the allocation plan, we accept their statement quoted above as true. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "prospective, open, randomised controlled clinical trial" Comment: Open trial with no blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: None obvious |
| Other bias | Low risk | Comment: None obvious |
| Methods | RCT Setting: Not reported Country: Not reported Duration of follow‐up: Not reported Duration of treatment: Not reported Funding source: Not reported Unit of analysis: Not reported | |
| Participants | Inclusion criteria: Patients with foot ulcers with bone and tendon exposure and diabetes. Exclusion criteria: None noted. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = not reported) Iodine gauze (in the control group, iodine gauze dressings were applied at the time of skin graft and changed 3 times a day thereafter). Group 2: (n = not reported) Hydrofiber dressing with silver (changed every 24 hours). Additional comments: All foot ulcers were surgically debrided prior to initiation of the Hydrofiber dressing with silver or gauze treatment. | |
| Outcomes | Primary review outcomes: None reported. Secondary review outcomes: None reported. | |
| Notes | Conference abstract; no outcome data clearly reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty patients were randomised into either the experimental hydrofibre dressing with silver* group or control iodine gauze group" Comment: Insufficient information to make a low‐risk assessment |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No information available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: No information available |
| Selective reporting (reporting bias) | Unclear risk | Comment: No information available |
| Other bias | Unclear risk | Comment: No information available |
| Methods | RCT Setting: Department of General Surgery, Pakistan and Bhatti International Trust (BIT) Hospital Country: Pakistan Duration of follow‐up: 17 weeks Duration of treatment: Not reported Funding source: Not reported Unit of analysis: Not reported | |
| Participants | Inclusion criteria: All patients > 18 years of age with diabetic foot ulcer (Wagner grade I or II) were selected. Exclusion criteria: Patients with Wagner grade III to V, ankle‐brachial pressure index < 7, venous ulcer or malignant ulcer, uncontrolled diabetes (glycated haemoglobin > 7%), patients with > 1 ulcers, patients with haemoglobin < 10 g/dL, and patients with local signs of infection (presence of pus, initial culture positive) in the wound were excluded from the study. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = 180) Treated with normal saline dressing. Group 2: (n = 195) Treated with honey dressing. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to wound healing (partially reported) Secondary review outcomes: Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "grouping was done by simple randomization method (computer‐generated random numbers)" Comment: Adequate method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 16 people dropped out of honey dressing group, and 11 dropped out of saline group. Although not included in the final analysis, the proportion of dropout was balanced between groups and did not compromise the statistical power to detect any potential difference between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: None obvious |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: Office of study author (no further details) Country: Not reported Duration of follow‐up: 6 weeks Duration of treatment: 6 weeks Funding source: Not reported Unit of analysis: Participant | |
| Participants | 40 participants Inclusion criteria: Wagner grade I or II ulcerations of the foot. Study authors note that all patients included in the study presented with ulcers that were 3 centimetres in diameter or less on the plantar aspect of the foot (unclear if inclusion criteria or not). Currently under care for diabetes. Exclusion criteria: Glycated haemoglobin greater than 10%. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not clearly reported Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = 20) Silver sulphadiazine cream (no further details). Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulfadiazine cream. Additional comments: All participants were treated by off‐loading of weight bearing and shoe pressure from the area of ulceration. Debridement with a scalpel was performed as determined for each participant. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed (referred to as "resolved" by study authors) Secondary review outcomes: None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A research coordinator randomly assigned patients to receive ..." Comment: No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: As above |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "This was a blinded study" Comment: No further details provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: As above |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Low risk | Comment: It seems from report that all participants were followed up, as results table contains data for all 40 randomised participants. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: Hospital, 9 centres Country: UK Duration of follow‐up: 24 weeks Duration of treatment: 24 weeks or until healing Funding source: Not‐for‐profit Unit of analysis: Participant | |
| Participants | 317 participants Inclusion criteria: Type 1 or 2 diabetes; 18 years of age or older; a foot ulcer present for at least 6 weeks with a cross‐sectional area of between 25 and 2500 mm²; able and willing to give informed consent; reasonably accessible by car to the hospital base; under routine review by the multidisciplinary clinic. Exclusion criteria: Those with a known allergy to any of the trial preparations (including iodine); any ulcer on either foot extending to tendon, periosteum, or bone, infection of bone, soft‐tissue infection requiring treatment with systemic antibiotics; an ulcer on a limb being considered for revascularisation; those chosen for management with a non‐removable cast without a dressing window; gangrene on the affected foot; eschar that was not removable by clinical debridement; those with evidence of a sinus or deep track; those in whom the hallux had been amputated on the affected side (preventing the measurement of toe pressure); those with an ankle‐brachial pressure index of less than 0.7 or toe systolic pressure less than 30 mmHg; ulceration judged to be caused primarily by disease other than diabetes; patients with any other serious disease likely to compromise the outcome of the trial; patients with critical renal disease (creatinine greater than 300 mmol/L); those receiving immunosuppressants, systemic corticosteroid therapy (other than by inhalation), or any other preparation that, in the opinion of the supervising clinician, could have interfered with wound healing; those living at such a distance (generally further than 10 miles) from the clinic as would have made frequent assessment visits inappropriately expensive or impractical, or both; those who withheld consent. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not clear | |
| Interventions | Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Additional comments: Dressings were changed daily, on alternate days or 3 times a week according to need or availability of professional staff, or both. Participants were advised to have a bath or shower as often as they wished, provided the ulcer could be redressed afterwards, and provided the ulcerated foot was not immersed in water for more than 5 minutes. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed | |
| Notes | Randomisation was stratified by both centre and size, using a block size of 9. Randomisation was stratified across the whole population by ulcer area into 3 groups: 25 to 100 mm², 101 to 250 mm², and 251 to 2500 mm². | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation lists were created using SPSS (SPSS Inc., Version 14), using blinded dressing codes." Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "The lists were held at Cardiff University and each recruiting centre telephoned a designated number during working hours. They were required to identify the centre and size of wound only." Comment: Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Comment: The study was not blinded to personnel and participants. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Dressings were removed prior to examination by assessors who were not involved in the conduct of the trial and who were blind to the randomisation group." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear if infection assessment was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear if adverse event data collection was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Intention to treat analysis was carried out using the last value carried forward method, with strict adherence to the protocol such that only those who attended for a healing verification visit and reported as still healed at 28 days have been coded as ‘healed’ for the outcome classification." |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: 18 centres ‐ settings not clear Country: UK, France, Germany, and Sweden Duration of follow‐up: 8 weeks Duration of treatment: 8 weeks or until healed Funding source: For‐profit Unit of analysis: Participant | |
| Participants | 134 participants. Ulcer characteristics at baseline (e.g. anatomic site, size, number of ulcers, presence of infection, duration of ulceration where reported): Group 1: Ulcer duration (years) 1.4 (SD 2.6); ulcer area (cm²) 4.2 (SD 7.8) Group 2: Ulcer duration (years) 1.2 (SD 2.1); ulcer area (cm²) 4.2 (SD 4.1) Infection status at baseline: Mixed: 22 participants had clinically infected ulcers at baseline, 13 in Group A and 9 in Group B. On enrolment antibiotics were prescribed to 8 participants in Group A and 13 in Group B. | |
| Interventions | Group 1: (n = 67) Calcium‐alginate dressing (Algosteril, Smith & Nephew). Manufacturer's instructions were followed, and dressing was moistened before use on dry wounds, and changed on leakage or at evaluation or every 7 days as indicated (except for infected wounds, for which the dressing was changed daily). Group 2 (n = 67): Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec). Left in place and changed on leakage or at evaluation or every 7 days as indicated. In both groups, ulcers were cleansed using sterile saline; each dressing was covered with a sterile, non‐adherent foam dressing. Additional comments: Accommodative footwear for non‐plantar ulcers and off‐loading for plantar ulcers delivered as required. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed (number of ulcers healed); time to healing (only partially reported) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible individuals were randomly assigned to receive one of the two dressings according to instructions in a sealed envelope and stratified according to whether or not systemic antibiotics were being administered for treatment of the studied ulcer" Comment: No detailed information provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: See above Comment: It is unclear how allocation was conducted. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label" Comment: The study was not blinded to personnel and participants. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information is provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information is provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information is provided. |
| Incomplete outcome data (attrition bias) | Low risk | 65 out of the 67 participants in each study group were rated for wound condition at final evaluation. All included participants were evaluated for safety. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
| Methods | RCT Setting: Patients were managed initially on inpatient and then on outpatient basis Country: India Duration of follow‐up: Until healing > 8 weeks Duration of treatment: 10 weeks Funding source: Unclear: the study notes that "financial support was provided by dr. Ram Manohar Lohia Hospital, New Dehli" Unit of analysis: Participant | |
| Participants | 60 participants Inclusion criteria: Diabetic foot ulcer of at least 8 weeks' duration ‐ stage III and IV, absence of vascular insufficiency involving large‐ and medium‐sized arteries proximal to the ulcer demonstrated by Doppler study, age ≥ 18 years with Type 1 or 2 diabetes. Exclusion criteria: Patients with uncontrolled diabetes, foot ulcer with established gangrene, compromised vascularity of the particular limb, associated osteomyelitis at site of ulcers, pregnant and lactating females, neoplasm at the local site, patients on any immunosuppressive agents, presence of multiple ulcers, patient HIV seropositive, patients with known drug allergy, presence of concomitant life‐threatening infections, chronic renal insufficiency (serum creatinine > 3 mg/dL), when ear cannot equalise the pressure when congested with cold/hay fever, patients with perforation of ear drum. High‐risk case, i.e. bronchial asthma/emphysema Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported | |
| Interventions | Group 1: (n = 20) Hyperbaric oxygen therapy. Therapy delivered at 2.5 atmospheres absolute for 60 min per sitting for a total of 30 sittings or until the ulcer had healed. Sittings were distributed over a period of 10 weeks. Patients were given either daily or alternate‐day therapy depending on the availability of slot in the facility. The patients in this group were also debrided from time to time but dressed only with normal saline. No antiseptics were used (group not considered further in review). Group 2: (n = 20) Recombinant human platelet‐derived growth factor. The patients in this group were initially Group 3: (n = 20) Antiseptic treatments (Edinburgh University Solution of Lime (EUSOL), hydrogen peroxide, and povidone iodine). The foot was soaked in EUSOL for 30 min, followed by use of hydrogen peroxide and povidone iodine (no details about concentration). | |
| Outcomes | Primary review outcome: Proportion of ulcers healed; time to healing (partially reported) Secondary outcomes: None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation methods not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information is provided. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study Comment: Blinding unfeasible due to the differences in setting and formulation of the interventions. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information is provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | High risk | Quote: "6 patients in group 1 (30%), 5 in group 2 (25%) and 1 (5%) in group 3 lost to follow up" Comment: Systematic differences in withdrawal from the study among groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: The outcomes reported in the results are not the same as specified in the Material and Methods section. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: 16 centres, but outpatient or inpatient setting is not specified Country: USA Duration of follow‐up: 4 weeks Duration of treatment: 10 days Funding source: For‐profit funding; "This project was supported by a research grant from Oculus Innovative Sciences." Unit of analysis: Participant | |
| Participants | 67 participants Inclusion criteria: > 18 years of age with diabetes mellitus (Type 1 or 2) and a mild diabetic foot infection. Eligible foot ulcers involved skin and deeper soft tissue and were classified by Infectious Diseases Society of America guidelines as mildly infected and by the University of Texas Classification as 1B. Ulcers could be located on the foot and malleolar areas, measured 1 to 9 cm², and were accessible for culture. Adequate circulation to the foot was required. Exclusion criteria: Antibiotic treatment for more than 24 hours within 72 hours of study entry; necrotising fasciitis, deep abscesses in the soft tissue, sinus tracts, gas gangrene, or infected burns, superinfected eczema or other chronic medical conditions; ulcers located on the stump of an amputated extremity; ulcers having a non‐diabetic aetiology; infections complicated by the presence of prosthetic materials and osteomyelitis; pregnancy or risk of pregnancy, breastfeeding; liver disease; neutropenia; hypersensitivity to chlorine or quinolones; patients receiving glucocorticoid or adjuvant therapy with hyperbaric oxygen or topical formulations containing growth factors, antimicrobials, enzymatic debriders, or granulation promoters; disorders of immune function and any medical condition that, in the investigator’s opinion, would require dose modification of levofloxacin to less than 750 mg/d or who had received an investigational agent within 1 month before the baseline evaluation. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected ulcers | |
| Interventions | Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day. Group 2: (n = 21) Topical Microcyn therapy once per day (not considered in review). Group 3 (n = 25) Topical Microcyn therapy plus 750 mg levofloxacin once per day. Additional comments: Wound cleaning and coverage was performed once a day with 30 mL of either Microcyn Rx or saline. Sterile gauze was saturated with approximately 25 mL of Microcyn Rx or saline, and the excess solution was wrung out. Working from the inside out, the wound was scrubbed gently to remove drainage and exudates. Once the wound bed was prepared, another sterile gauze pad was saturated with an additional 5 mL of Microcyn Rx or saline. Enough of the soaked gauze was applied to fill, but not tightly pack, the wound. The wound was covered with an occlusive dressing after each dressing change. Where necessary, off‐loading was achieved with fixed ankle boots or healing sandals, as indicated by the investigator. Debridement procedures were limited to 3 for the duration of the study. | |
| Outcomes | Primary review outcomes: Resolution of infection (defined in the paper as "cure" ‐ resolution of all signs and symptoms, including the presence of culturable exudates, warmth, erythema, induration, tenderness, pain, swelling, and a healing wound (as determined by the investigator) after 5 or more days of treatment). Secondary review outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was accomplished at each study site by using a manual system and stratified by site". "Envelopes containing group designations opened sequentially" Comment: It is not clear how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Envelopes containing group designations opened sequentially" Comment: It is unclear if the allocation was foreseeable with this method; numbering envelopes would have added extra rigour, and it is not clear if this was done. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label Comment: Blinding unfeasible due to the differences in setting and formulation of the interventions. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: The study was conducted on an ITT basis with missing = failure; 66 out of 67 participants randomised were evaluated. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: Predominantly outpatients (with some inpatients) (from study author) Country: USA Duration of follow‐up: 28 to 42 days Duration of treatment: 14 to 28 days Funding source: For‐profit; "Magainin Pharmaceuticals sponsored the studies" Unit of analysis: Participant | |
| Participants | 493 participants Inclusion criteria: Men or women aged > 18 years with diabetes mellitus and an infected wound below the malleoli that exceeded 0.5 cm² in area after appropriate debridement. Wounds had to be full‐thickness (i.e. through the epidermis and into or through the dermis, but not involving tendon, bone, or joint capsule). Exclusion criteria: Patients were excluded if they had: an abscess; extensive gangrene; an imminently limb‐threatening infection; evidence of systemic infection (e.g. fever, chills, or hypotension); plain radiograph findings suggestive of osteomyelitis; no palpable dorsalis pedis or posterior tibial pulse or a pedal systolic pressure (by Doppler) of ≤ 40 mmHg on the affected limb; requirement for renal dialysis; need for immunosuppressive medication; or hypersensitivity to either study medication. Infection was defined by the presence of purulent drainage or ≥ 2 of the following: erythema, warmth, pain or tenderness, or oedema or induration. The diabetic foot infection had to be severe enough to require antibiotic therapy, but it had to be amenable to outpatient treatment. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer area (mm²) 131.5 (no SD reported) Group 2: Ulcer area (mm²) 117.3 (no SD reported) Infection status at baseline: Infected ulcers | |
| Interventions | Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream. Additional comments: Investigators performed appropriate local wound care, including any necessary debridement and pressure off‐loading of the infected site, and they obtained wound tissue specimens for aerobic and anaerobic culture at enrolment, and at follow‐up, when material was available. Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets. Each treatment administered twice daily. | |
| Outcomes | Primary outcomes: None reported in useable format Secondary outcomes: Surgical resection; adverse events | |
| Notes | Some information about study methods was available from corresponding author. Classed as study 303 in paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Computer generated random sequence" (from study author) Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "After completion of required screening procedures eligible patients received a sequentially assigned randomization number. Each investigational centre received a unique set of randomization numbers." (from study author) Comment: Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind. Patients were instructed to take 2 tablets (either 200 mg of active ofloxacin orally twice daily and to apply a cream (either active pexiganan acetate or placebo, sufficient to form a dime thick layer) twice daily directly onto the ulcer and to dress the wound with sterile, dry gauze. Patients randomised to treatment with pexiganan received placebo tablets, and those randomised to ofloxacin treatment received placebo cream" Comment: Blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Figure in paper shows that all participants had clinical data analysed in ITT. There were more missing data for microbial analysis, but we did not consider these in the review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Healing data did not seem to be reported. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: Predominantly outpatients (with some inpatients) (from study author) Country: USA Duration of follow‐up: 28 to 42 days Duration of treatment: 14 to 28 days Funding source: For‐profit Unit of analysis: Participant | |
| Participants | 342 participants Inclusion criteria: Men or women aged > 18 years with diabetes mellitus and an infected wound below the malleoli that exceeded 0.5 cm² in area after appropriate debridement. Wounds had to be full‐thickness (i.e. through the epidermis and into or through the dermis, but not involving tendon, bone, or joint capsule). Exclusion criteria: Patients were excluded if they had an abscess, extensive gangrene, an imminently limb‐threatening infection, evidence of systemic infection (e.g. fever, chills, or hypotension), plain radiograph findings suggestive of osteomyelitis, no palpable dorsalis pedis or posterior tibial pulse or a pedal systolic pressure (by Doppler) of ≤ 40 mmHg on the affected limb, requirement for renal dialysis, need for immunosuppressive medication, or hypersensitivity to either study medication. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected ulcers | |
| Interventions | Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream. Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets. Additional comments: Investigators performed appropriate local wound care, including any necessary debridement and pressure off‐loading of the infected site, and they obtained wound tissue specimens for aerobic and anaerobic culture at enrolment, and at follow‐up, when material was available. Each treatment administered twice daily. | |
| Outcomes | Primary outcomes: None reported in useable format Secondary outcomes: Surgical resection; adverse events | |
| Notes | Some information about study methods was available from corresponding author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Computer generated random sequence" (from study author) Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "20 or more clinical study centres received sufficient randomization numbers to complete approximately 224 patients at each site. The investigators were blinded as to the treatment group assignment throughout the study. After completion of required screening procedures eligible patients received a sequentially assigned randomization number. Each investigational centre received a unique set of randomization numbers." (from study author) Comment: Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind. Patients were instructed to take 2 tablets (either 200 mg of active ofloxacin orally twice daily and to apply a cream (either active pexiganan acetate or placebo, sufficient to form a dime thick layer) twice daily directly onto the ulcer and to dress the wound with sterile, dry gauze. Patients randomised to treatment with pexiganan received placebo tablets, and those randomised to ofloxacin treatment received placebo cream" Comment: Blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Figure in paper shows that all participants had clinical data analysed in ITT. There were more missing data for microbial analysis, but we did not consider these in the review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Healing data did not seem to be reported. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT (2:1 randomisation ratio) Setting: Diabetic foot clinics (mainly inpatients) (from study author) Country: USA and UK Duration of follow‐up: Outcome assessment planned for 2 weeks after cessation of treatment for a total study duration of 42 days Duration of treatment: At least 7 days and for a maximum of 28 days Funding source: For‐profit; "This study was funded in whole by Innocoll Technologies Ltd." Unit of analysis: Participant | |
| Participants | 56 participants Inclusion criteria: Diabetic patients aged 18 to 80 years with a single, moderately infected lower extremity ulcer. Exclusion criteria: Patients who had received any antimicrobial therapy in the preceding 2 weeks; those with ischaemia of the lower limb; and, at institutional review board request, patients with a glycated haemoglobin level of ≥ 10.0%. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected ulcers | |
| Interventions | Group 1: (n = 38) Systemic antibiotic therapy alone (a daily oral or intravenous dose of 750 mg of levofloxacin or alternative antimicrobial therapy, as determined by susceptibility testing). Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy (a daily oral or intravenous dose of 750 mg of levofloxacin or alternative antimicrobial therapy, as determined by susceptibility testing). Additional comments: Participants in both arms also received standard diabetic wound management, including sharp surgical debridement at each visit where appropriate, pressure off‐loading as applicable, and daily dressing changes using a non‐adherent, moisture‐permeable gauze dressing followed by a second saline‐moistened gauze dressing. | |
| Outcomes | Primary review outcomes: Resolution of infection Secondary review outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: No information presented. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised in a 2:1 ratio to the treatment or control group using a interactive voice response system" Comment: Confirmed centralised randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label" Comment: The authors chose not to administer placebo collagen sponges to participants in the control group due to the concern that a placebo sponge could potentially harbour bacteria and bias the results in favour of the active treatment. Consequently, to reduce the complexity of this pilot study, they chose an open‐label design. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Incomplete outcome data (attrition bias) | High risk | Quote "of the 56 randomised subjects, 20 (12 in group 1 and 8 in group 2) were deemed ineligible; three more subjects in the study group discontinued (1 because of adverse events, 1 because of protocol non‐compliance and 1 lost to follow up)." "we defined a modified ITT population to use of efficacy analyses to include only the 36 eligible patients" Comment: 41% of participants were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: Outpatient clinic Country: Mexico Duration of follow‐up: 20 weeks Duration of treatment: Minimum of 10 days Funding source: Not reported Unit of analysis: Participant | |
| Participants | 45 participants Inclusion criteria: Type 2 diabetes; older than 18 years of age; infected, deep wounds at or distal to the malleoli; presence of malodour, active periwound cellulites; loss of protective sensation; and at least 1 Dopplerable pedal pulse. Exclusion criteria: Severe arterial disease; ankle‐brachial index below 0.5; a diagnosis of osteomyelitis; total gangrene of the study foot or forefoot; severe cardiovascular or renal failure; and severe neurological problems. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer duration (weeks) 15.1 (SD 16.3) Group 2: Ulcer duration (weeks) 13.7 (SD 24.0) Infection status at baseline: Infected ulcers | |
| Interventions | Group 1: (n = 16) Povidone iodine and saline. Povidone iodine was used after debridement. When the infection resolved and formation of granulation tissue was observed, the participant was switched to a surgical soap (Dermo Clean) with saline rinse to minimise the cytotoxic effects of povidone iodine. If clinical signs of infection returned, the use of povidone iodine was resumed. Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution. Participants received an initial 15‐ to 20‐minute immersion of the affected foot. Following appropriate debridement, the affected foot soak was repeated either weekly or biweekly. Additional comments: All participants were treated using an outpatient ambulatory model, which included appropriate surgical debridement, administration of aggressive parenteral/intramuscular broad‐spectrum antibiotic therapy, appropriate off‐loading, and strict glycaemic control. Systemic antibiotics were given for a minimum of 10 days to all participants in both groups. Antibiotics were used for more that 10 days if clinical signs of infection continued to be present. All participants received pentoxyphylline at a dose of 1200 mg/day as a haemorheologic. All participants in both groups were instructed to reduce weight bearing on the affected foot by using a wheelchair or crutches and by resting as much as possible. | |
| Outcomes | Primary review outcomes: Resolution of infection Secondary review outcomes: None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patient randomised using randomly alternate assignment" Comment: It was not clear whether process was random ‐ could also describe alternation. |
| Allocation concealment (selection bias) | High risk | Quote: "patient randomised using randomly alternate assignment" Comment: The description is not entirely clear, but allocation could have been foreseeable. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients were blinded about [sic] the differences in treatment." Comment: Adequate blinding for participants but not personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Outcomes reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT Setting: Diabetic foot clinic Country: Mexico Duration of follow‐up: 20 weeks Duration of treatment: Until healing Funding source: Indas S. A. Laboratory, distributor of Cicactiv (zinc hyaluronate) Unit of analysis: Participant | |
| Participants | 50 participants Inclusion criteria: People with foot ulcer and diabetes Exclusion criteria: Not reported Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported as a summary measure Infection status at baseline: Not reported (based on translated material) | |
| Interventions | Group 1 (n = 25): Conventional treatment (no further details provided) Group 2 (n = 25): Zinc hyaluronate Additional comments: Not reported (based on translated material) | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to healing (partially reported) Secondary review outcomes: None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... were assigned randomly ..." Comment: No further information reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open label" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "open label" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Figure in paper shows that all participants had clinical data analysed in ITT. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None obvious |
| Methods | RCT Setting: Hospital university centre Country: Malaysia Duration of follow‐up: Not stated Duration of treatment: Between 7 and 26 days Funding source: Not reported Unit of analysis: Participant | |
| Participants | 30 participants. Non‐insulin‐dependent diabetes mellitus patients with Wagner grade II ulcers admitted to the hospital for surgery Inclusion criteria: age between 35 and 65 years, transcutaneous oxygen tension of more than 30 mmHg, and serum albumin level of more than 35 g/dL. Exclusion criteria: Multiple medical comorbidity, corticosteroid therapy, neutrophil count less than 2000/mm₃. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not stated Infection status at baseline: Not reported | |
| Interventions | Group 1 (n = not stated): Standard dressing group (povidone iodine solution 10%). Group 2 (n = not stated): Honey dressing group. Additional comments: 30 consecutive patients were randomised, but number of participants in each group not reported. | |
| Outcomes | Primary review outcomes: Time to healing (partially reported) Secondary review outcomes: None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised into two study arms" Comment: Randomisation methods were not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomised into two study arms" Comment: Randomisation methods were not specified. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all the wounds were assessed every other day by a surgeon blinded to the material of dressing" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 30 participants were randomised, but no further information on the number of participants in each group and for each outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None noted. |
| Methods | RCT; prospective, randomised, double‐blind, placebo‐controlled clinical trial Setting: Foot clinic at the Veterans Affairs Medical Center, San Diego Country: USA Duration of follow‐up: 16 weeks Duration of treatment: 4 weeks Funding source: Supported by OrthoNeutrogena Unit of analysis: Some participants had more than 1 ulcer (22 participants with 24 ulcers) | |
| Participants | 24 participants Inclusion criteria: Lower extremity ulcer and a diagnosis of diabetes mellitus Exclusion criteria: Patients unable to give informed consent; had a known bleeding disorder; were pregnant at the time of enrolment; had infected ulcers or nearby tissues; or had lower extremity ulcers due to large artery disease (by clinical examination or abnormal ankle‐brachial index, or both) Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not infected | |
| Interventions | Group 1 (n = 11): Placebo (normal saline solution that was coloured the same as the topical tretinoin). Group 2 (n = 13): Topical 0.05% tretinoin solution (Retin‐A; Ortho Pharmaceutical Corp, Raritan, NJ). The randomly assigned solution was applied directly to the wound bed and left in contact for 10 minutes every day; it was then rinsed off with normal saline. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to healing (partially reported) Secondary review outcomes: None | |
| Notes | 24 participants included, 22 participants analysed (13 + 11 ulcers) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by an uninvolved third party who used a computer‐generated random sequence to balance the numbers of the 2 treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each newly enrolled patient was assigned a topical solution in ascending order" Comment: Not clear if the sequence was foreseeable with this method |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "all dispensed bottles of solutions were identical in appearance (identified by number only), and neither the investigators nor the patients were aware of the treatment group to which patients were assigned until the study was completed" Comment: The double‐blind appears to have been respected. |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Selective reporting (reporting bias) | Low risk | Comment: 24 participants were randomised; 22 completed the study and were considered for the outcomes, 20 with 1 foot ulcer and 2 with 2 foot ulcers. |
| Other bias | High risk | Comment: Some participants had multiple ulcers, but this was not accounted for. |
| Methods | RCT; randomised, controlled, open study Setting: Unclear Country: India Duration of follow‐up: Not reported (not clear) Duration of treatment: 2 months Funding source: Not stated Unit of analysis: Not stated | |
| Participants | 4 participants Inclusion criteria: People with diabetes having grade I or II foot ulcer Exclusion criteria: Unclear Infection at baseline: Unclear | |
| Interventions | Group 1 (n = 2): Povidone iodine and metronidazole 1% gel dressing. Group 2 (n = 2): Honey and metronidazole 1% gel dressing. | |
| Outcomes | Primary outcomes: Proportion of ulcers healed Secondary outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomised, controlled, open study" Comment: Unclear how randomisation was achieved |
| Allocation concealment (selection bias) | Unclear risk | Quote: "open study" Comment: No mention of allocation concealment process |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Open study, no blinding |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Open study, no blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | High risk | Not relevant |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: None obvious |
| Other bias | Low risk | Comment: None observed. |
| Methods | RCT; single‐centre, open‐label, phase III, comparative study Setting: Diabetes Research Centre Country: India Duration of follow‐up: 20 weeks Duration of treatment: Not clear Funding source: Cholayil Products and Services, Koyambedu, Chennai, India provided the polyherbal cream with their formulation. Unit of analysis: Participant Participants enrolled between August 2008 and February 2009 | |
| Participants | 40 participants Inclusion criteria: Consecutive Type 2 diabetes patients who presented with an ulcer up to Wagner's grade III classification (grade I, superficial ulcer; grade II, deep ulcer probing to tendon, capsule, or bone; grade III, deep ulcer with abscess, osteomyelitis, or joint sepsis). Exclusion criteria: People who had clinical signs of severe infection; wound that had exposed bone; unwillingness to participate in the study were excluded. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): "There was no significant difference in the location of the wound between the groups. The distribution of ulcers according to Wagner's grade was also similar in both the study groups. Wagner grade I and II foot ulcers were viable and grade III ulcers were non‐viable tissues" Infection status at baseline: Unclear; severe infections were excluded | |
| Interventions | Group 1 (n = 20): Polyherbal formulation wound cream: Glycyrrhiza glabra, Musa × paradisiaca,Curcuma longa,Pandanus,Aloe vera,Cocos nucifera oil. Group 2 (n = 20): Silver sulphadiazine cream. | |
| Outcomes | Primary review outcomes: Proportion of ulcers healed (partially reported); time to healing (partially reported) Secondary review outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) | High risk | Comment: Rate of ulcer healing not reported. |
| Selective reporting (reporting bias) | Low risk | Quote: "Of the 40 patients enrolled in this study, 38 adhered to the protocol (group 1; n = 19 and group 2; n = 19). One patient in group 1 was excluded from the study because of severe infection and one patient in group 2 died during the study period (unrelated cause)" |
| Other bias | Low risk | Comment: None noted. |
ITT: intention‐to‐treat
PP: per protocol
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not relevant intervention (hyperbaric oxygen therapy) | |
| Not relevant study population (mixed wounds) | |
| Not relevant intervention (all antibiotics were administered intervenously) | |
| Not relevant study population | |
| Not RCT | |
| Not relevant study population (mixed wound types) | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not relevant study population (mixed wound types) | |
| Not relevant intervention | |
| Not RCT | |
| No outcome data available on request | |
| Not RCT | |
| Not relevant study population | |
| Not RCT | |
| Not relevant study population | |
| Not RCT | |
| Not relevant intervention | |
| Not relevant intervention (no topical treatment tested) | |
| Not relevant intervention | |
| Not relevant intervention (no topical treatment tested) | |
| Not RCT | |
| Not relevant intervention (BioLight, combination of pulsating monochromatic light) | |
| Not relevant intervention (no topical treatment tested) | |
| Not RCT | |
| Not relevant intervention (ozone therapy) | |
| Not relevant intervention (no topical treatment tested) | |
| Not relevant intervention (phototherapy) | |
| Not relevant intervention (photosensitiser compound) | |
| Not relevant intervention (cationic photosensitisers) | |
| Not relevant study population (mixed wound types) | |
| Not RCT | |
| Not RCT | |
| Not relevant study population (mixed population; only 18 diabetic patients included, separate data not available) | |
| Not RCT | |
| Not relevant study population | |
| Not relevant study population | |
| Not relevant study population (mixed population, number with diabetes and foot ulcers not reported) | |
| Not relevant intervention | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not relevant study population (mixed population, number with diabetes and foot ulcers not reported) | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not relevant study population (mixed population, data for people with diabetes and foot ulcers not available) | |
| Not RCT | |
| Antimicrobial treatment not the only systematic difference between trial arms | |
| Not relevant study population | |
| Not relevant intervention (ozone therapy) |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT Setting: Hospital Country: India Duration of follow‐up: Not reported Duration of treatment: 14 days Funding source: Not reported Unit of analysis: Participant |
| Participants | 50 participants Inclusion criteria: Diabetic ulcer of the foot Exclusion criteria: Not reported Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported |
| Interventions | Group 1: (n = 25) 5% w/v povidone iodine solution twice daily for 14 days. Group 2: (n = 25) Phenytoin‐soaked suspension (20 mg/cm²) total body surface area; frequency not reported. Additional comments: After 14 days all participants were subject to split‐thickness skin graft. |
| Outcomes | Primary review outcomes: Time to healing Secondary review outcomes: None reported |
| Notes | Available only as a conference abstract (authors contacted for further information; awaiting response) |
| Methods | RCT Setting: Surgical unit, hospital, 1 centre Country: Pakistan Duration of follow‐up: 2 weeks Duration of treatment: 2 weeks Funding source: Not reported Unit of analysis: Participant |
| Participants | 60 participants Inclusion criteria: Wagner grade I or II diabetic ulcers. Exclusion criteria: Patients not consenting to study, having features of systemic infection and other comorbidities. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported)): Size of ulcer after surgical debridement measured at baseline, but data not reported. Infection status at baseline: Not reported |
| Interventions | Group 1: Honey‐soaked dressing (local honey used ‐ no further information). Group 2: Povidone iodine/normal saline dressing. Additional comments: Dressing changed once a day. |
| Outcomes | Primary review outcomes: Proportion of ulcers healed Secondary review outcomes: None |
| Notes | Contacted author for randomisation methods |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | Double‐blind, multicentre trial |
| Participants | Diabetic patients with foot ulcers |
| Interventions | Normal diabetic socks versus (experimental group) copper‐impregnated socks |
| Outcomes | Quality of life, healing, odour |
| Starting date | |
| Contact information | |
| Notes | ABSTRACT ONLY, ONGOING |
| Trial name or title | A randomized, double‐blind, multicenter, superiority, placebo‐controlled phase 3 study of pexiganan cream 0.8% applied twice daily for 14 days in the treatment of adults with mild infections of diabetic foot ulcers |
| Methods | Interventional RCT |
| Participants | Mild infected ulcer in diabetic patients, full‐ or partial‐thickness ulcer on the foot distal to the malleoli with a surface area ≥ 1 cm² after the wound has undergone appropriate debridement |
| Interventions | Pexiganan versus placebo |
| Outcomes | 28 days clinical response, 28 days microbiological response, incidence and severity of adverse events |
| Starting date | 2014 |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 945 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.45] |
| Analysis 1.1 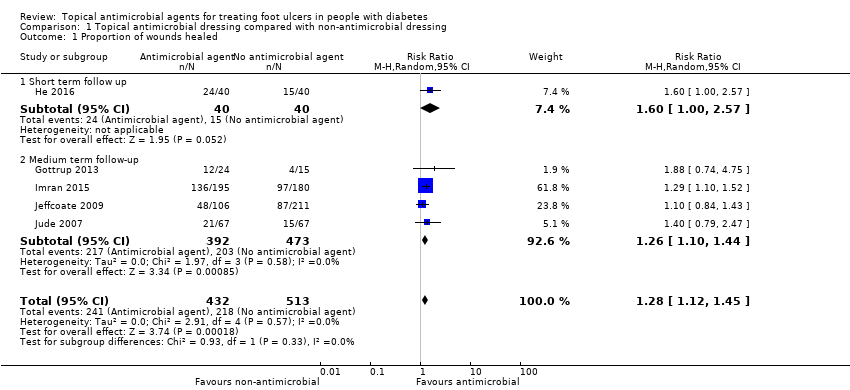 Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 1 Proportion of wounds healed. | ||||
| 1.1 Short term follow up | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [1.00, 2.57] |
| 1.2 Medium term follow‐up | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.44] |
| 2 Incidence of infection: medium term follow‐up Show forest plot | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.10] |
| Analysis 1.2  Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 2 Incidence of infection: medium term follow‐up. | ||||
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.72] |
| Analysis 1.3 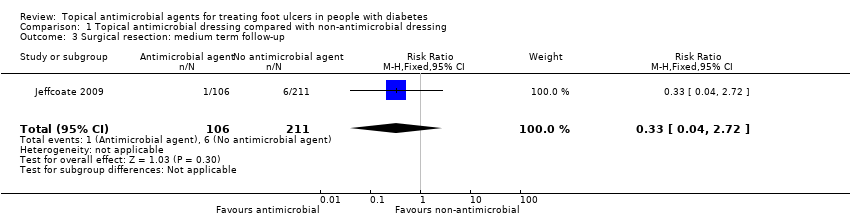 Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 3 Surgical resection: medium term follow‐up. | ||||
| 4 Adverse events Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.48] |
| Analysis 1.4 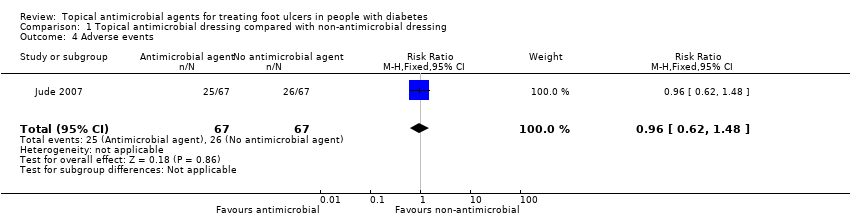 Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 4 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: medium term follow‐up Show forest plot | 3 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.56, 14.23] |
| Analysis 2.1  Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 1 Proportion of wounds healed: medium term follow‐up. | ||||
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.51] |
| Analysis 2.2  Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 2 Resolution of infection: medium term follow‐up. | ||||
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.47, 5.90] |
| Analysis 2.3  Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 3 Surgical resection: medium term follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 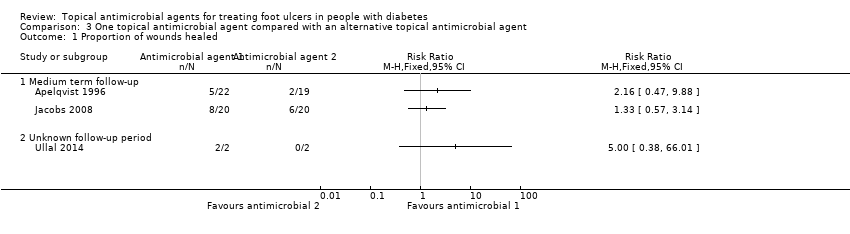 Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 1 Proportion of wounds healed. | ||||
| 1.1 Medium term follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Unknown follow‐up period | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 2 Resolution of infection: medium term follow‐up. | ||||
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.53, 7.03] |
| Analysis 3.3 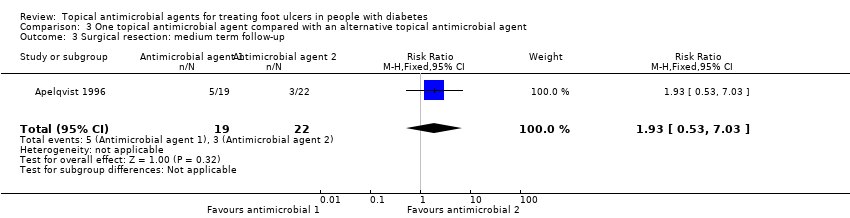 Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 3 Surgical resection: medium term follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of infection Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.91, 2.49] |
| Analysis 4.1  Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 1 Resolution of infection. | ||||
| 1.1 Short‐term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.69, 3.45] |
| 1.2 Medium term follow‐up | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 2 Surgical resection: medium term follow‐up Show forest plot | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.51, 2.91] |
| Analysis 4.2  Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 2 Surgical resection: medium term follow‐up. | ||||
| 3 Adverse events Show forest plot | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| Analysis 4.3 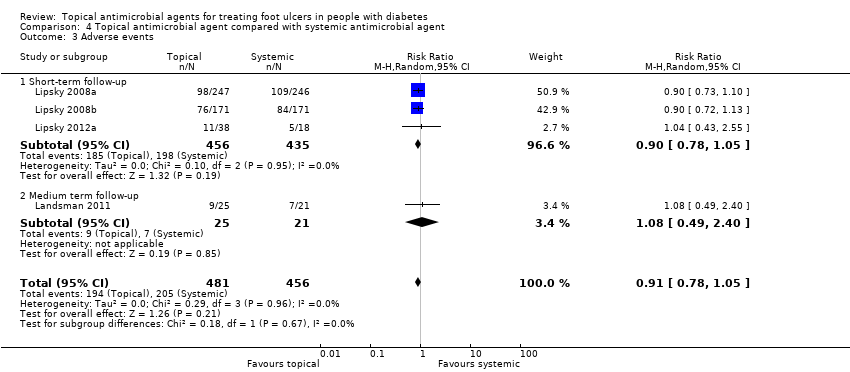 Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 3 Adverse events. | ||||
| 3.1 Short‐term follow‐up | 3 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 3.2 Medium term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: Medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.28, 0.89] |
| Analysis 5.1  Comparison 5 Topical antimicrobial agent compared with growth factor, Outcome 1 Proportion of wounds healed: Medium term follow‐up. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
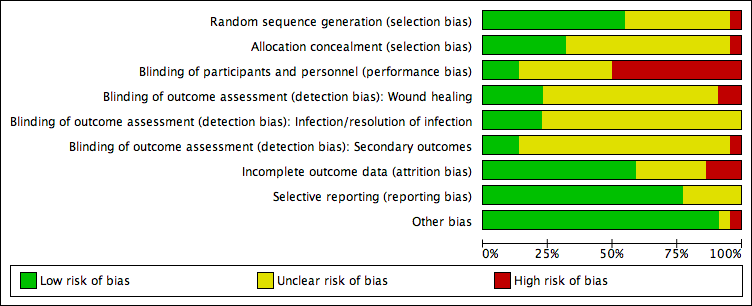
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 1 Proportion of wounds healed.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 2 Incidence of infection: medium term follow‐up.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 3 Surgical resection: medium term follow‐up.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 4 Adverse events.

Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 1 Proportion of wounds healed: medium term follow‐up.
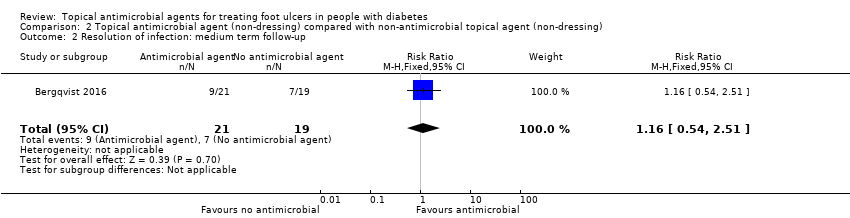
Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 2 Resolution of infection: medium term follow‐up.

Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 3 Surgical resection: medium term follow‐up.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 1 Proportion of wounds healed.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 2 Resolution of infection: medium term follow‐up.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 3 Surgical resection: medium term follow‐up.

Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 1 Resolution of infection.
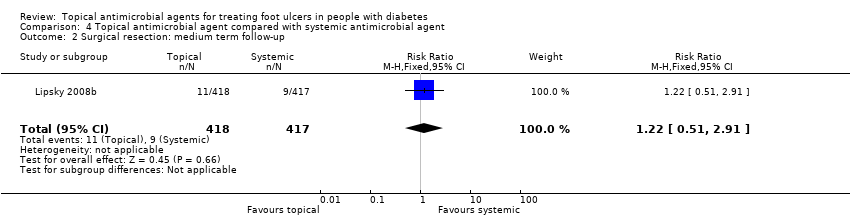
Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 2 Surgical resection: medium term follow‐up.

Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 3 Adverse events.
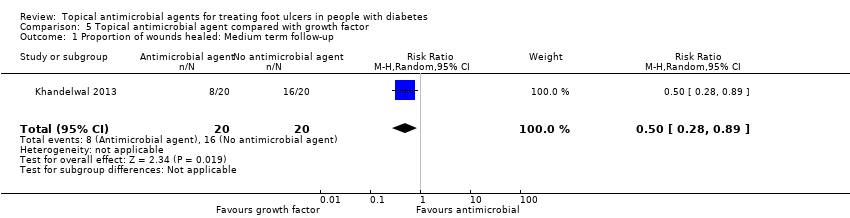
Comparison 5 Topical antimicrobial agent compared with growth factor, Outcome 1 Proportion of wounds healed: Medium term follow‐up.
| Antimicrobial dressings compared with non‐antimicrobial dressings | ||||||
| Patient or population: Foot ulcers in people with diabetes Settings: Mixed Intervention: Antimicrobial dressings Comparison: Standard dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard dressings | Risk with antimicrobial dressings | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 425 per 1000 | 544 per 1000 | RR 1.28 (1.12 to 1.45) | 945 participants (5 studies) | ⊕⊕⊝⊝ | On average, use of an antimicrobial dressing compared with a non‐antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period. |
| Risk difference: 119 more healed wounds per 1000 (51 more to 191 more) | ||||||
| Incidence of infection Up to 24 weeks' follow‐up | 183 per 1000 | 62 per 100 (7 to 567) | RR 0.34 (0.04 to 3.10) | 173 participants (2 studies) | ⊕⊝⊝⊝ | On average, it is unclear whether or not use of an antimicrobial dressing compared with a non‐antimicrobial dressing reduces the incidence of ulcer infection over a medium‐term follow‐up period. |
| Risk difference: 121 fewer infections per 1000 (176 fewer to 384 more) | ||||||
| Resolution infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Adverse events Up to 24 weeks' follow‐up | 388 per 1000 | 373 per 1000 (241 to 574) | RR 0.96 (0.62 to 1.48) | 134 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether use of an antimicrobial dressing affects the risk of adverse events compared with use of a non‐antimicrobial dressing over a medium‐term follow‐up period. |
| Risk difference: 16 fewer adverse events per 1000 (147 fewer to 186 more) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias due to one study (with the highest weighting in the meta‐analysis) being at unclear risk of selection bias and three studies being at high risk of performance bias (36% weighting in analysis), although the studies were at unclear or low risk of detection bias for this outcome. | ||||||
| Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with non‐antimicrobial treatment | Risk with topical antimicrobial treatment | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 241 per 1000 | 679 per 1000 (135 to 1000) | RR 2.82 (0.56 to 14.23) | 112 participants (3 studies) | ⊕⊝⊝⊝ | The average effect of antimicrobial agents compared with non‐antimicrobial treatment is uncertain over a medium‐term follow‐up period. |
| Risk difference: 438 more healed wounds per 1000 (106 fewer to 1000 more) | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 368 per 1000 | 427 per 1000 (199 to 925) | RR 1.16 (0.54 to 2.51) | 40 participants (1 study) | ⊕⊕⊝⊝ | It is unclear whether use of an antimicrobial topical agent has an effect on risk of infection over a medium‐term follow‐up period. |
| Risk difference: 59 more resolved infections per 1000 (169 fewer to 556 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 81 participants (2 studies) | ⊕⊝⊝⊝ | 2 studies reported adverse event data. We were unable to extract per‐participant data for 1 study. The second study stated that no adverse events were reported in each arm. We judged this as very low‐certainty evidence. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias with two studies at high risk of detection bias, which is of particular concern when healing is being assessed, and one study not accounting for a small number of participants with multiple ulcers in their trial. Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for inconsistency: one small study reported all wounds healed in one arm and few wounds healed in the other. These data are adding heterogeneity to the analysis. | ||||||
| One topical antimicrobial agent compared with another topical antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical antimicrobial agent | Risk with alternative topical antimicrobial agent | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | Data were not pooled due to the 3 studies evaluating different interventions. | N/A | 85 participants (3 studies) | ⊕⊝⊝⊝ | It is generally uncertain whether 1 topical treatment has an increased likelihood of healing compared with the alternative treatment. We judged this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias. | |
| Incidence of infection Up to 24 weeks' follow‐up | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 625 per 1000 | 906 per 1000 (606 to 1000) | RR 1.45 (0.97 to 2.17) | 37 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether 1 specific type of topical antimicrobial agent has a different effect on resolution of infection than another over a medium‐term follow‐up period. |
| Risk difference: 281 more resolved infections per 1000 (19 fewer to 731 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 41 participants (1 study) | ⊕⊝⊝⊝ | The 1 study noted that no events were reported in either group. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded for risk of performance and detection bias. | ||||||
| Topical antimicrobial agent compared with systemic antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic antibiotic agent | Risk with topical antimicrobial agent | |||||
| Proportion of wounds healed | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | 333 per 1000 | 503 per 1000 (303 to 830) | RR 1.51 (0.91 to 2.49) | 102 participants (2 studies) | ⊕⊝⊝⊝ | It is uncertain whether the effects of topical antimicrobial treatment on resolution of infection differ from those of systemic antibiotics. |
| Risk difference: 170 more resolved infections per 1000 (30 fewer to 497 more) | ||||||
| Adverse events | 450 per 1000 | 409 per 1000 (351 to 477) | RR 0.91 (0.78 to 1.06) | 937 participants (4 studies) | ⊕⊕⊕⊝ moderate2 | On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments compared here. |
| Risk difference: 40 fewer adverse events per 1000 (99 fewer to 27 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for risk of performance bias. | ||||||
| Topical antimicrobial agent compared with growth factor | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with growth factor | Risk with topical antimicrobial | |||||
| Proportion of wounds healed | 800 per 1000 | 400 per 1000 (224 to 712) | RR 0.50 (0.28 to 0.89) | 40 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether treatment with growth factor affects the risk of healing when compared with antiseptic dressing. |
| Risk difference: 400 fewer resolved infections 576 fewer to 88 fewer | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Adverse events | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision: small sample size and small number of events ‐ optimal information size not met and results are fragile. Downgraded twice for risk of performance and attrition bias. | ||||||
| Clinical manifestation of infection | PEDIS grade | IDSA infection |
| No symptoms or signs of infection | 1 | Uninfected |
| Infection present, as defined by the presence of at least 2 of the following items:
| ||
| Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). If erythema, must be > 0.5 cm to ≤ 2 cm around the ulcer. | 2 | Mild |
| Local infection (as described above) with erythema > 2 cm, or involving structures deeper than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic arthritis, fasciitis), and no systemic inflammatory response signs (as described below) | 3 | Moderate |
| Local infection (as described above) with the signs of SIRS, as manifested by ≥ 2 of the following:
| 4 | Severe* |
| Abbreviations: IDSA, Infectious Diseases Society of America; PaCO2, partial pressure of arterial carbon dioxide; PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation; SIRS, systemic inflammatory response syndrome *Ischaemia may increase the severity of any infection, and the presence of critical ischaemia often makes the infection severe. Systemic infection may sometimes manifest with other clinical findings, such as hypotension, confusion, vomiting, or evidence of metabolic disturbances, such as acidosis, severe hyperglycaemia, and new‐onset azotaemia. | ||
| Product and formulations | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Acetic acid | 0.25%, 0.5%, and 1% solutions | Bactericidal against most gram‐positive and gram‐negative organisms, including Pseudomonas aeruginosa | Inexpensive; shown to eliminate P aeruginosa colonisation from burn | Cytotoxic in vitro although maybe not in vivo; limited activity against biofilm | $ | No longer as widely used as in the past |
| Cadexomer iodine | Gel,c ointment, and dressing | Polysaccharide starch lattice; active agent is slowly released free iodine; broad spectrum of activity (same as iodine) | Reduced local toxicity compared to iodine; elemental iodine released on exposure to exudate | Application may cause stinging and erythema, but less tissue damage than other iodine products; effect may not persist, and efficacy may be reduced in body fluids. | $$ | Indicated for use in cleaning wet ulcers and wounds and reducing microbial load in the wound environment |
| Cetrimide | Solution, 40% | Active against bacteria and fungi; not active against P aeruginosa | May be less toxic to wound tissues than other antiseptics | May be corrosive and is potentially harmful if swallowed | $ | Not available in the USA |
| Chlorhexidine gluconate | Solution, 2% and 4%; liquid, 2% and 4%; hand rinse, 0.5%; wipes, 0.5%; sponge/brush, 4%; and foam, 4% | Active against gram‐positive bacteria (e.g. Staphylococcus aureus) and gram‐negative bacteria, including P aeruginosa | Persistent activity up to 6 h after application; few adverse effects | Hypersensitivity, including anaphylaxis, generalised urticaria, bronchospasm, cough, dyspnoea, wheezing, and malaise; may cause serious injury to the eye and middle ear; avoid contact with face or head; some resistance reported | $ | 2% chlorhexidine indicated as surgical hand scrub, hand wash, skin and wound cleanser; polyhexanide is a similar, newer biguanide. |
| Hexachlorophene | Liquid, 3%; foam, 0.23% with 56% alcohol | Biguanide that is bacteriostatic against Staphylococcus species and other gram‐positive bacteria | May retain residual effect on skin for several days | Rapidly absorbed and may result in toxic blood levels; application to burns has resulted in neurotoxicity and death; may cause central nervous system stimulation and convulsions, dermatitis, and photosensitivity reactions | $$$ | Not recommended for routine use on wounds due to potential toxicity |
| Iodine compounds and iodine tincturec | Solution (aqueous) 2% and 2.4%; and tincture (44% to 50% alcohol) 2% and 2.4% | Microbicidal against bacteria, fungi, viruses, spores, protozoa, and yeasts | Broad spectrum | Highly toxic if ingested or significantly absorbed; do not use with occlusive dressings; causes pain and stains skin and clothing; use cautiously in people with thyroid disorders | $ | Iodine compounds are now rarely used for wound management; cadexomer iodine and povidone iodine products are less toxic. |
| Povidone iodinec | Ointment, 1%, 4.7%, 10%; solution, 1% and 10%; also wash, scrub, cleanser, gel, aerosol, gauze pad, swab, and other forms | Broad spectrum includes S aureus and enterococci; active ingredient is liberated free iodine; shares spectrum but is less potent than iodine | Less irritating to skin and allergenic than iodine. Can be covered with dressings. Clinically significant resistance very rare | Antibacterial action requires at least 2 min contact; may cause stinging and erythema; effect may not persist, and efficacy may be reduced in body fluids; prolonged use may cause metabolic acidosis; stains skin and clothing; possible interaction with starches in dressings | $ | Indicated for perioperative skin cleansing and for cleansing and prevention of infection in superficial burns, incisions, and other superficial wounds |
| Sodium hypochlorite (Dakin’s solution and EUSOL) | Solution, 0.0125%, 0.125%, 0.25%, and 0.5% | Vegetative bacteria, viruses, and some spores and fungi | Inexpensive | No known systemic toxicity. May require prolonged contact for antibacterial action; inactivated by pus; toxic to fibroblasts and keratinocytes, and may cause pain or lyse blood clots | $ | A concentration of 0.025% is both bactericidal and non‐toxic to tissues (Heggers 1991). |
| Hydrogen peroxidec | Solution, 1% and 3%; and cream, 1% | Oxidizing agent active against many gram‐positive and gram‐negative bacteria | Broad‐spectrum, bactericidal, inexpensive; no known 1q11 | May cause some discomfort | $ | Commonly used, but few clinical studies |
| Silver nitrate | Solution 0.5%, 10%, 25%, and 50%; ointment, 10%; and swabs, 25% to 50% | Silver ions are bactericidal against a broad spectrum of gram‐positive and gram‐negative bacteria. | Low cost; easily applied | Painful on application; stains tissues; may delay healing; concentrations 10.5% cause cauterisation; inactivated by wound exudates and chlorine | $ | Previously widely used, but now largely replaced by other compounds, including newer silver dressings |
| Silver dressings | At least 6 approved products with different properties | Slowly released silver ions have broad spectrum, including MRSA and VRE. | Provide sustained levels of active silver ions; microbial resistance is rare; less painful and few adverse effects than silver nitrate; variety of products adaptable to different types of wounds; infrequent application required | Levels of silver ions at wound interface not well defined; may cause silver staining of tissues; may delay epithelialisation; relatively expensive; few published comparative trials | $$ | Should not substitute for non‐medicated dressings for uninfected wounds; may be useful for subclinically infected, highly colonised wounds or for wounds being prepared for skin grafting |
| Abbreviations: EUSOL, Edinburgh University Solution of Lime; MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococci. aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. | ||||||
| Product and | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Bacitracin c | Ointment, 500 units/g; and powder combinations with neomycin, polymyxin B, and zinc | Many gram‐positive organisms, including aerobic staphylococci and streptococci, corynebacteria, anaerobic cocci, and clostridia; inactive against most gram‐negative organisms | Activity not impaired by blood, pus, necrotic tissue, or large bacterial inocula; resistance is rare but increasing among staphylococci; no cross‐resistance with other antibiotics; minimal absorption | May cause allergic reactions, contact dermatitis, and (rarely) anaphylactic reactions; may lead to overgrowth of drug‐resistant organisms, including fungi | $ | Widely used for many years; indicated for prevention of infection in minor skin wounds |
| Fusidic acid | Cream, 2%; ointment, 2%; and gel, 2% | Staphylococcus aureus, streptococci (in topical concentrations), corynebacteria, and clostridia | Penetrates intact and damaged skin as well as crust and cellular debris | Occasional hypersensitive reactions; resistance among staphylococci is emerging; must apply 3 times daily | $$ | Not available in the USA |
| Gentamicin | Cream, 0.1%; and ointment, 0.1% | Streptococci, staphylococci, Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae | Broad spectrum; inexpensive | Must be applied 3 to 4 times daily; may drive resistance to an agent used systemically | $ | Indicated for primary skin infections (pyodermas) and secondary skin infections, including infected excoriations, and for bacterial superinfections |
| Mafenide acetate | Solution, 5%; and cream, 85 mg/g | A sulfonamide that is bacteriostatic against many gram‐negative organisms, including P aeruginosa, and some gram‐positive organisms, but minimal activity against staphylococci and some obligate anaerobes | Remains active in the presence of pus and serum, and its activity is not affected by acidity of environment | Systemic absorption may occur; drug and metabolites may inhibit carbonic anhydrase, potentially causing metabolic acidosis; use cautiously in patients with renal impairment; pain on application; hypersensitive reactions. | $$$ | Indicated as adjunctive therapy in second‐ and third‐degree burns; may be used in rapidly progressing bacterial necrotising fasciitis; limited use in other wounds |
| Metronidazole | Cream, 0.75%; gel, 1%; lotion, 0.75% | Many clinically important anaerobic bacteria | May reduce odour associated with anaerobic infections; application only 1 to 2 times daily | Relatively expensive; systemic formulations available; could drive resistance to these | $–$$ | Indicated for inflammatory papules and pustules of rosacea |
| Mupirocin and mupirocin calcium | Ointment, 2%; for mupirocin calcium, cream, 2.15%; and nasal ointment, | Gram‐positive aerobes, including S aureus (most MRSA), Staphylococcus epidermidis, Staphylococcus saprophyticus, and streptococci (groups A, B, C, and G) but not enterococci, some gram‐negative aerobes (not P aeruginosa), corynebacteria, and obligate anaerobes | Minimal potential for allergic reactions | Rare local burning and irritation; applying ointment to large wounds in azotaemic patients can cause accumulation of polyethylene glycol; long‐term use can lead to resistance among staphylococci, which is increasing | $$ | Indicated for topical treatment of impetigo and eradication of nasal colonisation with S aureus |
| Neomycin sulfatec | Powder; cream, 0.5%; combinations with polymyxin B and pramoxine, and ointment, 0.5%; combinations with bacitracin, polymyxin B, lidocaine, and pramoxine | Good for gram‐negative organisms but not P aeruginosa; active against some gram‐positive | Low cost; applied only 1 to 3 times daily; may | Topical powder in wound irrigating solution | $ | Use of topical powder alone or in solution is not recommended; cream and ointment, in combination with other agents, are indicated for prevention of infection in minor skin injuries. |
| Nitrofurazone | Solution, 0.2%; ointment, 0.2%; and cream, 0.2% | Broad gram‐positive and gram‐negative activity, | Used mainly for burn wounds | Hypersensitive reactions; polyethylene glycols (in some formulations) may be absorbed and can cause problems in azotaemic patients | $$ | Indicated as adjunctive to prevent infections in people with second‐ and third‐degree |
| Polymyxin Bc | Cream, 5000 units/g or | Bactericidal against many gram‐negative organisms, | Inexpensive | Some hypersensitive and neurological or | $ | Only available in combination with other agents, including bacitracin and neomycin; |
| Retapamulin | Ointment, 1% | Active against staphylococci (but uncertain | May be active against some mupirocin‐resistant S aureus strains; broader activity than mupirocin | Not evaluated for use on mucosal surfaces; may cause local irritation | $$$ | Indicated for impetigo due to S aureus (methicillin‐susceptible only) or Streptococcus pyogenes |
| Silver sulphadiazine | Cream, 1% | A sulfonamide; the released silver ions are the primary active ingredient; active against many gram‐positive and gram‐negative organisms, including P aeruginosa | Applied only once or twice daily; soothing | Potential cross‐reaction with other sulphonamides; may rarely cause skin staining | $ | Indicated as adjunctive treatment to prevent |
| Sulfacetamide Na+ | Lotion, 10% | Bacteriostatic against many gram‐positive and gram‐negative pathogens | Broad spectrum; can be combined with sulphur | Systemic absorption and rarely severe side | $$$ | Indicated for secondary bacterial skin infections |
| There are no published studies supporting the use of topical erythromycin, clindamycin, aminoglycosides other than neomycin, gramicidin, or tetracyclines for treating chronically infected wounds. Abbreviations: FDA, US Food and Drug Administration; MRSA, methicillin‐resistant Staphylococcus aureus. aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. | ||||||
| Title (comparator) | Current status | Relevant outcomes listed | Database | Results (# enrolled) | Listed contact | Company and any further information received |
| Phase IIa Randomised, Placebo Controlled Trial to Investigate Antimicrobial Photodynamic Therapy in Chronic Leg Ulcers and Diabetic Foot Ulcers (placebo = “cream”) | Prematurely ended (date unclear) | Photodynamic therapy using the combined effect of 3,7‐bis(N,N‐dibutylamino) phenothiazin‐5‐ium bromide (PPA904) and light; measure reduction of bacterial content of diabetic foot ulcers | ClincialTrialsRegister.eu EudraCT number: 2005‐001363‐58 | None (not listed) | None listed. | Photopharmacia |
| Pexiganan Versus Placebo Control for the Treatment of Mild Infections of Diabetic Foot Ulcers (OneStep‐1 and 2) | Completed (August 2016) | 1°: clinical response (resolution of infection); 2°: microbiological response; safety | ClinicalTrials.gov; NCT01594762 | No results (200 for each of the 2 trials) reported on website. | Robert Deluccia, Dipexium | Dipexium Pharmaceuticals, Inc. Multicentre study; all sites outpatient centre in USA |
| Comparison of Resin Salve and Octenidine in Patients with Neuropathic Diabetic Foot Ulcers (comparator: octenidine dihydrochloride‐impregnated gauze) | Completed (May 2015) | Investigate healing rate and healing time of neuropathic diabetic foot ulcer in people suffering from infected fore‐ or mid‐foot ulceration. 2°: eradication of bacteria; wound healing and infection | ClinicalTrials.gov; NCT02169167 | No results on website (n = 35) (see addendum in “comments”) | Janne J Jokinen | Salve prepared from Norway spruce (Repolar Ltd.) |
| Clinical Outcomes for Diabetic Foot Ulcers Treated With Clostridial Collagenase (SANTYL®) Ointment or With a Comparator Product Containing Silver (investigator choice of silver) | Running until January 2017 (last updated November 2016) | Randomly assigned to apply SANTYL or a topical treatment containing silver to their to foot ulcer. 1°: mean change in ulcer area at end of treatment; 2°: target ulcer infection rate | ClinicalTrials.gov; NCT02581488 | No results (102) | Jaime E Dickerson, PhD (Smith & Nephew) | (Smith & Nephew) Information from the sponsor received end of December 2016 stated that the trial is not yet complete but last participant out will be achieved in the next week. The trial enrolled its target number of participants, with the last participant completed December 2016. The evaluability will be carried out prior to the scheduled database lock in January 2017. As intention‐to‐treat is the analysis set for primary inference, it is anticipated that all participants will be included. Final study report is timed for April 2017 (15 December 2016). Waiting for further information to assess eligibility for review |
| Randomized, Controlled Study to Investigate the Efficacy and Safety of a Topical Gentamicin‐Collagen Sponge in Combination with Systemic Antibiotic Therapy in Diabetic Patients With a Moderate or Severe Foot Ulcer Infection | Recruiting (as of September 2013) | 1°: "clinical cure" at the test of cure; 2°: clinical response; time to clinical cure; eradication of baseline pathogen | ClinicalTrials.gov; NCT01951768 | No results (estimate 144) | Ilker Uckay, MD; Hospital of the University of Geneva | Innocoll, Inc. |
| Comparison of the Efficacy of Standard Treatment Associated with Phage Therapy Versus Standard Treatment Plus Placebo for Diabetic Foot Ulcers Monoinfected by Staphylococcus aureus: a Randomized, Multi‐centre, Controlled, 2‐parallel‐group, Double‐blind, Superiority Trial | Starting January 2017 | 1°: reduction in wound surface area; 2°: safety; changes in resistance and virulence of S aureus isolates; production of anti‐phage antibodies | ClinicalTrials.gov; NCT026647401 | No results (estimate 60) | Albert Sotto, MD, PhD +33.(0)6.09.56.66.55 | Centre Hospitalier Universitaire de Nīmes; Pherecydes Pharma. Per correspondence from Prof Sotto on 8 January 2017, National Agency for the Safety of Medicines and Health Products requested “pre‐clinical phase complements”, causing a postponement of the start of the clinical trial. |
| A Phase I/IIa, Randomized Double Blind, Placebo‐Controlled, Dose Escalating Study to Evaluate the Safety and Tolerability of Topically Applied Bisphosphocin Nu‐3 on Infected Diabetic Ulcers of Subjects With Type I or II Diabetes Mellitus (placebo) | Enrolling by invitation only (last verified April 2016) | Diabetic foot ulcers; infection localised to area of ulcer and mild. 1° outcome: treatment‐related adverse events, safety 2°: microbiological activity evaluated by wound assessments, presence of pathogenic bacteria | ClinicalTrials.gov; NCT02737722 | No results (estimate 30) | Paul DiTullio, MSc | Lakewood‐Amedex, Inc. |
| A Phase II, Randomized, Parallel, Double‐blind, Placebo‐controlled Study to Assess Prevention of Infection Using a Topical Gentamicin‐Collagen Sponge in Diabetic Patients With Uninfected Lower Extremity Skin Ulcers (placebo sponge) | Terminated (last verified March 2012) | 1° outcome: uninfected diabetic foot ulcers that remain free of signs/symptoms of infection to end of study 2°: days to wound closure; time to any signs/symptoms of infection; decrease in wound area; pathogen burden in infected wounds | ClinicalTrials.gov; NCT00658957 | No results (49) | David Prior, PhD; Chesapeake Foot and Ankle Center, Pasadena (MD), USA | Innocoll Pharmaceuticals |
| A Phase 3 Randomized, Placebo‐Controlled, Blinded Study to Investigate the Safety and Efficacy of a Topical Gentamicin‐Collagen Sponge in Combination With Systemic Antibiotic Therapy in Diabetic Patients With an Infected Foot Ulcer (COACT 1 and 2) (placebo is no sponge) | Last updated June 2016 | Sponge is adjunctive treatment to systemic antibiotic therapy. 1° outcome: per cent of participants with a clinical outcome of clinical cure (resolution of all clinical signs and symptoms of infection) ˜10 days after end of treatment; 2° outcomes: baseline pathogen eradication; re‐infection; time to clinical cure; amputation; ulcer closure | ClinicalTrials.gov: NCT02447172 | No results posted. | Nigel Jones, VP, Global Clinical Operations, Innocoll Pharmaceuticals | Innocoll Pharmaceuticals |
| Study of the Efficacy of Topical Application of Royal Jelly and Panthenol (PedyPhar® Ointment) on the Diabetic Foot Ulcers, an Open Label, Randomized, Non‐placebo‐controlled Study (active comparator panthenol ointment) | Terminated; (last updated February 2015) | Diabetic foot ulcers at any stage after proper surgical treatment (if needed) 1° outcome: healing of ulcer; 2°: reduction of infection in ulcer site; local reaction possibly related to study drug | ClinicalTrials.gov; NCT01531517 | No results (estimate 120; 47 enrolled) | (?) | European Egyptian Pharmaceutical Industries |
| Platelet Rich Fibrin in Combination With Topical Antibiotics or Antiseptics in the Treatment of Chronic Wounds ‐ a Prospective, Randomized, Active Controlled, Double Blind Pilot Trial With an Observer‐blinded Control Group (3 platelet rich fibrin arms & 1 active comparator (Acticoat)) | Recruiting (last verified January 2016) | People with infected chronic wounds (unclear if diabetic foot) 1° outcome: reduction of wound area; 2°: number requiring systemic antimicrobial therapy; C‐reactive protein level; wound volume; occurrence of drug‐resistant bacteria | ClinicalTrials.gov; NCT02652169 | No results (estimate 120) | Florian Thalhammer, Medical University of Vienna; 0043140400 ext 44400; [email protected] | Medical University of Vienna |
| Double Blind, Randomized, Placebo Controlled Clinical Trial for the Treatment of Diabetic Foot Ulcers, Using a Nitric Oxide Releasing Patch: PATHON | Completed (last verified November 2012) | 1° outcome: per cent reduction in ulcer size; 2°: complete cure of any infection; development of infection during treatment; adverse events | ClinicalTrials.gov; NCT00428727 | No results (?) | Fundación Cardiovascular de Colombia | (?) |
| A Phase I/II, Open Label, Controlled Study to Evaluate the Safety and Efficacy of AppliGel‐G (Gentamicin Sulfate Topical Gel) for Treatment of Mild to Moderately Infected Diabetic Foot Ulcers in Patients With Type 1 and Type 2 Diabetes (comparator oral ciprofloxacin and doxycycline alone) | Terminated (last verified May 2015) | For mild to moderately infected diabetic foot ulcers 1°: complete wound clearing of infection 2°: incidence infection cleared; wound volume and area change | ClinicalTrials.gov; NCT02036528 | No results | Royer Biomedical, Inc. | Royer Biomedical, Inc. |
| A Randomised, Double‐blind, Dose‐response, Placebo‐controlled, Multicenter, Phase IIA Clinical Study to Evaluate the Efficacy and Safety of Topical Application of G.68.y/EtOH in Patients with Type 1 or Type 2 Diabetes With Infected Foot Ulcers (placebo topical gel) | Completed | Enrolling patients with infected “grade 2 PEDIS” diabetic foot ulcers 1°: reduction of bacterial load 2°: maintenance of efficacy; tolerability and safety | EudraCT number: 2010‐019598‐13 | No results (plan for 60) | Molteni | |
| Trial to Assess Safety and Efficacy of Topical MBN‐101 (BisEDT ) in Patients With Moderate/ Severe Diabetic Foot Infections (placebo – vehicle‐controlled) | Not yet open for participant recruitment (last update March 2016) | Part I, participants will be enrolled into 1 of 3 escalating dose cohorts at a ratio of 3:1 (active to placebo). In Part II, participants will be randomised in a 1:1 ratio (active to placebo) based on the optimal dose demonstrated in Part I. People with infected foot ulcer | ClinicalTrials.gov; NCT02723539 | No results (plan for 88) | Department of Vascular Surgery, Rigshospitalet Copenhagen, Denmark, 2100 | Microbion Corporation |
| Abbreviations: PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation | ||||||
| Intervention 1 | Intervention 2 | Foot ulcer grade | Infection status at baseline | Follow‐up | Review‐relevant outcomes with reportable data | |
| Group 1: (n = 30) Pyodine bath and saline and vaseline gauze dressing | Group 2: (n = 30) Phenytoin powder | Grade I or II | Not reported | 8 weeks | None reported | |
| Group 1: (n = 19) Gentamicin solution | Group 2: (n = 22) Cadexomer iodine ointment | Grade I or II | Not reported | 12 weeks |
| |
| Group 1: (n = 19) Standard care | Group 2: (n = 21) Chloramine plus standard care | Not reported | Infected | 24 weeks |
| |
| Group 1: (n = 10) Saline solution | Group 2: (n = 10) Super‐oxidised aqueous solution | Grade I or II | Not infected | 4 weeks |
| |
| Group 1: (n = 15) Foam dressing | Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing | Grade II or III | Not infected | 14 weeks |
| |
| Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improve microcirculation | Group 2: (n = 40) Silver ion dressing plus standard care | Not reported | Not reported | 4 weeks |
| |
| Group 1: (n = not reported) Iodine gauze | Group 2: (n = not reported) Hydrofiber dressing with silver | Ulcers with bone and tendon exposure | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Saline dressing | Group 2: (n = 195) Honey dressing | Grade I or II | Not reported | 17 weeks |
| |
| Group 1: (n = 20) Silver sulphadiazine cream | Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream | Grade I or II | Not reported | 6 weeks |
| |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing | Group 3: (n = 106) Iodine‐containing dressing | Not reported | Not reported | 24 weeks |
| |
| Group 1: (n = 67) Calcium‐alginate dressing | Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver | Grade I or II | Mixed infected and not infected | 8 weeks |
| |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered further) Group 2: (n = 20) Recombinant human platelet‐derived growth factor | Group 3: (n = 20) Antiseptic treatments (EUSOL, hydrogen peroxide, and povidone iodine) | Grade III or IV | Not reported | More than 8 weeks |
| |
| Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day Group 2: (n = 21) Super‐oxidised aqueous solution (topical Microcyn) alone (not considered) | Group 3: (n = 21) super‐oxidised aqueous solution (topical Microcyn) therapy plus 750 mg levofloxacin once per day | Eligible foot ulcers involved skin and deeper soft tissue | Infected | 4 weeks |
| |
| Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets | Full‐thickness wounds | Infected | Up to 42 days |
| |
| Group 1: (n = 38) Systemic antibiotic therapy alone | Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 16) Povidone iodine and saline | Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution | Not reported | Infected | 20 weeks |
| |
| Group 1: (n = 25) Conventional treatment (no further details translated) | Group 2: (n = 25) Zinc hyaluronate | Not reported | Unclear | 20 weeks |
| |
| (30 participants randomised, but number in each group not specified) | Group 1: Standard‐dressing group (povidone iodine solution 10%) (n not reported) | Group 2: Honey dressing group (n not reported) | Grade II | Not reported | Not reported | No useable data |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) | Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | 16 weeks |
| |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing | Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Grade I and II | Not reported | Not reported |
| |
| Group 1: (n = 19) Polyherbal formulation | Group 2: (n = 19) silver sulphadiazine cream | Grade I, II, and III | Unclear | 20 weeks | No useable data | |
| Abbreviations: EUSOL, Edinburgh University Solution of Lime | ||||||
| Resolution of infection | Incidence of wound infection | Time to healing | Proportion of wounds healed | Microbial counts | Health‐related quality of life | Need for surgical resection, including partial or complete lower limb amputation | Safety (adverse events) | |
| Group 1: (n = 30) Povidone iodine bath and saline Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder plus povidone iodine bath and saline Vaseline gauze dressing Not infected at baseline | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Gentamicin solution Group 2: (n = 22) Cadexomer iodine ointment Baseline infection status not reported. | Not reported | Not reported | Not reported | Group 1: 2/18 Group 2: 5/17 | Not reported | Not reported | Surgical resection was reported: Group 1: 5/19 Group 2: 3/22 | Study reports that no adverse reactions related to the topical treatment were documented. |
| Group 1: (n = 19) Standard care alone Group 2: (n = 21) Chloramine plus standard care Infected at baseline | Group 1: 7/15 Group 2: 9/13 | Not reported | Time‐to‐event data presented with no reported hazard ratio. Given the small number of participants and events, no further attempts were made to calculate time‐to‐event values. | Healed at 24 weeks Group 1: 9/17 Group 2: 10/17 | Not reported | Not reported | Vascular procedure or amputation Group 1: 3/17 Group 2: 5/17 | Adverse event data reported but unable to get a per‐participant value, as it is noted that some participants had more than 1 event. |
| Group 1: (n = 10) Saline solution Group 2: (n = 10) Super‐oxidised aqueous solution Not infected at baseline | Not reported | Not reported | Not reported | Study notes that 15% of the study ulcers were healed, but this information not reported by group. | The bacterial load in the wound bed was defined as scattered (0/+), light (+), medium (++), or heavy (+++). At week 4 there was a reduction of 33% in the bacterial load versus baseline. Figure presented but difficult to interpret data by group. | Not reported | Not reported | No safety concerns were reported in either the super‐oxidised aqueous solution group or the saline group; no adverse reactions were recorded. |
| Group 1: (n = 15) Foam dressing Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing Not infected at baseline | Not reported | Wound infection Group 1: 4/13 Group 2: 0/23 | Not reported | Healed by week 14 Group 1: 4/13 Group 2: 12/23 | Not reported | Not reported | Not reported | Limited details of adverse events (in addition to infection data already recorded). There were no reported adverse events related to the use of collagen/oxidised regenerated cellulose/silver dressing, and 5 cases of adverse events (no further details) related to foam dressing. |
| Group 1: (n = 40) Routine debridement plus standard care Group 2: (n = 40) Silver ion dressing plus standard care Baseline infection status not reported. | Not reported | Not reported | Mean wound healing time in days: Group 1: 47.4 ± 11.5 Group 2: 31.3 ± 8.2 Mean granulation tissue occurrence time in days: Group 1: 10.8 ± 1.9 Group 2: 6.4 ± 0.72 | Group 1: 15/40 Group 2: 24/40 | Not reported | Not reported | Not reported | Not reported |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Treated with normal saline dressing Group 2: (n = 195) Treated with honey dressing | Not reported | Not reported | Median healing time in honey group is 18 days (IQR is 6 to 120), and in the saline group is 29 days (IQR 7 to 120). Data do not seem to have been calculated using correct time‐to‐event approaches and were not considered further. | Group 1: 97/169 Group 2: 136/179 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 20) Silver sulphadiazine Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream Baseline infection status not reported. | Not reported | Not reported | Not reported | Healed by week 6 Group 1: 6/20 Group 2: 8/20 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Baseline infection status not reported. | Not reported | Number of infected ulcers at 24 weeks: not reported by group Study reports the number of episodes of infection listed as serious adverse events, but it is unclear if foot infections, and not clear how many people had how many infection events. | Mean time to healing in days (SD) (fixed at max of 168 days) Group 2: 125.8 (55.9) Group 3: 127.8 (54.2) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed at 24 weeks: Group 2: 46/103 Group 3: 48/106 | Not reported | Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group 1: Physical functioning: 68.9 (19.1). Social functioning: 69.8 (23.5). Well‐being: 50.2 (21.1) Group 2: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well‐being: 53.1 (19.9) Other | Minor amputations (below ankle): Group 2: 1/103 n not clear; assumed to be all participants | Non‐serious adverse events Group 2: 227/103 Group 3: 239/106 Serious adverse events Group 2: 28/103 Group 3: 37/106 Not clear how many participants had how many events, but seems to be more than 1 per person; data not analysed further |
| Group 1: (n = 67) Calcium‐alginate dressing Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver Mixed wound infection status at baseline | Not reported | Group 1: 11/67 Group 2: 8/67 | Time to 100% healing also reported, but this is only for a subset of those that healed, so not a useful pan‐study measure. Not reported Mean time to healing in days Group 1: 52.6 ± 1.8 Group 2: 57.7 ± 1.7 | Number of ulcers healed in 8 weeks | Not reported | Not reported | Not reported | Group 1: 26/67 participants experienced adverse event. Death = 1; Infection = 8. 13 participants discontinued treatment due to adverse event. Group 2: 25/67 participants experienced 1 or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to adverse event. |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered in review) Group 2: (n = 20) Recombinant human platelet‐derived Group 3: (n = 20) Antiseptic dressings | Not reported | Not reported | Mean time to healing in weeks (standard error) Group 1: 6.83 (2.5) Group 2: 7.6 (2.5) Group 3: 6.75 (2.7) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed Group 1: 12/20 Group 2: 16/20 Group 3: 8/20 Review authors calculated figures from graph. | Not reported | Not reported | Not reported | Not recorded |
| Group 1: (n = 21) Levofloxacin plus saline Group 2: (n = 21) Super‐oxidised aqueous solution alone (not considered) Group 3: (n = 25) Levofloxacin plus super‐oxidised aqueous solution Ulcers infected at baseline. | Group 1: 6/21 Group 2: 11/21 Group 3: 11/25 | Not reported | Not reported | Mentioned, but data not presented. | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more event) Group 1: 7/21 Group 2: 7/21 Group 3: 9/25 |
| Group 1: (n = 246) Ofloxacin Group 2: (n = 247) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | See below ‐ results presented by study authors cumulatively for these 2 studies only. | Adverse events (number of participants with > 1 adverse event) Group 1: 109/246 Group 2: 98/247 |
| Group 1: (n = 171) Ofloxacin Group 2: (n = 171) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | Group 1: 9/417 Group 2: 11/418 (cumulative of two RCTs reported in single paper) | Adverse events (number of participants with > 1 adverse event) Group 1: 84/171 Group 2: 76/171 |
| Group 1: (n = 18) Systemic antibiotic therapy alone Group 2: (n = 38) Topical application of the gentamicin‐collagen sponge + systemic antibiotic therapy Ulcers infected at baseline. | Resolution of infection by 7 days Group 1: 7/18 Group 2: 22/38 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more events) Group 1: 5/18 Group 2: 11/38 |
| Group 1: (n = 16) Standard management with chemical antiseptics such as soap or povidone iodine Group 2: (n = 21) Super‐oxidised aqueous solution | Advances from infection to granulating tissue: Group 1: 10/16 Group 2: 19/21 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 25) Conventional treatment (no further details translated) Group 2: (n = 25) Zinc hyaluronate | Not reported/translated | Not reported/translated | Mean time to healing in weeks (not clear if standard deviation or standard error presented) Group 1: Only 2 ulcers healed; no time‐to‐event data reported Group 2: 7.80 (3.49) with all ulcers healing | Group 1: 2/25 Group 2: 25/25 | Not reported/translated | Not reported/translated | Not reported/translated | Not reported/translated |
| Group 1: Standard‐dressing group (povidone iodine solution 10%) Group 2: Honey dressing group 30 participants randomised, but number in each group not specified. | Not reported | Not reported | Time to healing in days Group 1: 15.4 days (range 9 to 36 days) Group 2: 14.4 days (range 7 to 26 days) Comment: mean and range, but no measure of variation provided. Unclear how many participants in each group and how many ulcers healed, thus if this measure is a valid time‐to‐healing measure | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | Data presented as time‐to‐event figure with no further data. Given the small number of participants and events, we have not tried to analyse further. | 16 weeks Group 1: 2/10 Group 2: 6/12 Unclear if ulcers were healed in the same or different participants; for the analysis we have assumed in different participants | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Not reported | Not reported | Not reported | Group 1: 0/2 Group 2: 2/2 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Polyherbal formulation Group 2: (n = 19) Silver sulphadiazine cream | Not reported | Not reported | "Number of days taken for healing of the wound: Group 1: 43.1 ± 26.8 Group 2: 43.6 ± 30.7" Not clear what sort of analysis was conducted | Healing was defined as complete epithelialisation either by secondary intention or by split skin graft. However, figures are not reported. | "the microbiological investigations were not done" | Not reported | Not reported | "There were no adverse events reported in both the groups." |
| Abbreviations: IQR, interquartile range; SD, standard deviation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 945 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.45] |
| 1.1 Short term follow up | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [1.00, 2.57] |
| 1.2 Medium term follow‐up | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.44] |
| 2 Incidence of infection: medium term follow‐up Show forest plot | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.10] |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.72] |
| 4 Adverse events Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: medium term follow‐up Show forest plot | 3 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.56, 14.23] |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.51] |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.47, 5.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Medium term follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Unknown follow‐up period | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.53, 7.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of infection Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.91, 2.49] |
| 1.1 Short‐term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.69, 3.45] |
| 1.2 Medium term follow‐up | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 2 Surgical resection: medium term follow‐up Show forest plot | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.51, 2.91] |
| 3 Adverse events Show forest plot | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| 3.1 Short‐term follow‐up | 3 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 3.2 Medium term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: Medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.28, 0.89] |

