نقش عوامل ضد‐میکروبی موضعی در درمان زخمهای پا در افراد مبتلا به دیابت
چکیده
پیشینه
افراد مبتلا به دیابت در معرض خطر بالای ابتلا به زخمهای پا قرار دارند که اغلب عفونت میکنند. این زخمها، به ویژه هنگامی که عفونی میشوند، منجر به موربیدیتی قابل توجهی میشوند. درمان زخم باید بهمنظور کاهش نشانهها، پیشرفت التیام، و جلوگیری از بروز پیامدهای جانبی، به ویژه قطع عضو اندام تحتانی، انجام شوند. درمان ضد‐میکروبی موضعی در زخمهای پای دیابتی، بهعنوان درمانی برای زخمهای عفونی شده از نظر بالینی، یا برای جلوگیری از عفونت در زخمهای بالینی غیر‐عفونی استفاده شدهاند.
اهداف
ارزیابی اثرات درمان با داروهای ضد‐میکروبی موضعی بر: از بین بردن علائم و نشانههای عفونت؛ التیام زخمهای پای دیابتی عفونی شده؛ و جلوگیری از عفونت و بهبود التیام در زخمهای پای دیابتی بالینی غیر‐عفونی.
روشهای جستوجو
پایگاه ثبت تخصصی گروه زخم در کاکرین؛ CENTRAL؛ Ovid MEDLINE؛ Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations)؛ Ovid Embase؛ و EBSCO CINAHL Plus را در آگوست 2016 جستوجو کردیم. همچنین در پایگاه ثبت کارآزماییهای بالینی برای یافتن مطالعات در حال انجام و منتشر نشده جستوجو کرده، و فهرست منابع را برای شناسایی مطالعات بیشتر کنترل کردیم. هیچگونه محدودیتی را از نظر زبان، تاریخ انتشار، یا شرایط مطالعه اعمال نکردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده انجام شده را در هر محیطی (بستری یا سرپایی) وارد کردیم که به مقایسه درمان موضعی با هر نوع داروی ضد‐میکروبی جامد یا مایع (به عنوان مثال، کرم، ژل، پماد)، از جمله ضد‐عفونی کنندهها (آنتیسپتیکها)، آنتیبیوتیکها، و پانسمانهای ضد‐میکروبی، در افراد مبتلا به دیابت ملیتوس پرداختند که مبتلا به زخم یا زخم باز پا، عفونی شده از نظر بالینی یا غیر‐عفونی، بودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم انتخاب مطالعه، ارزیابی «خطر سوگیری (bias)» و استخراج دادهها را انجام دادند. اختلافات اولیه از طریق بحث و تبادل نظر، یا در صورت لزوم با انتخاب نویسنده سوم مطالعه حل شد.
نتایج اصلی
ما 22 کارآزمایی را یافتیم که با مجموع بیش از 2310 شرکتکننده (یک مطالعه تعداد شرکتکنندگان را گزارش نکرد) معیار ورود ما را داشتند. مطالعات وارد شده اغلب تعداد کمی شرکتکننده (از 4 تا 317) و دورههای پیگیری نسبتا کوتاهی داشتند (4 تا 24 هفته). در ابتدای کار، شش کارآزمایی فقط افراد مبتلا به زخمهایی را انتخاب کردند که از نظر بالینی عفونی بودند؛ یک کارآزمایی افراد مبتلا به زخمهای عفونی و غیر‐عفونی را انتخاب کرد؛ دو کارآزمایی افراد مبتلا به زخمهای غیر‐عفونی را وارد کردند؛ و 13 مطالعه باقی مانده وضعیت عفونت را گزارش نکردند.
مطالعات وارد شده درمانهای ضد‐میکروبی موضعی مختلفی را به کار گرفتند، از جمله پانسمانهای ضد‐میکروبی (مانند نقره، یدید (iodides))، محلولهای آب سوپر اکسید شده (super‐oxidised aqueous solutions)، هیالورونات زینک (zinc hyaluronate)، سولفادیازین نقره (silver sulphadiazine)، ترتینوئین (tretinoin)، کرم پکسیگانان (pexiganan cream)، و کلورامین (chloramine). پنج مقایسه زیر را بر اساس مطالعات وارد شده انجام دادیم:
پانسمانهای ضد‐میکروبی در مقایسه با پانسمانهای غیر ضد‐میکروبی: دادههای ترکیب شده از پنج کارآزمایی با مجموع 945 شرکتکننده (بر اساس میانگین تاثیر درمان به دست آمده از مدل اثر تصادفی) نشان میدهند، هنگامی که زخمها با پانسمانهای ضد‐میکروبی درمان شوند، در مقایسه با پانسمانهای غیر ضد‐میکروبی، زخمهای بیشتری ممکن است التیام یابند: خطر نسبی (RR): 1.28؛ 95% فاصله اطمینان (CI): 1.12 تا 1.45. این نتایج متناظر بود با 119 رویداد التیام یافته در بازوی پانسمان ضد‐میکروبی در هر 1000 شرکتکننده (95% CI؛ 51 تا بیش از 191). این شواهد با قطعیت پائین هستند (دو سطح به علت خطر سوگیری کاهش یافت). شواهد مربوط به عوارض جانبی یا سایر پیامدها نامطمئن بودند (شواهد با اطمینان بسیار پائین، اغلب به علت خطر سوگیری و عدم دقت کاهش یافت).
درمانهای موضعی ضد‐میکروبی (بدون پانسمان) در مقایسه با درمان موضعی غیر ضد‐میکروبی (بدون پانسمان): در این مقایسه چهار کارآزمایی با 132 شرکتکننده وجود داشت که به صورت متفاوتی به برآورد دادههای پیامد کمک میکردند. شواهد بهطور کلی قطعیت پائین یا بسیار پائینی داشتند، و فواصل اطمینان شامل منفعت و آسیب بودند: نسبت زخمهای بهبود یافته: RR: 2.82؛ 95% CI؛ 0.56 تا 14.23؛ 112 شرکتکننده؛ 3 کارآزمایی؛ شواهد با اطمینان بسیار پائین؛ بهبود عفونت RR: 1.16؛ 95% CI؛ 0.54 تا 2.51؛ 40 شرکتکننده؛ 1 کارآزمایی؛ شواهد با اطمینان پائین؛ جراحی قطع عضو RR: 1.67؛ 95% CI؛ 0.47 تا 5.90؛ 40 شرکتکننده؛ 1 کارآزمایی؛ شواهد با اطمینان پائین؛ و وجود یک عارضه جانبی (بدون عوارض در بازوها؛ 81 شرکتکننده؛ 2 کارآزمایی؛ شواهد با اطمینان بسیار پائین.
مقایسه درمانهای ضد‐میکروبی موضعی مختلف: هشت مطالعه را با مجموع 250 شرکتکننده وارد کردیم، اما همه مقایسهها متفاوت بودند و نتوانستیم هیچ دادهای را به درستی ترکیب کنیم. دادههای گزارش شده از پیامد محدود بودند و در مورد اثرات نسبی داروهای ضد‐میکروبی موضعی مربوط به هر یک از پیامدهای مطالعه مروری ما در این مقایسه، که شامل التیام زخم، برطرف شدن عفونت، رزکسیون جراحی، و عوارض جانبی بود، نامطمئن بودیم (تمام شواهد دارای اطمینان بسیار پائین).
ضد‐میکروبهای موضعی در مقایسه با آنتیبیوتیکهای سیستمیک: چهار مطالعه را با مجموع 937 شرکتکننده وارد کردیم. این مطالعات دادههای مربوط به التیام زخم را گزارش نکردند، و شواهد مربوط به اثرات نسبی بهبود عفونت در زخمهای عفونی و رزکسیون جراحی نامطمئن بودند (قطعیت بسیار پائین). بهطور متوسط، احتمالا تفاوت کمی از نظر خطر عوارض جانبی بین درمانهای ضد‐میکروبی موضعی در مقایسه با آنتیبیوتیکهای سیستمیک وجود دارد: (RR: 0.91؛ 95% CI؛ 0.78 تا 1.06؛ شواهد با اطمینان متوسط؛ یک سطح به دلیل تناقض کاهش یافت).
داروهای ضد‐میکروبی موضعی در مقایسه با فاکتور رشد: یک مطالعه را با 40 شرکتکننده انتخاب کردیم. تنها پیامد مرتبط با مطالعه مروری گزارش شده، تعداد زخمهای بهبود یافته بود، و این دادهها نامطمئن بودند (شواهد با اطمینان بسیار پائین).
نتیجهگیریهای نویسندگان
دادههای به دست آمده از کارآزمایی تصادفیسازی و کنترل شده درباره اثربخشی و ایمنی درمانهای ضد‐میکروبی موضعی بر زخمهای پای دیابتی، به دلیل در دسترس بودن کارآزماییهای نسبتا اندک، اغلب کوچک، و اغلب با طراحی ضعیف، محدود هستند. بر اساس این مرور سیستماتیک و تجزیهوتحلیل منابع علمی، پیشنهاد میکنیم که: 1) استفاده از یک پانسمان ضد‐میکروبی به جای پانسمان غیر ضد‐میکروبی، ممکن است تعداد زخمهای پای دیابتی را که در طول یک دوره پیگیری متوسط بهبود یافتند افزایش دهد (شواهد با اطمینان پائین)؛ و 2) بر اساس مطالعات موجود احتمالا تفاوت کمی از نظر خطر عوارض جانبی مرتبط با درمان بین آنتیبیوتیکهای سیستمیک و درمانهای ضد‐میکروبی موضعی وجود دارد (شواهد با اطمینان متوسط). برای هر کدام از پیامدهای دیگری که بررسی کردیم، دادهای گزارش نشد یا دادهای وجود نداشت، و ما را در مورد اینکه تفاوتی بین درمانهای مقایسه شده وجود دارد یا خیر، ناامید کرد. با توجه به فراوانی بالا و رو به افزایش زخمهای پای دیابتی، محققان را تشویق میکنیم تا کارآزماییهای تصادفیسازی و کنترل شده را با طراحی مناسب در این زمینه انجام دهند تا اثرات درمانهای ضد‐میکروبی موضعی را هم بر پیشگیری و هم بر درمان عفونت این زخمها و در نهایت اثرات آن بر التیام زخم بررسی کنند.
PICO
خلاصه به زبان ساده
عوامل ضد‐میکروبی موضعی (محصولات آنتیباکتریال که بهطور مستقیم روی زخمها استعمال میشوند) برای درمان زخمهای پا در افراد مبتلا به دیابت
سوال مطالعه مروری
ما شواهد مربوط به اینکه مصرف موضعی (که بهطور مستقیم روی منطقه آسیب دیده استعمال میشود) عوامل ضد‐میکروبی (محصولات آنتیباکتریال) میتوانند از عفونتهای پا در افراد مبتلا به دیابت جلوگیری کرده یا آن را درمان کنند یا خیر، مورد بررسی قرار دادیم. ما میخواستیم بدانیم که درمانهای آنتیباکتریال میتوانستند به التیام زخمهای عفونی و غیر‐عفونی کمک کرده، و از عود عفونت زخمهای غیر‐عفونی جلوگیری کنند یا خیر.
پیشینه
افراد مبتلا به دیابت در معرض خطر بالای گسترش زخمهای پا قرار دارند. این زخمها ممکن است ایجاد ناراحتی کرده و اغلب عفونت کنند. زخمهای پای دیابتی که بهبود نمییابند ممکن است منجر به قطع قسمتی از یا تمام پا یا حتی اندام تحتانی شود. داروهای ضد‐میکروبی مانند ضد‐عفونی کنندهها و آنتیبیوتیکها باعث از بین رفتن یا پیشگیری از رشد باکتریها میشوند، و گاهی اوقات برای درمان زخمهای پای دیابتی استفاده میشوند. ضد‐میکروبها ممکن است برای کاهش عفونت یا پیشرفت التیام در زخمهای عفونی، یا برای جلوگیری از عفونت یا پیشرفت التیام در زخمهایی که عفونت در آنها شناسایی نشده، استفاده شوند. ما میخواستیم بدانیم که درمانهای ضد‐میکروبی در هر یک از این موارد موثر بودند یا خیر؛ کدام درمان موثرتر بود؛ و اگر درمان شدهاند، دچار عوارض جانبی مضری میشوند یا خیر.
ویژگیهای مطالعه
در آگوست 2016، کارآزماییهای تصادفیسازی و کنترل شدهای را جستوجو کردیم که از هر گونه درمان ضد‐میکروبی برای زخمهای پا یا سایر زخمهای باز پا برای افراد مبتلا به دیابت استفاده کرده بودند. ما در مجموع 22 کارآزمایی را شامل بیش از 2310 شرکتکننده بزرگسال یافتیم (یک کارآزمایی تعداد شرکتکنندگان خود را گزارش نکرد). تعداد شرکتکنندگان در هر کارآزمایی بین 4 تا 317 شرکتکننده متغیر بود و زمان پیگیری حین و پس از درمان بین 4 تا 24 هفته گزارش شد. بعضی از کارآزماییها شرکتکنندگان مبتلا به زخمهای عفونی را انتخاب کردند، در حالیکه سایر کارآزماییها شرکتکنندگان مبتلا به زخمهای غیر‐عفونی را وارد کردند. این کارآزماییها انواع مختلف پانسمانهای ضد‐میکروبی، محلولها، ژلها، کرمها، یا پمادها را مقایسه کردند.
نتایج اصلی
بسیاری از کارآزماییها دادههای مهمی را گزارش نکردند، یعنی اعتبار نتایج نامطمئن هستند. نتایج حاصل از پنج کارآزمایی شامل 945 شرکتکننده نشان میدهد که استفاده از بعضی از انواع پانسمانهای ضد‐میکروبی ممکن است تعداد زخمهای بهبود یافته را در پیگیریهای میانمدت (4 تا 24 هفته) در مقایسه با پانسمانهای غیر ضد‐میکروبی افزایش دهد (شواهد با اطمینان پائین). با توجه به اطلاعات محدود موجود، قادر به ارزیابی اثربخشی درمانها در پیشگیری یا برطرف کردن عفونت زخم نبودیم. چهار کارآزمایی شامل 937 شرکتکننده به مقایسه آنتیبیوتیکهای سیستمیک (خوراکی یا تزریقی، از راه جریان خون در سراسر بدن پخش میشود)، با درمانهای ضد‐میکروبی که مستقیما روی زخم استعمال میشوند، پرداختند. این کارآزماییها دادههای مربوط به التیام یا عفونت را ارائه نکردند، اما به نظر میرسد که تفاوتی در اثرات جانبی شرکتکنندگانی که زخمهای آن بهطور سیستمیک یا موضعی درمان شد، وجود نداشت (شواهد با اطمینان متوسط).
کیفیت شواهد
بهطور کلی، اطمینان شواهد ارائه شده توسط کارآزماییها بسیار پائینتر از آن بود که بتوانیم از منافع و آسیبهای درمانهای ضد‐میکروبی موضعی برای درمان زخمهای پا در افراد مبتلا به دیابت اطمینان حاصل کنیم. در این زمینه باید کارآزماییهای تصادفیسازی و کنترل شده بیشتر، بزرگتر و با طراحی بهتر انجام شود.
Authors' conclusions
Summary of findings
| Antimicrobial dressings compared with non‐antimicrobial dressings | ||||||
| Patient or population: Foot ulcers in people with diabetes Settings: Mixed Intervention: Antimicrobial dressings Comparison: Standard dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard dressings | Risk with antimicrobial dressings | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 425 per 1000 | 544 per 1000 | RR 1.28 (1.12 to 1.45) | 945 participants (5 studies) | ⊕⊕⊝⊝ | On average, use of an antimicrobial dressing compared with a non‐antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period. |
| Risk difference: 119 more healed wounds per 1000 (51 more to 191 more) | ||||||
| Incidence of infection Up to 24 weeks' follow‐up | 183 per 1000 | 62 per 100 (7 to 567) | RR 0.34 (0.04 to 3.10) | 173 participants (2 studies) | ⊕⊝⊝⊝ | On average, it is unclear whether or not use of an antimicrobial dressing compared with a non‐antimicrobial dressing reduces the incidence of ulcer infection over a medium‐term follow‐up period. |
| Risk difference: 121 fewer infections per 1000 (176 fewer to 384 more) | ||||||
| Resolution infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Adverse events Up to 24 weeks' follow‐up | 388 per 1000 | 373 per 1000 (241 to 574) | RR 0.96 (0.62 to 1.48) | 134 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether use of an antimicrobial dressing affects the risk of adverse events compared with use of a non‐antimicrobial dressing over a medium‐term follow‐up period. |
| Risk difference: 16 fewer adverse events per 1000 (147 fewer to 186 more) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias due to one study (with the highest weighting in the meta‐analysis) being at unclear risk of selection bias and three studies being at high risk of performance bias (36% weighting in analysis), although the studies were at unclear or low risk of detection bias for this outcome. | ||||||
| Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with non‐antimicrobial treatment | Risk with topical antimicrobial treatment | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 241 per 1000 | 679 per 1000 (135 to 1000) | RR 2.82 (0.56 to 14.23) | 112 participants (3 studies) | ⊕⊝⊝⊝ | The average effect of antimicrobial agents compared with non‐antimicrobial treatment is uncertain over a medium‐term follow‐up period. |
| Risk difference: 438 more healed wounds per 1000 (106 fewer to 1000 more) | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 368 per 1000 | 427 per 1000 (199 to 925) | RR 1.16 (0.54 to 2.51) | 40 participants (1 study) | ⊕⊕⊝⊝ | It is unclear whether use of an antimicrobial topical agent has an effect on risk of infection over a medium‐term follow‐up period. |
| Risk difference: 59 more resolved infections per 1000 (169 fewer to 556 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 81 participants (2 studies) | ⊕⊝⊝⊝ | 2 studies reported adverse event data. We were unable to extract per‐participant data for 1 study. The second study stated that no adverse events were reported in each arm. We judged this as very low‐certainty evidence. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias with two studies at high risk of detection bias, which is of particular concern when healing is being assessed, and one study not accounting for a small number of participants with multiple ulcers in their trial. Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for inconsistency: one small study reported all wounds healed in one arm and few wounds healed in the other. These data are adding heterogeneity to the analysis. | ||||||
| One topical antimicrobial agent compared with another topical antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical antimicrobial agent | Risk with alternative topical antimicrobial agent | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | Data were not pooled due to the 3 studies evaluating different interventions. | N/A | 85 participants (3 studies) | ⊕⊝⊝⊝ | It is generally uncertain whether 1 topical treatment has an increased likelihood of healing compared with the alternative treatment. We judged this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias. | |
| Incidence of infection Up to 24 weeks' follow‐up | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 625 per 1000 | 906 per 1000 (606 to 1000) | RR 1.45 (0.97 to 2.17) | 37 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether 1 specific type of topical antimicrobial agent has a different effect on resolution of infection than another over a medium‐term follow‐up period. |
| Risk difference: 281 more resolved infections per 1000 (19 fewer to 731 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 41 participants (1 study) | ⊕⊝⊝⊝ | The 1 study noted that no events were reported in either group. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded for risk of performance and detection bias. | ||||||
| Topical antimicrobial agent compared with systemic antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic antibiotic agent | Risk with topical antimicrobial agent | |||||
| Proportion of wounds healed | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | 333 per 1000 | 503 per 1000 (303 to 830) | RR 1.51 (0.91 to 2.49) | 102 participants (2 studies) | ⊕⊝⊝⊝ | It is uncertain whether the effects of topical antimicrobial treatment on resolution of infection differ from those of systemic antibiotics. |
| Risk difference: 170 more resolved infections per 1000 (30 fewer to 497 more) | ||||||
| Adverse events | 450 per 1000 | 409 per 1000 (351 to 477) | RR 0.91 (0.78 to 1.06) | 937 participants (4 studies) | ⊕⊕⊕⊝ moderate2 | On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments compared here. |
| Risk difference: 40 fewer adverse events per 1000 (99 fewer to 27 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for risk of performance bias. | ||||||
| Topical antimicrobial agent compared with growth factor | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with growth factor | Risk with topical antimicrobial | |||||
| Proportion of wounds healed | 800 per 1000 | 400 per 1000 (224 to 712) | RR 0.50 (0.28 to 0.89) | 40 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether treatment with growth factor affects the risk of healing when compared with antiseptic dressing. |
| Risk difference: 400 fewer resolved infections 576 fewer to 88 fewer | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Adverse events | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision: small sample size and small number of events ‐ optimal information size not met and results are fragile. Downgraded twice for risk of performance and attrition bias. | ||||||
Background
Worldwide, there are currently over 415 million adults with diabetes mellitus (5 million of whom die of the disease annually), and the prevalence of diabetes is expected to reach over 640 million (1 in 10) by 2040 (IDF 2015). Furthermore, treating diabetes accounts for 12% of global health expenditure (USD 673 billion). Skin wounds, particularly chronic ulcers, commonly develop in the feet of people with diabetes mellitus, usually related to neuropathy (nerve damage), as well as arterial (blood vessel) disease or trauma (Davies 2007; Lipsky 2009). Peripheral neuropathy (damage to the nerves to the feet), peripheral arterial disease, or both develop over time in most people with diabetes (American Diabetes Association 2003). Many people with diabetes also have as‐yet poorly defined defects in immune responses that impair their ability to resist or overcome infection (Delamaire 1997). These factors put diabetic patients at high risk of developing foot ulcers, most of which become infected. The estimated lifetime risk of a foot ulcer in a person with diabetes is 25%, at a cost (in Europe in 2008) of EUR 10,000 for an uninfected ulcer and EUR 17,000 for an infected ischaemic ulcer (Markakis 2016). These wounds, especially those that become clinically infected, cause substantial morbidity. Estimates are that somewhere in the world a person with diabetes undergoes a lower extremity amputation every 20 seconds. (IWGDF 2016). Infection of a diabetic foot wound is defined as the presence of at least two of the classic signs or symptoms of inflammation (pain or tenderness, warmth, redness, swelling) or purulent secretions (pus). Foot problems, especially when complicated by infection, are now responsible for more days of hospitalisation than any other complication of diabetes (Pecoraro 1990; Singh 2005). Diabetic foot infections, in particular those that contiguously spread to underlying bone, are also the main precipitating factor for lower extremity amputation, which is associated with substantial financial cost, reduced quality of life, and early mortality (Lipsky 2012b; Lipsky 2016). To avoid these adverse outcomes it is crucial to prevent foot infections, or failing that, to optimally treat the infected wounds. Treatment of infection almost always requires antimicrobial therapy, which may be given systemically (to the whole body via the oral or parenteral (i.e. intravenous or intramuscular) route) or topically (i.e. locally, through application of antiseptic, antibiotic, or other antimicrobial preparations (e.g. solutions, creams, gels, ointments)). Sometimes it is difficult for the clinician to tell if a diabetic foot wound is infected, especially if the patient has peripheral neuropathy or arterial disease. Furthermore, the mere presence of micro‐organisms, especially if they are virulent or present in high numbers, may also impair wound healing in clinically uninfected wounds. Thus some advocate prescribing antimicrobial therapy (especially topically) for high‐risk clinically uninfected wounds to reduce the bacteria 'bioburden' and potentially accelerate healing or avoid overt infection.
Description of the condition
Micro‐organisms rapidly colonise virtually all open wounds; this usually has no apparent consequences in the absence of clinical evidence of infection, and healing occurs as expected (White 2006). However, some wounds exhibit a host response (usually manifested by inflammation or tissue damage) to the organisms they harbour, suggesting that they are clinically infected (Cutting 2005). The likelihood of a wound becoming infected increases directly with the size of its microbial inoculum, the virulence of the specific colonising organisms, and the level of diminution of the host’s local and systemic immunological resistance (Heinzelmann 2002). For the clinician, characterising a wound as infected or not is a key clinical challenge. Published studies show that almost half of all people with a diabetic foot ulcer have no clinical signs of infection; these people do not usually need to have cultures taken from their wound, as they generally do not require antimicrobial therapy (Lavery 2006; Prompers 2007).
Many classification schemes have been proposed for diabetic foot wounds, but most categorise infection only as being either 'present' or 'absent', and do not specify infection severity or how to define its presence. Classification systems that provide more information on infection have been developed by the International Working Group on the Diabetic Foot (IWGDF) and the Infectious Diseases Society of America (IDSA) (Table 1) (IWGDF 2015; Lipsky 2012b). These classifications, which are nearly identical, have been validated as predictive of the patient’s need for hospitalisation and for lower extremity amputation. As they also provide a way for a clinician to communicate key information to others caring for the wound, guidelines recommend that clinicians routinely use them to classify the presence and clinical severity of diabetic foot infections (Lipsky 2012b).
| Clinical manifestation of infection | PEDIS grade | IDSA infection |
| No symptoms or signs of infection | 1 | Uninfected |
| Infection present, as defined by the presence of at least 2 of the following items:
| ||
| Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). If erythema, must be > 0.5 cm to ≤ 2 cm around the ulcer. | 2 | Mild |
| Local infection (as described above) with erythema > 2 cm, or involving structures deeper than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic arthritis, fasciitis), and no systemic inflammatory response signs (as described below) | 3 | Moderate |
| Local infection (as described above) with the signs of SIRS, as manifested by ≥ 2 of the following:
| 4 | Severe* |
Abbreviations: IDSA, Infectious Diseases Society of America; PaCO2, partial pressure of arterial carbon dioxide; PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation; SIRS, systemic inflammatory response syndrome
*Ischaemia may increase the severity of any infection, and the presence of critical ischaemia often makes the infection severe. Systemic infection may sometimes manifest with other clinical findings, such as hypotension, confusion, vomiting, or evidence of metabolic disturbances, such as acidosis, severe hyperglycaemia, and new‐onset azotaemia.
In light of the high prevalence of infection in foot wounds in people with diabetes, it is important for clinicians to consider this possibility when such patients present for care. Clinicians should generally define infection by the presence of at least two of the classic symptoms or signs of inflammation, that is erythema (redness), calor (warmth), tumour (swelling or induration), dolour (pain or tenderness), or purulent secretions (pus). As the presence of neuropathy or arterial or immunological diseases may obscure these findings, some authorities accept additional "secondary" or "intermediate" signs of infection (Cutting 2005; Gardner 2001; Lipsky 2012b).
Cultures of specimens from acutely infected wounds (especially in patients from high‐income Western countries who have not recently been on antibiotic therapy) usually grow bacteria classified as aerobic gram‐positive cocci. In this situation these are generally the only bacteria against which clinicians need target their antimicrobial therapy. However, in chronic wounds, or when a patient has recently been treated with antibiotics, other bacteria (especially aerobic gram‐negative rods and obligate anaerobes) often accompany these gram‐positive cocci, necessitating broader‐spectrum antibiotic therapy. Recently, molecular diagnostic studies of wounds have shown that they harbour an even greater variety of organisms than had previously been recognised (Davies 2004; James 2008), but the clinical importance of this finding is as yet unclear (Lipsky 2013). Furthermore, in many chronic wounds bacteria persist as so‐called "small colony variants" (von Eiff 2006), which are both more difficult to culture and to eradicate. Finally, micro‐organisms in chronic wounds often exist in states or communities that are particularly difficult to treat, such as in an adhesive, polymeric matrix called biofilm, which induces chronic inflammation, delays healing, and protects the organism from the effects of antimicrobial therapy (Rhoads 2008).
Given the problems associated with treating diabetic foot infections, treatment with topical antimicrobials has potential benefits, for example it could result in very high drug levels at the infected site (with little or none at other sites) and may allow the use of agents that cannot be given systemically (Lipsky 2009). These findings, combined with a wish to avoid systemic antibiotic therapy where possible, have led many clinicians to consider using topical antimicrobial therapy for open infected wounds, especially those that fail to heal despite apparently appropriate treatment. It was thus important to determine if this route of therapy is safe and effective.
Description of the intervention
Clinically infected wounds virtually always require antibiotic therapy, whereas clinically uninfected wounds that are healing normally do not (Lipsky 2009). Of note, some superficial infections (e.g. impetigo, fungal dermatitis) may respond to first‐line topical antimicrobial therapy alone, without recourse to systemic therapy. However, controversy exists over how to treat poorly healing wounds that display 'secondary' signs suggestive of infection and that may benefit from topical antimicrobial agents. The rationale for using a topical antimicrobial is to kill, or at least halt, the replication of pathogenic micro‐organisms on the skin, mucosae, or in a wound, without causing clinically significant damage to the host cells. Topical antimicrobials may be used on their own or in combination with other topical or systemic antimicrobial agents.
There are several classes of topical agents that inhibit or kill micro‐organisms (Lipsky 2009).
-
Disinfectants are non‐specific agents with activity against virtually all disease‐causing micro‐organisms, including those in a spore state. Since these may be toxic to host tissues, they are used primarily for sterilising inanimate surfaces and not for topical treatment of wounds.
Most topical antimicrobials for clinical use belong to one of two major groups:
-
Antiseptics: These are usually a type of disinfectant that can be used on intact skin and some open wounds to kill or inhibit micro‐organisms. They often have multiple microbial targets, a broad antimicrobial spectrum, and residual anti‐infective activity. Unfortunately, they may be toxic to one or more types of host cells or tissues (e.g. fibroblasts, keratinocytes, and possibly leukocytes). Topical antiseptic agents used in the past (e.g. hexachlorophene and iodines) are used less frequently today because of concerns about toxicity to host cells and the availability of safer agents. Chlorhexidine and povidone iodine are older agents that have been (and continue to be) widely used as wound antiseptics. Recently, a variety of products that release silver ions have been approved and are being promoted for management of wound micro‐organisms.
-
Antibiotics: These are chemicals produced either naturally (by a micro‐organism) or synthetically that, in dilute solution, inhibit or kill other micro‐organisms. They usually act on one specific cell target, have a narrower spectrum of activity than antiseptics, are relatively non‐toxic, and are more susceptible to losing their effectiveness as bacteria develop resistance. Most agents that are used exclusively as topical antibiotics have efficacy against gram‐positive bacteria (e.g. bacitracin, mupirocin, retapamulin), with a smaller number demonstrating efficacy against gram‐negative bacteria (e.g. neomycin, silver sulphadiazine). Some antibiotics that are used systemically (e.g. gentamicin, metronidazole, clindamycin) have also been formulated for topical use.
Below, we have provided a summary of the principal characteristics of currently available antiseptics (Table 2) and topical antibiotics (Table 3).
| Product and formulations | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Acetic acid | 0.25%, 0.5%, and 1% solutions | Bactericidal against most gram‐positive and gram‐negative organisms, including Pseudomonas aeruginosa | Inexpensive; shown to eliminate P aeruginosa colonisation from burn | Cytotoxic in vitro although maybe not in vivo; limited activity against biofilm | $ | No longer as widely used as in the past |
| Cadexomer iodine | Gel,c ointment, and dressing | Polysaccharide starch lattice; active agent is slowly released free iodine; broad spectrum of activity (same as iodine) | Reduced local toxicity compared to iodine; elemental iodine released on exposure to exudate | Application may cause stinging and erythema, but less tissue damage than other iodine products; effect may not persist, and efficacy may be reduced in body fluids. | $$ | Indicated for use in cleaning wet ulcers and wounds and reducing microbial load in the wound environment |
| Cetrimide | Solution, 40% | Active against bacteria and fungi; not active against P aeruginosa | May be less toxic to wound tissues than other antiseptics | May be corrosive and is potentially harmful if swallowed | $ | Not available in the USA |
| Chlorhexidine gluconate | Solution, 2% and 4%; liquid, 2% and 4%; hand rinse, 0.5%; wipes, 0.5%; sponge/brush, 4%; and foam, 4% | Active against gram‐positive bacteria (e.g. Staphylococcus aureus) and gram‐negative bacteria, including P aeruginosa | Persistent activity up to 6 h after application; few adverse effects | Hypersensitivity, including anaphylaxis, generalised urticaria, bronchospasm, cough, dyspnoea, wheezing, and malaise; may cause serious injury to the eye and middle ear; avoid contact with face or head; some resistance reported | $ | 2% chlorhexidine indicated as surgical hand scrub, hand wash, skin and wound cleanser; polyhexanide is a similar, newer biguanide. |
| Hexachlorophene | Liquid, 3%; foam, 0.23% with 56% alcohol | Biguanide that is bacteriostatic against Staphylococcus species and other gram‐positive bacteria | May retain residual effect on skin for several days | Rapidly absorbed and may result in toxic blood levels; application to burns has resulted in neurotoxicity and death; may cause central nervous system stimulation and convulsions, dermatitis, and photosensitivity reactions | $$$ | Not recommended for routine use on wounds due to potential toxicity |
| Iodine compounds and iodine tincturec | Solution (aqueous) 2% and 2.4%; and tincture (44% to 50% alcohol) 2% and 2.4% | Microbicidal against bacteria, fungi, viruses, spores, protozoa, and yeasts | Broad spectrum | Highly toxic if ingested or significantly absorbed; do not use with occlusive dressings; causes pain and stains skin and clothing; use cautiously in people with thyroid disorders | $ | Iodine compounds are now rarely used for wound management; cadexomer iodine and povidone iodine products are less toxic. |
| Povidone iodinec | Ointment, 1%, 4.7%, 10%; solution, 1% and 10%; also wash, scrub, cleanser, gel, aerosol, gauze pad, swab, and other forms | Broad spectrum includes S aureus and enterococci; active ingredient is liberated free iodine; shares spectrum but is less potent than iodine | Less irritating to skin and allergenic than iodine. Can be covered with dressings. Clinically significant resistance very rare | Antibacterial action requires at least 2 min contact; may cause stinging and erythema; effect may not persist, and efficacy may be reduced in body fluids; prolonged use may cause metabolic acidosis; stains skin and clothing; possible interaction with starches in dressings | $ | Indicated for perioperative skin cleansing and for cleansing and prevention of infection in superficial burns, incisions, and other superficial wounds |
| Sodium hypochlorite (Dakin’s solution and EUSOL) | Solution, 0.0125%, 0.125%, 0.25%, and 0.5% | Vegetative bacteria, viruses, and some spores and fungi | Inexpensive | No known systemic toxicity. May require prolonged contact for antibacterial action; inactivated by pus; toxic to fibroblasts and keratinocytes, and may cause pain or lyse blood clots | $ | A concentration of 0.025% is both bactericidal and non‐toxic to tissues (Heggers 1991). |
| Hydrogen peroxidec | Solution, 1% and 3%; and cream, 1% | Oxidizing agent active against many gram‐positive and gram‐negative bacteria | Broad‐spectrum, bactericidal, inexpensive; no known 1q11 | May cause some discomfort | $ | Commonly used, but few clinical studies |
| Silver nitrate | Solution 0.5%, 10%, 25%, and 50%; ointment, 10%; and swabs, 25% to 50% | Silver ions are bactericidal against a broad spectrum of gram‐positive and gram‐negative bacteria. | Low cost; easily applied | Painful on application; stains tissues; may delay healing; concentrations 10.5% cause cauterisation; inactivated by wound exudates and chlorine | $ | Previously widely used, but now largely replaced by other compounds, including newer silver dressings |
| Silver dressings | At least 6 approved products with different properties | Slowly released silver ions have broad spectrum, including MRSA and VRE. | Provide sustained levels of active silver ions; microbial resistance is rare; less painful and few adverse effects than silver nitrate; variety of products adaptable to different types of wounds; infrequent application required | Levels of silver ions at wound interface not well defined; may cause silver staining of tissues; may delay epithelialisation; relatively expensive; few published comparative trials | $$ | Should not substitute for non‐medicated dressings for uninfected wounds; may be useful for subclinically infected, highly colonised wounds or for wounds being prepared for skin grafting |
Abbreviations: EUSOL, Edinburgh University Solution of Lime; MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococci.
aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15.
bUS Food and Drug Administration–approved indications.
cAvailable without prescription. Modified from Lipsky 2009.
| Product and | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Bacitracin c | Ointment, 500 units/g; and powder combinations with neomycin, polymyxin B, and zinc | Many gram‐positive organisms, including aerobic staphylococci and streptococci, corynebacteria, anaerobic cocci, and clostridia; inactive against most gram‐negative organisms | Activity not impaired by blood, pus, necrotic tissue, or large bacterial inocula; resistance is rare but increasing among staphylococci; no cross‐resistance with other antibiotics; minimal absorption | May cause allergic reactions, contact dermatitis, and (rarely) anaphylactic reactions; may lead to overgrowth of drug‐resistant organisms, including fungi | $ | Widely used for many years; indicated for prevention of infection in minor skin wounds |
| Fusidic acid | Cream, 2%; ointment, 2%; and gel, 2% | Staphylococcus aureus, streptococci (in topical concentrations), corynebacteria, and clostridia | Penetrates intact and damaged skin as well as crust and cellular debris | Occasional hypersensitive reactions; resistance among staphylococci is emerging; must apply 3 times daily | $$ | Not available in the USA |
| Gentamicin | Cream, 0.1%; and ointment, 0.1% | Streptococci, staphylococci, Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae | Broad spectrum; inexpensive | Must be applied 3 to 4 times daily; may drive resistance to an agent used systemically | $ | Indicated for primary skin infections (pyodermas) and secondary skin infections, including infected excoriations, and for bacterial superinfections |
| Mafenide acetate | Solution, 5%; and cream, 85 mg/g | A sulfonamide that is bacteriostatic against many gram‐negative organisms, including P aeruginosa, and some gram‐positive organisms, but minimal activity against staphylococci and some obligate anaerobes | Remains active in the presence of pus and serum, and its activity is not affected by acidity of environment | Systemic absorption may occur; drug and metabolites may inhibit carbonic anhydrase, potentially causing metabolic acidosis; use cautiously in patients with renal impairment; pain on application; hypersensitive reactions. | $$$ | Indicated as adjunctive therapy in second‐ and third‐degree burns; may be used in rapidly progressing bacterial necrotising fasciitis; limited use in other wounds |
| Metronidazole | Cream, 0.75%; gel, 1%; lotion, 0.75% | Many clinically important anaerobic bacteria | May reduce odour associated with anaerobic infections; application only 1 to 2 times daily | Relatively expensive; systemic formulations available; could drive resistance to these | $–$$ | Indicated for inflammatory papules and pustules of rosacea |
| Mupirocin and mupirocin calcium | Ointment, 2%; for mupirocin calcium, cream, 2.15%; and nasal ointment, | Gram‐positive aerobes, including S aureus (most MRSA), Staphylococcus epidermidis, Staphylococcus saprophyticus, and streptococci (groups A, B, C, and G) but not enterococci, some gram‐negative aerobes (not P aeruginosa), corynebacteria, and obligate anaerobes | Minimal potential for allergic reactions | Rare local burning and irritation; applying ointment to large wounds in azotaemic patients can cause accumulation of polyethylene glycol; long‐term use can lead to resistance among staphylococci, which is increasing | $$ | Indicated for topical treatment of impetigo and eradication of nasal colonisation with S aureus |
| Neomycin sulfatec | Powder; cream, 0.5%; combinations with polymyxin B and pramoxine, and ointment, 0.5%; combinations with bacitracin, polymyxin B, lidocaine, and pramoxine | Good for gram‐negative organisms but not P aeruginosa; active against some gram‐positive | Low cost; applied only 1 to 3 times daily; may | Topical powder in wound irrigating solution | $ | Use of topical powder alone or in solution is not recommended; cream and ointment, in combination with other agents, are indicated for prevention of infection in minor skin injuries. |
| Nitrofurazone | Solution, 0.2%; ointment, 0.2%; and cream, 0.2% | Broad gram‐positive and gram‐negative activity, | Used mainly for burn wounds | Hypersensitive reactions; polyethylene glycols (in some formulations) may be absorbed and can cause problems in azotaemic patients | $$ | Indicated as adjunctive to prevent infections in people with second‐ and third‐degree |
| Polymyxin Bc | Cream, 5000 units/g or | Bactericidal against many gram‐negative organisms, | Inexpensive | Some hypersensitive and neurological or | $ | Only available in combination with other agents, including bacitracin and neomycin; |
| Retapamulin | Ointment, 1% | Active against staphylococci (but uncertain | May be active against some mupirocin‐resistant S aureus strains; broader activity than mupirocin | Not evaluated for use on mucosal surfaces; may cause local irritation | $$$ | Indicated for impetigo due to S aureus (methicillin‐susceptible only) or Streptococcus pyogenes |
| Silver sulphadiazine | Cream, 1% | A sulfonamide; the released silver ions are the primary active ingredient; active against many gram‐positive and gram‐negative organisms, including P aeruginosa | Applied only once or twice daily; soothing | Potential cross‐reaction with other sulphonamides; may rarely cause skin staining | $ | Indicated as adjunctive treatment to prevent |
| Sulfacetamide Na+ | Lotion, 10% | Bacteriostatic against many gram‐positive and gram‐negative pathogens | Broad spectrum; can be combined with sulphur | Systemic absorption and rarely severe side | $$$ | Indicated for secondary bacterial skin infections |
There are no published studies supporting the use of topical erythromycin, clindamycin, aminoglycosides other than neomycin, gramicidin, or tetracyclines for treating chronically infected wounds.
Abbreviations: FDA, US Food and Drug Administration; MRSA, methicillin‐resistant Staphylococcus aureus.
aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15.
bFDA‐approved indications.
cAvailable without prescription.
How the intervention might work
For millennia healers have applied various compounds to infected wounds, some of which (e.g. silver, honey) are still in use today. Use of a topical application has many potential advantages compared with giving systemic antibiotic therapy, including: a high and sustained concentration of the antimicrobial agent at the site of infection; the need to use only a limited amount of the antimicrobial at the selected site; avoidance of potential toxicity associated with systemic treatment; ability to use novel agents not available for systemic use; easy application in the outpatient setting; and potentially better patient adherence to treatment. Topical treatments may also prove helpful in addressing the globally increasing problem of multidrug‐resistant organisms that are now untreatable with most systemic agents. For example, a study of 47 organisms from burn wounds that were multidrug‐resistant to systemic antibiotics were susceptible to 11 commonly used topical antibiotics and antiseptics, although the rates of resistance were higher than in non–multidrug‐resistant organisms (Neely 2009).
Topical antimicrobial therapy also has some potential disadvantages: few agents have been proven to be effective in clinical trials; almost all have minimal penetration of intact skin or soft tissue, limiting use to open wounds that do not have either cellulitis or deep soft‐tissue infection; systemic absorption of some agents may occur if used on large wounds; agents may induce local hypersensitivity or contact dermatitis reactions; some agents may interfere with normal wound‐healing processes; treatment may produce an alteration of normal cutaneous flora that may lead to other problems; topical applications are difficult to dose accurately; topical agents may require frequent applications; agents may be difficult to apply or aesthetically unacceptable to some patients; and agents in multiuse containers can become contaminated during repeated use (Gelmetti 2008; Lio 2004).
Topical antimicrobials have traditionally been formulated in one of two ways. As ointments, they are more occlusive, often contain petrolatum, and are best used for dry lesions. As creams, they are less occlusive, wash off with water, are less messy, and are best for moist lesions. Newer technologies have allowed incorporation of antimicrobials into dressings, such as alginates, foams, collagen and sponges, potentially allowing controlled release at the wound surface. One major problem with topical therapies is that internationally no official oversight agency has standardised and approved specific tests to establish the efficacy and safety of these agents (Cooper 2004).
Why it is important to do this review
A recent Cochrane review summarised and analysed the data on the effectiveness of systemic antibiotic therapy for diabetic foot infections (Selva Olid 2015). To date, however, the lack of available data has made it difficult to assess the efficacy of topical antimicrobials for diabetic foot ulcers (Drucker 2012; Lipsky 2009; Peters 2012). A systematic review of antimicrobial agents for various chronic wounds (including diabetic foot ulcers) concluded that few systemic agents improved outcomes, but hastened healing was associated with use of several topical substances (O'Meara 2001). A Cochrane systematic review of treatment with antibiotics or antiseptics for healing venous leg ulcers found some evidence supporting the use of cadexomer iodine but not the routine use of honey‐ or silver‐based products (O'Meara 2014); further evidence was required before conclusions could be made about other agents. A systematic review of the effectiveness of various interventions for enhancing the healing of chronic diabetic foot ulcers found limited evidence of benefit of any agents for healing of diabetic foot wounds (Game 2016). Another Cochrane review of treatment with silver‐based wound dressings or topical agents for diabetic foot ulcers found no randomised controlled trials reporting outcomes on healing rates or infection resolution (Bergin 2006). Likewise, a Cochrane review of silver‐containing dressings or topical agents for treating infected or contaminated chronic wounds concluded there was insufficient evidence, on the basis of three randomised trials, to recommend these treatments (Vermeulen 2007). An updated Cochrane systematic review on topical honey for treating wounds concluded that it may reduce healing time for mild‐to‐moderate superficial and partial‐thickness burns and infected postoperative wounds, but did not significantly hasten leg ulcer healing (Jull 2015). Finally, a recent systematic review of the effectiveness of interventions in the management of diabetic foot infections found six studies that investigated the use of topical agents (Peters 2016), but the methods and results did not allow the authors to draw any definitive conclusions. Among the two studies of topical antibiotics, one found that an antimicrobial peptide, pexiganan cream, was similar in effectiveness to a systemic antibiotic (ofloxacin) in the treatment of mildly infected diabetic foot ulcers, while another study of adjunctive therapy with a gentamicin‐collagen sponge (along with systemic antibiotic therapy) was difficult to interpret because of methodological problems (Peters 2016).
Clearly, the currently available literature does not provide an adequate overview as to whether topical antimicrobial therapy is safe or effective for foot ulcers in people with diabetes. Given the high frequency of these wounds, their potentially serious adverse outcomes, and the possibility of benefit in preventing or curing infection or accelerating wound healing and of reducing unnecessary use of systemic antibiotics, we considered a systematic review of all the available evidence of the use of topical antimicrobial agents for preventing or treating infection in diabetic foot ulcers to be both timely and important.
Objectives
To evaluate the effects of treatment with topical antimicrobial agents on: the resolution of signs and symptoms of infection; the healing of infected diabetic foot ulcers; and preventing infection and improving healing in clinically uninfected diabetic foot ulcers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) conducted in any setting (e.g. inpatient/institutional or outpatient/ambulatory).
Types of participants
People with diabetes mellitus (as defined by the study authors) diagnosed with an ulcer of the foot (i.e. below the malleoli, the bony prominences on each side of the ankle), whether clinically infected or uninfected. We only included a study that enrolled a mixed population of participants if some of those enrolled had a foot ulcer and diabetes, and if the randomisation to treatment was stratified by wound type. We otherwise excluded studies with partial trial data, as this approach is akin to a subgroup analysis. We also included studies that had a mixed population if more than 80% of participants were people with diabetes and a foot ulcer.
Types of interventions
We reviewed studies evaluating treatment with any type of solid (liquid, gel, ointment, cream) topical antimicrobial agent, including antiseptics and antibiotics. We did not include any studies of antimicrobial agents that were in a 'gaseous' form (e.g. local oxygen), or that relied on phototherapy.
Specific comparisons included one or more of the following:
-
a topical antimicrobial agent plus standard care (e.g. cleansing, debridement, wound dressings, pressure off‐loading) compared with standard care alone, or combined with a placebo;
-
two or more different topical antimicrobial agents;
-
a topical antimicrobial agent (with or without a systemic antimicrobial agent) compared with a systemic antimicrobial agent alone (or with a topical placebo).
Types of outcome measures
Our primary and secondary outcomes are listed below. If a study was otherwise eligible (i.e. it had the correct study design, population, and intervention/comparator) but did not report a listed outcome, we attempted to contact the study authors to establish whether or not they had measured an outcome of interest to us that they did not report.
We defined follow‐up as the time from participant randomisation to outcome measurement. We reported outcome measures at the latest time point available (assumed to be length of follow‐up if not otherwise specified) or the time point specified in the methods as being of primary interest to the authors (if this was different from latest time point available).
Primary outcomes
For studies of wounds that were clinically infected or clinically uninfected, our primary outcome was as follows.
-
Complete ulcer healing. We included this outcome (complete epithelialisation of the ulcer), seeking the following as measures:
-
time to complete ulcer healing (correctly analysed using survival, time‐to‐event approaches, ideally with adjustment for relevant covariates, such as baseline size);
-
the proportion of people with an ulcer that completely healed.
-
Where both of these outcomes were reported, our plan was to present all data in a summary outcome table for reference, but give 'time to complete ulcer healing' primacy; however, no study reported time‐to‐event data that was analysable. As planned, when time was analysed as a continuous measure, but it was not clear whether all ulcers had healed, we documented the use of this outcome in the study but did not extract, summarise, or otherwise use the data in any meta‐analysis.
For studies involving wounds that were clinically infected at baseline, a second primary outcome for this review was as follows.
-
Resolution of infection. We accepted the investigators' assessment of resolution of infection, e.g. diminution or disappearance of clinical findings such as erythema (redness), warmth, pain or tenderness, induration (swelling), or purulent secretions (Table 1).
For studies involving wounds that were clinically uninfected at baseline, a second primary outcome for the review was as follows.
-
Incidence of infection. We accepted the investigators' assessment of the development of infection in a diabetic foot wound, e.g. by the appearance of new clinical findings, such as erythema (redness), warmth, pain or tenderness, induration (swelling), or purulent secretions (Table 1) (Lipsky 2012b).
Secondary outcomes
For both clinically infected and clinically uninfected wounds, we reported the following outcomes, when available.
-
Microbial counts, usually defined as bacterial colony forming units/gram of tissue or semiquantitative counts of number of colonies on a culture plate (typically graded from 1 to 4).
-
Health‐related quality of life, if it was reported using global measures of a validated scale (e.g. SF‐36 or EQ‐5D) or a validated disease‐specific questionnaire (e.g. Cardiff Wound Impact Schedule). These reported data were adjusted for the baseline score. We did not include ad hoc measures of quality of life that are unlikely to be validated and would not be common to multiple trials.
-
Risk of surgical resection of the foot wound, including partial or complete lower limb amputation.
-
Adverse events, defined and grouped together, as 'adverse events' where the study provided a clear methodology for the collection of these data. This would include making it clear whether (i) events were reported at the participant level or if multiple events per person were reported; and (ii) that an appropriate adjustment was made for data clustering. Where available, we extracted data on all serious and all non‐serious adverse events. We anticipated that adverse events for topical treatments would be likely to be similar to those for conventional treatments (e.g. wound deterioration, maceration, pruritis). We also recorded information about study authors' assessment of the treatment‐related nature of adverse events. (Nebeker 2004).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant RCTs:
-
the Cochrane Wounds Specialised Register (searched 15 August 2016);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library) (2016, Issue 7, searched 15 August 2016);
-
Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and Epub Ahead of Print) (1946 to 15 August 2016);
-
Ovid Embase (1974 to 15 August 2016);
-
EBSCO CINAHL Plus (1937 to 15 August 2016).
The full search strategies for CENTRAL, Ovid MEDLINE, Ovid Embase, and EBSCO CINAHL Plus are shown in Appendix 1.
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase randomised trials filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the Cumulative Index to Nursing and Allied Health Literature (CINAHL) search with the randomised trials filter terms developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). We used no restrictions with respect to an article's language, date of publication, or study setting.
We also searched the following clinical trials registries (19th December 2016) for additional eligible studies:
-
ClinicalTrials.gov (www.clinicaltrials.gov);
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/Default.aspx);
-
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
For studies that met our criteria we emailed any listed contact person to seek any available results of the study.
Searching other resources
In addition to the searches described above, we checked the reference lists of all relevant trials identified and retrieved by the above methods. We originally planned to contact other authors and trialists who work in the area, but did not do so.
Data collection and analysis
We summarised our data using standard Cochrane methodologies (Higgins 2011). Data collection and analysis were carried out according to methods stated in the published protocol (Lipsky 2014), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed each reference identified by the search against our inclusion criteria. We retrieved full copies of those references that appeared potentially eligible, and two review authors independently assessed each of these papers. Any disagreements were resolved through discussion, or by consultation with a third review author if required.
Data extraction and management
One review author extracted data from the included trials using a piloted form, and another review author checked the entered data.
We extracted the following data when available:
-
trial identification (first author's surname and year of main publication);
-
setting of care;
-
participant eligibility criteria;
-
participant demographics (age, sex, country);
-
total number of participants recruited;
-
number of participants per group;
-
characteristics of the foot ulcers (e.g. anatomic site, size, number of ulcers, presence/absence of infection, duration of ulceration);
-
ulcer treatments (antimicrobial and other);
-
details of concurrent interventions (e.g. off‐loading, debridement);
-
duration of antimicrobial treatment;
-
duration of follow‐up;
-
outcomes, as defined above, at the end of therapy and at last follow‐up post‐therapy; and
-
withdrawals and losses to follow‐up, with reasons, by treatment group.
The review authors discussed any discrepancies and achieved a final consensus.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study following the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions (Appendix 2) (Higgins 2011). They discussed any discrepancies and achieved consensus on the final assessment.
The Cochrane 'Risk of bias' tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias (Appendix 2).
We have presented our assessment of risk of bias using two 'Risk of bias' summary figures:
-
a summary of bias for each item across all studies; and
-
a cross‐tabulation of each trial by all of the 'Risk of bias' items. We classified studies judged to be at high risk of selection bias, detection bias, or attrition bias as being at overall high risk of bias (for the specified outcome for that study).
Measures of treatment effect
We reviewed the evidence separately for each of the different types of topical antimicrobial agents.
For each binary (yes/no) outcome (e.g. wound healed, lower extremity amputation, adverse event) we calculated the risk ratio (RR) with 95% confidence intervals (CI). In this review we only reported continuous data for the quality of life outcome, which we presented as mean differences (MD) with 95% CI. We were unable to present time‐to‐event data using hazard ratios with 95% CI, as these data were not available for any included study.
Unit of analysis issues
Our unit of analysis was the individual person: we collected and analysed a single measurement for each outcome from each participant. Where studies had unit of analysis issues that were not adequately handled, we noted this finding as part of our 'Risk of bias' assessment. We included three‐arm trials, but where possible we either combined control arms or included studies in multiple comparisons as required, but avoided double counting of data.
Dealing with missing data
Where data were missing that the review authors thought should be included in the analyses, we attempted to contact the relevant study authors to request any additional available data or information on the reasons for the missing data.
Where data remained missing for the primary outcome (proportion of ulcers healed and incidence/resolution of infection), we assumed participants did not achieve the outcome (i.e. they were considered in the denominator but not the numerator).
For continuous variables (e.g. quality of life), we presented available data from the study reports (and any additional information if provided by the study authors) and did not impute missing data.
For adverse events and all secondary dichotomous outcomes, we used an available‐case analysis, where possible. If this was not possible, we used whatever information the authors reported in the study.
Assessment of heterogeneity
To assess heterogeneity we did an initial assessment of clinical and methodological heterogeneity and then an assessment of the appropriateness of combining study results, that is the degree to which the included studies varied in terms of participants, interventions, outcomes, and characteristics such as length of follow‐up. We supplemented our assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity of the results, which we assessed using the Chi² test (at a significance level of P < 0.10) in conjunction with the I² measure (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). In general, I² values of 40% or less may mean a low/unimportant level of heterogeneity (Higgins 2003), and values of 75% or more indicate very high heterogeneity (Deeks 2011).
Assessment of reporting biases
We assessed studies for reporting biases, including publication bias and small‐study effects. As we did not conduct any meta‐analyses with 10 or more RCTs, we could not assess the possibility of small‐study effects using funnel plots.
We also considered the publication status of the studies and any information provided on how they were funded.
Data synthesis
We combined details of the included studies in the narrative review according to the type of comparator, and then by outcomes. We considered clinical and methodological heterogeneity, and undertook pooling when studies appeared appropriately similar in terms of types of wounds, interventions, and outcomes.
Our default approach for undertaking a meta‐analysis was to use the random‐effects model. We only used a fixed‐effect approach when we considered clinical heterogeneity to be minimal and statistical heterogeneity was not statistically significant for the Chi² value and 0% for the I² measure (Kontopantelis 2012). We adopted this approach because statistical assessments can miss potentially important between‐study heterogeneity in small samples, making the more conservative random‐effects model preferable (Kontopantelis 2012). Where we considered clinical heterogeneity to be acceptable we undertook a meta‐analysis, even when statistical heterogeneity was high. We attempted to interpret the causes for this heterogeneity, but did not have enough data to use meta‐regression for this purpose.
Where possible, we have presented our data using forest plots. We have presented the summary estimate as a RR with 95% CI for dichotomous outcomes. Where we measured continuous outcomes in the same way across studies, we planned to present a pooled MD with 95% CI. We planned to pool standardised mean difference estimates where studies measured the same outcome, but had to use different methods. Unfortunately it was not possible for us to plot (and, if appropriate, to pool) estimates of hazard ratios and 95% CIs for time‐to‐event data, as there were insufficient data presented in the study reports. Where time to healing was analysed as a continuous measure, but it was not clear if all wounds had healed, we documented use of the outcome in the study, but did not summarise or use these data in any meta‐analysis.
We obtained pooled estimates of the treatment effect using Cochrane Review Manager 5 software (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
As we anticipated clinical heterogeneity in the effects of the interventions, we planned to conduct the following subgroup analyses where data were available.
-
Severity and depth of the wound, using whatever severity classification the authors used in each of the included RCTs; we were unable to do this.
-
Duration of follow‐up, using that provided in each included study. We defined short‐term follow‐up as 1 to 4 weeks, medium‐term follow‐up as from > 4 weeks to 24 weeks, and longer‐term follow‐up as > 24 weeks.
-
Stratifying studies according to overall risk of bias (Higgins 2011); we were unable to conduct this analysis due to limitations of the included studies.
Sensitivity analysis
Due to limitations of the data reported in the included studies, we were unable to conduct a planned sensitivity analysis using an alternative imputation assumption (such as available‐case analysis) to consider the effect on risk of bias where the percentage of missing data varied widely between groups.
'Summary of findings' tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2008), and constructed a 'Summary of findings' table using GRADEpro GDT software (GradePro GDT 2015).
These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schünemann 2011b). We have presented the following outcomes in the 'Summary of findings' tables:
-
complete ulcer healing;
-
infection (either incidence of developing, or resolution of established);
-
adverse events.
For relevant outcomes reported for comparisons not listed above, we presented GRADE assessment without a 'Summary of findings' table.
When evaluating the 'Risk of bias' domain, we downgraded the GRADE assessment only when we classified a study as being at high risk of bias for one or more domains, or when the 'Risk of bias' assessment for selection bias was unclear (this was classified as unclear for either the generation of the randomisation sequence or the allocation concealment domain). We did not downgrade for unclear 'Risk of bias' assessments in other domains.
We selected an informal optimal information size of 300 for binary outcomes, following the GRADE default value (Guyatt 2011). We also followed GRADE guidance and downgraded twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciate harm.
Results
Description of studies
Results of the search
The electronic and manual searches yielded a total of 665 citations (Figure 1). After excluding 590 records that were not relevant to the scope of this review, we assessed 75 records for eligibility and discarded 53 for various reasons (see Figure 1 and Characteristics of excluded studies). A total of 22 trials (reported in 21 individual papers) met our inclusion criteria (see Characteristics of included studies). Two studies are awaiting assessment as based on the available data we are unsure whether they are randomised controlled trials; we have contacted the study authors for further information. We will contact these authors again at the next update of this review.

Study flow diagram.
We also located reports of 15 trials listed in various trial registries. Five studies were ongoing, but it was unclear if they met the inclusion criteria for this review. Eight studies were terminated or completed, but we were unable to locate any associated published data. We attempted to contact the designated person for each of these trials and succeeded with five trials; we obtained some information on these trials, but there were no published data. Based on the available information, we were unable to judge whether or not any of these studies might be eligible for the review (Table 4).
| Title (comparator) | Current status | Relevant outcomes listed | Database | Results (# enrolled) | Listed contact | Company and any further information received |
| Phase IIa Randomised, Placebo Controlled Trial to Investigate Antimicrobial Photodynamic Therapy in Chronic Leg Ulcers and Diabetic Foot Ulcers (placebo = “cream”) | Prematurely ended (date unclear) | Photodynamic therapy using the combined effect of 3,7‐bis(N,N‐dibutylamino) phenothiazin‐5‐ium bromide (PPA904) and light; measure reduction of bacterial content of diabetic foot ulcers | ClincialTrialsRegister.eu EudraCT number: 2005‐001363‐58 | None (not listed) | None listed. | Photopharmacia |
| Pexiganan Versus Placebo Control for the Treatment of Mild Infections of Diabetic Foot Ulcers (OneStep‐1 and 2) | Completed (August 2016) | 1°: clinical response (resolution of infection); 2°: microbiological response; safety | ClinicalTrials.gov; NCT01594762 | No results (200 for each of the 2 trials) reported on website. | Robert Deluccia, Dipexium | Dipexium Pharmaceuticals, Inc. Multicentre study; all sites outpatient centre in USA |
| Comparison of Resin Salve and Octenidine in Patients with Neuropathic Diabetic Foot Ulcers (comparator: octenidine dihydrochloride‐impregnated gauze) | Completed (May 2015) | Investigate healing rate and healing time of neuropathic diabetic foot ulcer in people suffering from infected fore‐ or mid‐foot ulceration. 2°: eradication of bacteria; wound healing and infection | ClinicalTrials.gov; NCT02169167 | No results on website (n = 35) (see addendum in “comments”) | Janne J Jokinen | Salve prepared from Norway spruce (Repolar Ltd.) |
| Clinical Outcomes for Diabetic Foot Ulcers Treated With Clostridial Collagenase (SANTYL®) Ointment or With a Comparator Product Containing Silver (investigator choice of silver) | Running until January 2017 (last updated November 2016) | Randomly assigned to apply SANTYL or a topical treatment containing silver to their to foot ulcer. 1°: mean change in ulcer area at end of treatment; 2°: target ulcer infection rate | ClinicalTrials.gov; NCT02581488 | No results (102) | Jaime E Dickerson, PhD (Smith & Nephew) | (Smith & Nephew) Information from the sponsor received end of December 2016 stated that the trial is not yet complete but last participant out will be achieved in the next week. The trial enrolled its target number of participants, with the last participant completed December 2016. The evaluability will be carried out prior to the scheduled database lock in January 2017. As intention‐to‐treat is the analysis set for primary inference, it is anticipated that all participants will be included. Final study report is timed for April 2017 (15 December 2016). Waiting for further information to assess eligibility for review |
| Randomized, Controlled Study to Investigate the Efficacy and Safety of a Topical Gentamicin‐Collagen Sponge in Combination with Systemic Antibiotic Therapy in Diabetic Patients With a Moderate or Severe Foot Ulcer Infection | Recruiting (as of September 2013) | 1°: "clinical cure" at the test of cure; 2°: clinical response; time to clinical cure; eradication of baseline pathogen | ClinicalTrials.gov; NCT01951768 | No results (estimate 144) | Ilker Uckay, MD; Hospital of the University of Geneva | Innocoll, Inc. |
| Comparison of the Efficacy of Standard Treatment Associated with Phage Therapy Versus Standard Treatment Plus Placebo for Diabetic Foot Ulcers Monoinfected by Staphylococcus aureus: a Randomized, Multi‐centre, Controlled, 2‐parallel‐group, Double‐blind, Superiority Trial | Starting January 2017 | 1°: reduction in wound surface area; 2°: safety; changes in resistance and virulence of S aureus isolates; production of anti‐phage antibodies | ClinicalTrials.gov; NCT026647401 | No results (estimate 60) | Albert Sotto, MD, PhD +33.(0)6.09.56.66.55 | Centre Hospitalier Universitaire de Nīmes; Pherecydes Pharma. Per correspondence from Prof Sotto on 8 January 2017, National Agency for the Safety of Medicines and Health Products requested “pre‐clinical phase complements”, causing a postponement of the start of the clinical trial. |
| A Phase I/IIa, Randomized Double Blind, Placebo‐Controlled, Dose Escalating Study to Evaluate the Safety and Tolerability of Topically Applied Bisphosphocin Nu‐3 on Infected Diabetic Ulcers of Subjects With Type I or II Diabetes Mellitus (placebo) | Enrolling by invitation only (last verified April 2016) | Diabetic foot ulcers; infection localised to area of ulcer and mild. 1° outcome: treatment‐related adverse events, safety 2°: microbiological activity evaluated by wound assessments, presence of pathogenic bacteria | ClinicalTrials.gov; NCT02737722 | No results (estimate 30) | Paul DiTullio, MSc | Lakewood‐Amedex, Inc. |
| A Phase II, Randomized, Parallel, Double‐blind, Placebo‐controlled Study to Assess Prevention of Infection Using a Topical Gentamicin‐Collagen Sponge in Diabetic Patients With Uninfected Lower Extremity Skin Ulcers (placebo sponge) | Terminated (last verified March 2012) | 1° outcome: uninfected diabetic foot ulcers that remain free of signs/symptoms of infection to end of study 2°: days to wound closure; time to any signs/symptoms of infection; decrease in wound area; pathogen burden in infected wounds | ClinicalTrials.gov; NCT00658957 | No results (49) | David Prior, PhD; Chesapeake Foot and Ankle Center, Pasadena (MD), USA | Innocoll Pharmaceuticals |
| A Phase 3 Randomized, Placebo‐Controlled, Blinded Study to Investigate the Safety and Efficacy of a Topical Gentamicin‐Collagen Sponge in Combination With Systemic Antibiotic Therapy in Diabetic Patients With an Infected Foot Ulcer (COACT 1 and 2) (placebo is no sponge) | Last updated June 2016 | Sponge is adjunctive treatment to systemic antibiotic therapy. 1° outcome: per cent of participants with a clinical outcome of clinical cure (resolution of all clinical signs and symptoms of infection) ˜10 days after end of treatment; 2° outcomes: baseline pathogen eradication; re‐infection; time to clinical cure; amputation; ulcer closure | ClinicalTrials.gov: NCT02447172 | No results posted. | Nigel Jones, VP, Global Clinical Operations, Innocoll Pharmaceuticals | Innocoll Pharmaceuticals |
| Study of the Efficacy of Topical Application of Royal Jelly and Panthenol (PedyPhar® Ointment) on the Diabetic Foot Ulcers, an Open Label, Randomized, Non‐placebo‐controlled Study (active comparator panthenol ointment) | Terminated; (last updated February 2015) | Diabetic foot ulcers at any stage after proper surgical treatment (if needed) 1° outcome: healing of ulcer; 2°: reduction of infection in ulcer site; local reaction possibly related to study drug | ClinicalTrials.gov; NCT01531517 | No results (estimate 120; 47 enrolled) | (?) | European Egyptian Pharmaceutical Industries |
| Platelet Rich Fibrin in Combination With Topical Antibiotics or Antiseptics in the Treatment of Chronic Wounds ‐ a Prospective, Randomized, Active Controlled, Double Blind Pilot Trial With an Observer‐blinded Control Group (3 platelet rich fibrin arms & 1 active comparator (Acticoat)) | Recruiting (last verified January 2016) | People with infected chronic wounds (unclear if diabetic foot) 1° outcome: reduction of wound area; 2°: number requiring systemic antimicrobial therapy; C‐reactive protein level; wound volume; occurrence of drug‐resistant bacteria | ClinicalTrials.gov; NCT02652169 | No results (estimate 120) | Florian Thalhammer, Medical University of Vienna; 0043140400 ext 44400; [email protected] | Medical University of Vienna |
| Double Blind, Randomized, Placebo Controlled Clinical Trial for the Treatment of Diabetic Foot Ulcers, Using a Nitric Oxide Releasing Patch: PATHON | Completed (last verified November 2012) | 1° outcome: per cent reduction in ulcer size; 2°: complete cure of any infection; development of infection during treatment; adverse events | ClinicalTrials.gov; NCT00428727 | No results (?) | Fundación Cardiovascular de Colombia | (?) |
| A Phase I/II, Open Label, Controlled Study to Evaluate the Safety and Efficacy of AppliGel‐G (Gentamicin Sulfate Topical Gel) for Treatment of Mild to Moderately Infected Diabetic Foot Ulcers in Patients With Type 1 and Type 2 Diabetes (comparator oral ciprofloxacin and doxycycline alone) | Terminated (last verified May 2015) | For mild to moderately infected diabetic foot ulcers 1°: complete wound clearing of infection 2°: incidence infection cleared; wound volume and area change | ClinicalTrials.gov; NCT02036528 | No results | Royer Biomedical, Inc. | Royer Biomedical, Inc. |
| A Randomised, Double‐blind, Dose‐response, Placebo‐controlled, Multicenter, Phase IIA Clinical Study to Evaluate the Efficacy and Safety of Topical Application of G.68.y/EtOH in Patients with Type 1 or Type 2 Diabetes With Infected Foot Ulcers (placebo topical gel) | Completed | Enrolling patients with infected “grade 2 PEDIS” diabetic foot ulcers 1°: reduction of bacterial load 2°: maintenance of efficacy; tolerability and safety | EudraCT number: 2010‐019598‐13 | No results (plan for 60) | Molteni | |
| Trial to Assess Safety and Efficacy of Topical MBN‐101 (BisEDT ) in Patients With Moderate/ Severe Diabetic Foot Infections (placebo – vehicle‐controlled) | Not yet open for participant recruitment (last update March 2016) | Part I, participants will be enrolled into 1 of 3 escalating dose cohorts at a ratio of 3:1 (active to placebo). In Part II, participants will be randomised in a 1:1 ratio (active to placebo) based on the optimal dose demonstrated in Part I. People with infected foot ulcer | ClinicalTrials.gov; NCT02723539 | No results (plan for 88) | Department of Vascular Surgery, Rigshospitalet Copenhagen, Denmark, 2100 | Microbion Corporation |
Abbreviations: PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation
Included studies
We have presented an overview of the 22 included trials in Table 5 and all outcome data in Table 6.
| Intervention 1 | Intervention 2 | Foot ulcer grade | Infection status at baseline | Follow‐up | Review‐relevant outcomes with reportable data | |
| Group 1: (n = 30) Pyodine bath and saline and vaseline gauze dressing | Group 2: (n = 30) Phenytoin powder | Grade I or II | Not reported | 8 weeks | None reported | |
| Group 1: (n = 19) Gentamicin solution | Group 2: (n = 22) Cadexomer iodine ointment | Grade I or II | Not reported | 12 weeks |
| |
| Group 1: (n = 19) Standard care | Group 2: (n = 21) Chloramine plus standard care | Not reported | Infected | 24 weeks |
| |
| Group 1: (n = 10) Saline solution | Group 2: (n = 10) Super‐oxidised aqueous solution | Grade I or II | Not infected | 4 weeks |
| |
| Group 1: (n = 15) Foam dressing | Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing | Grade II or III | Not infected | 14 weeks |
| |
| Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improve microcirculation | Group 2: (n = 40) Silver ion dressing plus standard care | Not reported | Not reported | 4 weeks |
| |
| Group 1: (n = not reported) Iodine gauze | Group 2: (n = not reported) Hydrofiber dressing with silver | Ulcers with bone and tendon exposure | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Saline dressing | Group 2: (n = 195) Honey dressing | Grade I or II | Not reported | 17 weeks |
| |
| Group 1: (n = 20) Silver sulphadiazine cream | Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream | Grade I or II | Not reported | 6 weeks |
| |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing | Group 3: (n = 106) Iodine‐containing dressing | Not reported | Not reported | 24 weeks |
| |
| Group 1: (n = 67) Calcium‐alginate dressing | Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver | Grade I or II | Mixed infected and not infected | 8 weeks |
| |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered further) Group 2: (n = 20) Recombinant human platelet‐derived growth factor | Group 3: (n = 20) Antiseptic treatments (EUSOL, hydrogen peroxide, and povidone iodine) | Grade III or IV | Not reported | More than 8 weeks |
| |
| Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day Group 2: (n = 21) Super‐oxidised aqueous solution (topical Microcyn) alone (not considered) | Group 3: (n = 21) super‐oxidised aqueous solution (topical Microcyn) therapy plus 750 mg levofloxacin once per day | Eligible foot ulcers involved skin and deeper soft tissue | Infected | 4 weeks |
| |
| Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets | Full‐thickness wounds | Infected | Up to 42 days |
| |
| Group 1: (n = 38) Systemic antibiotic therapy alone | Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 16) Povidone iodine and saline | Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution | Not reported | Infected | 20 weeks |
| |
| Group 1: (n = 25) Conventional treatment (no further details translated) | Group 2: (n = 25) Zinc hyaluronate | Not reported | Unclear | 20 weeks |
| |
| (30 participants randomised, but number in each group not specified) | Group 1: Standard‐dressing group (povidone iodine solution 10%) (n not reported) | Group 2: Honey dressing group (n not reported) | Grade II | Not reported | Not reported | No useable data |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) | Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | 16 weeks |
| |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing | Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Grade I and II | Not reported | Not reported |
| |
| Group 1: (n = 19) Polyherbal formulation | Group 2: (n = 19) silver sulphadiazine cream | Grade I, II, and III | Unclear | 20 weeks | No useable data |
Abbreviations: EUSOL, Edinburgh University Solution of Lime
| Resolution of infection | Incidence of wound infection | Time to healing | Proportion of wounds healed | Microbial counts | Health‐related quality of life | Need for surgical resection, including partial or complete lower limb amputation | Safety (adverse events) | |
| Group 1: (n = 30) Povidone iodine bath and saline Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder plus povidone iodine bath and saline Vaseline gauze dressing Not infected at baseline | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Gentamicin solution Group 2: (n = 22) Cadexomer iodine ointment Baseline infection status not reported. | Not reported | Not reported | Not reported | Group 1: 2/18 Group 2: 5/17 | Not reported | Not reported | Surgical resection was reported: Group 1: 5/19 Group 2: 3/22 | Study reports that no adverse reactions related to the topical treatment were documented. |
| Group 1: (n = 19) Standard care alone Group 2: (n = 21) Chloramine plus standard care Infected at baseline | Group 1: 7/15 Group 2: 9/13 | Not reported | Time‐to‐event data presented with no reported hazard ratio. Given the small number of participants and events, no further attempts were made to calculate time‐to‐event values. | Healed at 24 weeks Group 1: 9/17 Group 2: 10/17 | Not reported | Not reported | Vascular procedure or amputation Group 1: 3/17 Group 2: 5/17 | Adverse event data reported but unable to get a per‐participant value, as it is noted that some participants had more than 1 event. |
| Group 1: (n = 10) Saline solution Group 2: (n = 10) Super‐oxidised aqueous solution Not infected at baseline | Not reported | Not reported | Not reported | Study notes that 15% of the study ulcers were healed, but this information not reported by group. | The bacterial load in the wound bed was defined as scattered (0/+), light (+), medium (++), or heavy (+++). At week 4 there was a reduction of 33% in the bacterial load versus baseline. Figure presented but difficult to interpret data by group. | Not reported | Not reported | No safety concerns were reported in either the super‐oxidised aqueous solution group or the saline group; no adverse reactions were recorded. |
| Group 1: (n = 15) Foam dressing Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing Not infected at baseline | Not reported | Wound infection Group 1: 4/13 Group 2: 0/23 | Not reported | Healed by week 14 Group 1: 4/13 Group 2: 12/23 | Not reported | Not reported | Not reported | Limited details of adverse events (in addition to infection data already recorded). There were no reported adverse events related to the use of collagen/oxidised regenerated cellulose/silver dressing, and 5 cases of adverse events (no further details) related to foam dressing. |
| Group 1: (n = 40) Routine debridement plus standard care Group 2: (n = 40) Silver ion dressing plus standard care Baseline infection status not reported. | Not reported | Not reported | Mean wound healing time in days: Group 1: 47.4 ± 11.5 Group 2: 31.3 ± 8.2 Mean granulation tissue occurrence time in days: Group 1: 10.8 ± 1.9 Group 2: 6.4 ± 0.72 | Group 1: 15/40 Group 2: 24/40 | Not reported | Not reported | Not reported | Not reported |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Treated with normal saline dressing Group 2: (n = 195) Treated with honey dressing | Not reported | Not reported | Median healing time in honey group is 18 days (IQR is 6 to 120), and in the saline group is 29 days (IQR 7 to 120). Data do not seem to have been calculated using correct time‐to‐event approaches and were not considered further. | Group 1: 97/169 Group 2: 136/179 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 20) Silver sulphadiazine Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream Baseline infection status not reported. | Not reported | Not reported | Not reported | Healed by week 6 Group 1: 6/20 Group 2: 8/20 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Baseline infection status not reported. | Not reported | Number of infected ulcers at 24 weeks: not reported by group Study reports the number of episodes of infection listed as serious adverse events, but it is unclear if foot infections, and not clear how many people had how many infection events. | Mean time to healing in days (SD) (fixed at max of 168 days) Group 2: 125.8 (55.9) Group 3: 127.8 (54.2) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed at 24 weeks: Group 2: 46/103 Group 3: 48/106 | Not reported | Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group 1: Physical functioning: 68.9 (19.1). Social functioning: 69.8 (23.5). Well‐being: 50.2 (21.1) Group 2: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well‐being: 53.1 (19.9) Other | Minor amputations (below ankle): Group 2: 1/103 n not clear; assumed to be all participants | Non‐serious adverse events Group 2: 227/103 Group 3: 239/106 Serious adverse events Group 2: 28/103 Group 3: 37/106 Not clear how many participants had how many events, but seems to be more than 1 per person; data not analysed further |
| Group 1: (n = 67) Calcium‐alginate dressing Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver Mixed wound infection status at baseline | Not reported | Group 1: 11/67 Group 2: 8/67 | Time to 100% healing also reported, but this is only for a subset of those that healed, so not a useful pan‐study measure. Not reported Mean time to healing in days Group 1: 52.6 ± 1.8 Group 2: 57.7 ± 1.7 | Number of ulcers healed in 8 weeks | Not reported | Not reported | Not reported | Group 1: 26/67 participants experienced adverse event. Death = 1; Infection = 8. 13 participants discontinued treatment due to adverse event. Group 2: 25/67 participants experienced 1 or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to adverse event. |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered in review) Group 2: (n = 20) Recombinant human platelet‐derived Group 3: (n = 20) Antiseptic dressings | Not reported | Not reported | Mean time to healing in weeks (standard error) Group 1: 6.83 (2.5) Group 2: 7.6 (2.5) Group 3: 6.75 (2.7) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed Group 1: 12/20 Group 2: 16/20 Group 3: 8/20 Review authors calculated figures from graph. | Not reported | Not reported | Not reported | Not recorded |
| Group 1: (n = 21) Levofloxacin plus saline Group 2: (n = 21) Super‐oxidised aqueous solution alone (not considered) Group 3: (n = 25) Levofloxacin plus super‐oxidised aqueous solution Ulcers infected at baseline. | Group 1: 6/21 Group 2: 11/21 Group 3: 11/25 | Not reported | Not reported | Mentioned, but data not presented. | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more event) Group 1: 7/21 Group 2: 7/21 Group 3: 9/25 |
| Group 1: (n = 246) Ofloxacin Group 2: (n = 247) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | See below ‐ results presented by study authors cumulatively for these 2 studies only. | Adverse events (number of participants with > 1 adverse event) Group 1: 109/246 Group 2: 98/247 |
| Group 1: (n = 171) Ofloxacin Group 2: (n = 171) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | Group 1: 9/417 Group 2: 11/418 (cumulative of two RCTs reported in single paper) | Adverse events (number of participants with > 1 adverse event) Group 1: 84/171 Group 2: 76/171 |
| Group 1: (n = 18) Systemic antibiotic therapy alone Group 2: (n = 38) Topical application of the gentamicin‐collagen sponge + systemic antibiotic therapy Ulcers infected at baseline. | Resolution of infection by 7 days Group 1: 7/18 Group 2: 22/38 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more events) Group 1: 5/18 Group 2: 11/38 |
| Group 1: (n = 16) Standard management with chemical antiseptics such as soap or povidone iodine Group 2: (n = 21) Super‐oxidised aqueous solution | Advances from infection to granulating tissue: Group 1: 10/16 Group 2: 19/21 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 25) Conventional treatment (no further details translated) Group 2: (n = 25) Zinc hyaluronate | Not reported/translated | Not reported/translated | Mean time to healing in weeks (not clear if standard deviation or standard error presented) Group 1: Only 2 ulcers healed; no time‐to‐event data reported Group 2: 7.80 (3.49) with all ulcers healing | Group 1: 2/25 Group 2: 25/25 | Not reported/translated | Not reported/translated | Not reported/translated | Not reported/translated |
| Group 1: Standard‐dressing group (povidone iodine solution 10%) Group 2: Honey dressing group 30 participants randomised, but number in each group not specified. | Not reported | Not reported | Time to healing in days Group 1: 15.4 days (range 9 to 36 days) Group 2: 14.4 days (range 7 to 26 days) Comment: mean and range, but no measure of variation provided. Unclear how many participants in each group and how many ulcers healed, thus if this measure is a valid time‐to‐healing measure | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | Data presented as time‐to‐event figure with no further data. Given the small number of participants and events, we have not tried to analyse further. | 16 weeks Group 1: 2/10 Group 2: 6/12 Unclear if ulcers were healed in the same or different participants; for the analysis we have assumed in different participants | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Not reported | Not reported | Not reported | Group 1: 0/2 Group 2: 2/2 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Polyherbal formulation Group 2: (n = 19) Silver sulphadiazine cream | Not reported | Not reported | "Number of days taken for healing of the wound: Group 1: 43.1 ± 26.8 Group 2: 43.6 ± 30.7" Not clear what sort of analysis was conducted | Healing was defined as complete epithelialisation either by secondary intention or by split skin graft. However, figures are not reported. | "the microbiological investigations were not done" | Not reported | Not reported | "There were no adverse events reported in both the groups." |
Abbreviations: IQR, interquartile range; SD, standard deviation
Trial design and location of conduct
The included trials had a combined total of 2310 participants; one trial did not report the total number of participants, so we did not consider the data from this study (Hwang 2010). The sample size of individual trials varied widely, ranging from 4 to 317; 17 (77%) of the trials had fewer than 100 participants or did not clearly report this number. The duration of follow‐up of the studies ranged from 4 to 24 weeks.
Three included trials were designed as three‐arm trials. One trial had two arms in which participants received a non‐antimicrobial treatment (Jeffcoate 2009); we combined these for analysis and compared them with the third trial arm, which was a topical antimicrobial treatment. The three‐arm trials of Khandelwal 2013 and Landsman 2011 had one arm that was not relevant to this review, so we did not consider it further.
The included trials were conducted in at least 10 countries:
-
China (He 2016);
-
Denmark (Gottrup 2013);
-
India (Khandelwal 2013; Ullal 2014; Viswanathan 2011);
-
Malaysia (Shukrimi 2008);
-
Mexico (Martinez‐De Jesus 2007; Ramos Cuevas 2007);
-
Pakistan (Ahmed 2014; Imran 2015);
-
Sweden Apelqvist 1996; Bergqvist 2016);
-
UK (Bowling 2011; Jeffcoate 2009; Jude 2007);
-
USA (Landsman 2011; Lipsky 2008a; Lipsky 2008b; Tom 2005);
-
UK and USA (Lipsky 2012a);
-
Not reported (Hwang 2010; Jacobs 2008).
Trial participants
We required that all trial participants had both diabetes mellitus and a foot wound. Eight studies noted that they included participants with grade I and II ulcers (using various assessment tools; see Characteristics of included studies) (Ahmed 2014; Apelqvist 1996; Bowling 2011; Imran 2015; Jacobs 2008; Jude 2007; Shukrimi 2008; Ullal 2014); one study included grade I to III ulcers (Viswanathan 2011); one study included grade II and III ulcers (Gottrup 2013); and one study included grade III and IV ulcers (Khandelwal 2013). The remaining 10 studies did not clearly report a grade, precluding the conduct of our planned subgroup analysis on severity of wound.
Nine trials reported the clinical infection status of the wound. Six trials included only ulcers that were reported by the study authors to be infected at baseline (Bergqvist 2016; Landsman 2011; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a; Martinez‐De Jesus 2007). One study included both infected and uninfected ulcers (Jude 2007), and two trials included non‐infected ulcers at baseline (Bowling 2011; Gottrup 2013). The remaining 13 studies did not report the infection status of ulcers at baseline. We were unable to report data for infected and uninfected wounds in comparisons unless this information was specifically noted.
Interventions evaluated
The studies evaluated several different types of topical antimicrobial agents (see Characteristics of included studies and Table 5 for a full list) including antimicrobial dressings (Gottrup 2013; He 2016; Hwang 2010; Imran 2015; Jeffcoate 2009; Jude 2007; Shukrimi 2008; Ullal 2014), super‐oxidised aqueous solutions (Bowling 2011; Landsman 2011; Martinez‐De Jesus 2007), zinc hyaluronate (Ramos Cuevas 2007), tretinoin (Tom 2005), silver sulphadiazine (Viswanathan 2011), gentamicin‐collagen sponge (Lipsky 2012a), pexiganan cream (Lipsky 2008a; Lipsky 2008b), and chloramine (Bergqvist 2016).
Nine studies compared a topical antimicrobial agent with standard wound care or placebo (Bergqvist 2016; Bowling 2011; Gottrup 2013; He 2016; Imran 2015; Jeffcoate 2009; Jude 2007; Ramos Cuevas 2007; Tom 2005). Eight studies compared one topical agent against another (Ahmed 2014; Apelqvist 1996; Hwang 2010; Jacobs 2008; Martinez‐De Jesus 2007; Shukrimi 2008; Ullal 2014; Viswanathan 2011). Four studies compared a topical antimicrobial treatment with systemic antibiotic therapy (Landsman 2011; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). One trial compared a topical antimicrobial treatment with a growth factor cream (Khandelwal 2013).
Excluded studies
We excluded 53 of the assessed studies, most often because: the study was found not to be a RCT (n = 20); the intervention(s) being evaluated were not eligible (n = 14); and participants in the study population were not eligible (n = 14) (see Figure 1 and Characteristics of excluded studies).
Risk of bias in included studies
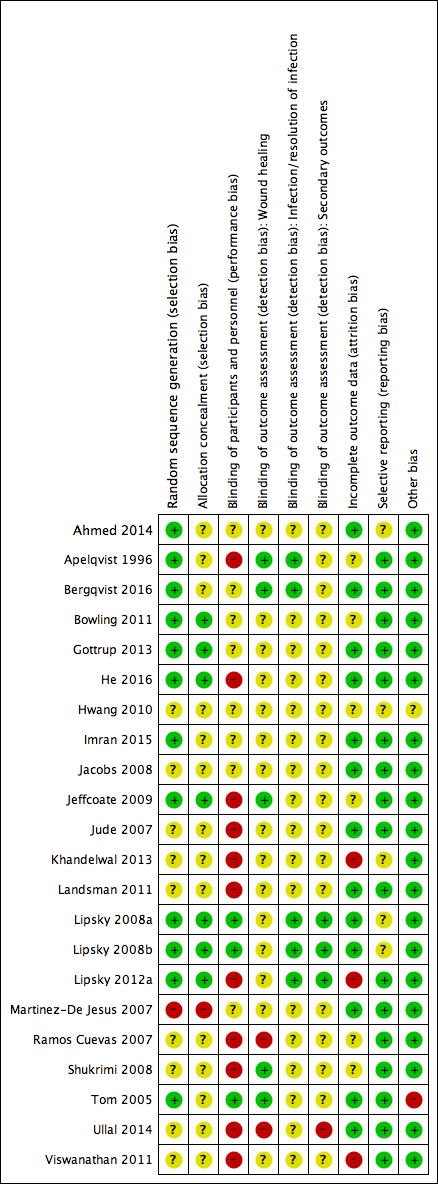
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We assessed no study as being at low risk of bias. We judged 12 studies (55%) as being at high risk of bias for one or more domains. We assessed the remaining 10 studies as being at unclear risk of bias for two or more domains.
Allocation
We assessed only one study as being at high risk of selection bias (Martinez‐De Jesus 2007), as the report's description of the randomisation process used was not clear and could have been alternation. We assessed seven studies as being at low risk of selection bias (Bowling 2011; Gottrup 2013; He 2016; Jeffcoate 2009; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a), and the reports of the remaining studies were unclear for random sequence generation or allocation concealment, or both.
Blinding
We assessed 11 studies as being at high risk of performance bias (Apelqvist 1996; He 2016; Jeffcoate 2009; Jude 2007; Khandelwal 2013; Landsman 2011; Lipsky 2012a; Ramos Cuevas 2007; Shukrimi 2008; Ullal 2014; Viswanathan 2011). In three studies the participants and staff did not know which of the treatments was being delivered (Lipsky 2008a; Lipsky 2008b; Tom 2005). In all of the other studies blinding status was unclear.
Five studies reported blinded outcome assessment for healing (Apelqvist 1996; Bergqvist 2016; Jeffcoate 2009; Shukrimi 2008; Tom 2005), and in two studies outcome assessment for healing was not blinded (Ramos Cuevas 2007; Ullal 2014). Detection bias (for wound healing) for the remaining studies was either unclear or not relevant, as the outcome was not reported.
Five studies were at low risk of bias for the reporting of infection data (Apelqvist 1996; Bergqvist 2016; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). Detection bias (infection status) for the remaining studies was either unclear or not relevant, as the outcome(s) was not reported.
Three studies were at low risk of detection bias for secondary outcomes (Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). Detection bias (for secondary outcomes) for the remaining studies was either unclear or not relevant, as relevant outcomes were not reported.
Incomplete outcome data
The risk of attrition bias was high in three studies (Khandelwal 2013; Lipsky 2012a; Viswanathan 2011), low in 13 studies, and unclear in the remaining studies.
Selective reporting
We judged five studies as at unclear risk of reporting bias, as we could not be certain if all outcomes had been reported (Ahmed 2014; Hwang 2010; Khandelwal 2013; Lipsky 2008a; Lipsky 2008b). We classified all other studies as being at low risk of reporting bias, although we did not obtain the full study protocol for any of these studies.
Other potential sources of bias
We judged all of the included studies as being at low risk of other sources of bias except for one, for which the limited available information precluded assessing this domain (Hwang 2010), and one judged at high risk of bias due to unit of analysis issues (Tom 2005).
Effects of interventions
See: Summary of findings for the main comparison Antimicrobial dressings compared with non‐antimicrobial dressings; Summary of findings 2 Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing); Summary of findings 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent; Summary of findings 4 Topical antimicrobial agent compared with systemic antimicrobial agent; Summary of findings 5 Topical antimicrobial agent compared with growth factor
For each comparison we have only listed outcomes for which there were reported data.
Comparison 1: Antimicrobial dressings compared with non‐antimicrobial dressings (standard care or placebo, or both) (5 trials; 945 participants)
See summary of findings Table for the main comparison.
Five trials met the criteria for this comparison: one with short‐term follow‐up, He 2016, and four with medium‐term follow‐up (Gottrup 2013; Imran 2015; Jeffcoate 2009; Jude 2007). Three studies evaluated silver‐containing dressings (Gottrup 2013; He 2016; Jude 2007), one a honey‐containing dressing (Imran 2015), and one an iodine‐containing dressing (Jeffcoate 2009). Wounds were not infected at baseline in one study (Gottrup 2013); mixed infected and not infected in one study (Jude 2007); and not reported in the remaining three studies.
Complete wound healing: proportion of ulcers healed (5 trials; 945 participants; 420 outcome events)
Using the average treatment effect from a random‐effects model, treatment with an antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period compared with non‐antimicrobial dressings: risk ratio (RR) 1.28, 95% confidence interval (CI) 1.12 to 1.45 (I² = 0%; low‐certainty evidence ‐ downgraded twice due to risk of bias) Analysis 1.1. This corresponds to an absolute risk (based on a combined event rate in the control arms of 425 per 1000) of 119 healing events per 1000 (95% CI from 51 more to 191 more). Where reported, the grade of ulcer in the studies ranged from I to III.
There was no evidence of a subgroup effect when studies were grouped based on their duration of follow‐up (test for subgroup differences P = 0.33, I² = 0%).
Incidence of infection (2 trials; 173 participants; 23 outcome events)
Using the average treatment effect from a random‐effects model, it is uncertain whether use of antimicrobial dressings reduces the incidence of an ulcer becoming clinically infected over a medium‐term follow‐up period when compared with non‐antimicrobial dressings: RR 0.34, 95% CI 0.04 to 3.10 (I² = 60%; very low‐certainty evidence ‐ downgraded twice due to imprecision, once due to inconsistency, and once due to risk of bias) Analysis 1.2.
Health‐related quality of life (1 trial; 317 participants)
One study measured health‐related quality of life (using the Cardiff Wound Impact Schedule and the SF‐36 at 24 weeks), presenting the data for each domain, but with no global summary score (Jeffcoate 2009). The study reported no significant difference between groups across domains. We have presented these data narratively (Table 6), but have not analysed them further.
Surgical resection (1 trial; 317 participants; 7 outcome events)
Based on data from only one study (Jeffcoate 2009), it is uncertain whether treatment with an antimicrobial dressing reduces the risk of amputation (minor or major) compared with a non‐antimicrobial dressing over a medium‐term follow‐up period: RR 0.33, 95% CI 0.04 to 2.72 (very low‐certainty evidence ‐ downgraded twice due to imprecision and once for risk of bias) Analysis 1.3.
Adverse events (1 trial with data analysed; 134 participants; 51 outcome events)
Whilst three studies reported adverse event data (Gottrup 2013; Jeffcoate 2009; Jude 2007), we analysed only the data from the one study that clearly reported rates per participant (Jude 2007).
It is uncertain whether antimicrobial dressings affect the risk of adverse events compared with non‐antimicrobial dressings over a medium‐term follow‐up period: RR 0.96, 95% CI 0.62 to 1.48 (very low‐certainty evidence ‐ downgraded twice due to imprecision and once for risk of bias) Analysis 1.4.
Comparison 1: Summary
Low‐certainty evidence suggests antimicrobial dressings probably increase the number of healing events in the medium term compared with non‐antimicrobial dressings. However, the effect of antimicrobial dressings on the incidence of infection, other outcomes, and adverse events is unclear (summary of findings Table for the main comparison).
Comparison 2: Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) (4 trials; 132 participants)
See summary of findings Table 2.
Four studies met the criteria for this comparison (Bergqvist 2016; Bowling 2011; Ramos Cuevas 2007; Tom 2005). Each study investigated a different non‐dressing topical treatment: chloramine (Bergqvist 2016), super‐oxidised aqueous solution (Bowling 2011), zinc hyaluronate (Ramos Cuevas 2007), and tretinoin (Ullal 2014). All studies had medium‐term follow‐up.
Complete wound healing: proportion of ulcers healed (3 trials; 112 participants; 54 outcome events)
Using the average treatment effect from a random‐effects model, the relative effect of non‐dressing antimicrobial treatments compared with non‐dressing non‐antimicrobial treatments is uncertain over a medium‐term follow‐up period: RR 2.82, 95% CI 0.56 to 14.23 (I² = 86%; very low‐certainty evidence ‐ downgraded twice for imprecision, once for inconsistency, and twice for risk of bias) Analysis 2.1.
Resolution of infection (1 trial; 40 participants; 16 outcome events)
One study reported data on resolution of clinical evidence of infection of ulcers during treatment (Bergqvist 2016). Of note, over half the participants in both treatment groups also received systemic antibiotic therapy during the study. It is unclear whether use of a non‐dressing antimicrobial topical treatment compared with non‐dressing non‐antimicrobial treatment affects the resolution of infection over a medium‐term follow‐up period: RR 1.16, 95% CI 0.54 to 2.51 (low‐certainty evidence ‐ downgraded twice for imprecision) Analysis 2.2.
Surgical resection (1 trial; 40 participants (data on 34 participants analysed); 8 outcome events)
One study reported data on the incidence of surgical resection (Bergqvist 2016). Data were missing in each arm for this outcome, although the report states that they conducted a complete‐case analysis. There was no clear evidence that use of a non‐dressing antimicrobial topical agent compared with non‐dressing non‐antimicrobial treatment affects the risk of surgical resection over a medium‐term follow‐up period: RR 1.67, 95% 0.47 to 5.90 (low‐certainty evidence ‐ downgraded twice for imprecision) Analysis 2.3.
Adverse events (2 trials; 81 participants; no trials reported data for analysis)
Two studies reported adverse event data over a medium‐term follow‐up period. We were unable to extract per‐participant values for one study (Bergqvist 2016), and the other study reported that no adverse events occurred in each arm (Bowling 2011). We considered this evidence to be of very low certainty.
Comparison 2: Summary
It is uncertain whether non‐dressing topical antimicrobial treatments affect wound healing, infection resolution, surgical resection, or adverse events compared with non‐dressing non‐antimicrobial treatments over a medium‐term follow‐up period. Data were available from only four small studies with limited outcome events, making them imprecise. The studies also evaluate a variety of treatment regimens (summary of findings Table 2).
Comparison 3: One topical antimicrobial agent compared with an alternative topical antimicrobial agent (8 trials; 250 participants (1 trial did not report number of participants))
See summary of findings Table 3.
Eight trials compared one topical antimicrobial agent with another (Ahmed 2014; Apelqvist 1996; Hwang 2010; Jacobs 2008Martinez‐De Jesus 2007; Shukrimi 2008; Ullal 2014; Viswanathan 2011). The comparisons varied, with no two comparisons the same (see Table 5). Reported outcome data were very limited, and we elected to present data from only four trials. All outcome data, including those that were not appropriate for analyses, are presented in Table 5.
Complete wound healing: proportion of ulcers healed (3 trials; 85 participants; 23 outcome events)
We included data from three studies in this analysis (Apelqvist 1996; Jacobs 2008; Ullal 2014). Due to the variation in treatments used in these studies, the data were not pooled.
Apelqvist 1996 (n = 41) compared treatment with a cadexomer iodine ointment to "standard treatment", which included a gentamicin solution, in people with a grade I or II ulcer and followed them for 12 weeks. It is uncertain whether there was a difference in the risk ratio of healing between these treatments: RR 2.16, 95% CI 0.47 to 9.88 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.1.
Jacobs 2008 (n = 40) compared silver sulphadiazine cream with a formulation containing benzoic acid, salicylic acid, and oak bark in people with a grade I or II ulcer and followed them for six weeks. It is uncertain whether there was a difference in the risk of healing between these treatments: RR 1.33, 95% CI 0.57 to 3.14 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.1.
Ullal 2014 (n = 4) compared treatment with a povidone iodine and metronidazole 1% gel dressing with a honey and metronidazole 1% gel dressing; neither the types of participants nor the duration of follow‐up were clearly reported. It is uncertain whether there was a difference in the risk of healing between these treatments: RR 5.00, 95% CI 0.38 to 66.01 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.1.
Resolution of infection (1 trial; 37 participants; 29 outcome events)
One study compared povidone iodine treatment with super‐oxidised aqueous solution (Martinez‐De Jesus 2007). All participants in both groups also received oral antibiotic therapy. It is uncertain whether there was a difference in the risk of infection resolution (defined largely by reduction in periwound cellulitis) between these treatments over a medium‐term follow‐up period: RR 1.45, 95% 0.97 to 2.17 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.2.
Surgical resection (1 trial; 41 participants; 8 outcome events)
One study that compared gentamicin solution with cadexomer iodine ointment reported data on the risk of surgical resection (Apelqvist 1996). It is uncertain whether there was a difference in the risk of surgical resection over a medium‐term follow‐up period: RR 1.93, 95% 0.53 to 7.03 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.3.
Adverse events (1 trial; 41 participants; no outcome events )
In the one study that reported this information there were no documented adverse reactions related to the topical treatment (Apelqvist 1996). We classified this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias.
Comparison 3: Summary
Whilst eight studies compared a variety of different antimicrobial topical agents against another, the outcome data were limited. Not all studies measured important outcomes, and the studies were small. We are uncertain about the relative effects of antimicrobial topical agents for all review outcomes, including wound healing and adverse events.
Comparison 4: Topical antimicrobial agents compared with systemic antimicrobials (4 trials; 937 participants)
See summary of findings Table 4.
Four studies compared therapy with a systemic (in all cases administered orally) antibiotic versus a topical antimicrobial treatment (Landsman 2011; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). Landsman 2011 compared levofloxacin (750 mg) and topical saline versus levofloxacin (750 mg) and super‐oxidised aqueous solution. Two studies (reported in one paper) compared ofloxacin (200 mg) with topical pexiganan cream (1%) (Lipsky 2008a; Lipsky 2008b), and one study compared systemic antibiotic therapy (largely levofloxacin) versus a gentamicin‐collagen sponge (Lipsky 2012a). We considered pooling the data to be appropriate, but given the different interventions in the classes of treatment being compared, we used a random‐effects model (although no there was no statistical heterogeneity and an I² of 0%).
Resolution of infection (2 trials; 102 participants; 46 outcome events)
It is uncertain whether or not, on average, topical antimicrobial treatment affects resolution of infection compared with systemic antibiotics in those with infected ulcers: RR 1.51, 95% CI 0.91 to 2.49 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 4.1. There was no evidence of a subgroup difference between one study that had short‐term follow‐up and one that had medium‐term follow‐up (P = 0.96; I² = 0%). The protocol we used for this review defined resolution of infection as the equivalent of clinical "cure". As two studies included participants with "improvement" along with "cure" (Lipsky 2008a; Lipsky 2008b), we could not use them for this comparison.
Surgical resection (2 trials (reported in 1 paper); 835 participants; 20 outcome events)
On average, there is no clear difference in the risk of surgical resection between treatments over a medium‐term follow‐up period: RR 1.22, 95% CI 0.51 to 2.91 (low‐certainty evidence ‐ downgraded twice for imprecision) Analysis 4.2.
Adverse events (4 trials; 937 participants; 399 outcome events)
On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments that were compared: RR 0.91, 95% CI 0.78 to 1.06 (moderate‐certainty evidence ‐ downgraded once for inconsistency) Analysis 4.3. There was no evidence of a subgroup difference for studies with short‐ versus medium‐term follow‐up (P = 0.67; I² = 0%).
Comparison 4: Summary
There is no evidence from RCTs on the relative effects of systemic antibiotics compared with topical antimicrobial agents on wound healing. Data on resolution of infection in infected wounds and on the need for surgical resection are limited, as studies are small with limited outcome events, resulting in low statistical power. On average, there is probably no difference in adverse events between the systemic and topical treatments we evaluated.
Comparison 5: Topical antimicrobial agents compared with growth factor (1 trial; 40 participants)
See summary of findings Table 5.
One study compared a topical application of growth factors versus antiseptic dressings (not described further in the study report) in people with a grade III or IV ulcer (Khandelwal 2013); the duration of follow‐up was described only as more than eight weeks.
Complete wound healing: proportion of ulcers healed (1 trial; 40 participants; 24 outcome events)
It is uncertain whether treatment with growth factors affects the risk of healing when compared with an antiseptic dressing: RR 0.50, 95% 0.28 to 0.89 (very low‐certainty evidence ‐ downgraded once for imprecision and twice for risk of bias) Analysis 5.1.
Comparison 5: Summary
In terms of healing, the relative effect of topical antimicrobial agents compared with growth factors remains uncertain. No other RCT data were available concerning any of the other outcomes relevant to this review.
Discussion
Summary of main results
This review includes 22 RCTs with a cumulative total of over 2310 participants (one trial did not report the number of study participants). These studies were grouped into five comparisons, as summarised below. The certainty of the available evidence was largely of low or very low.
Pooled data for non‐antimicrobial dressings compared with antimicrobial dressings suggests (based on the average treatment effect from a random‐effects model) that more wounds in people treated with antimicrobial dressings may completely heal. In absolute terms, the results correspond to an additional 119 healing events in the antimicrobial‐dressing arm per 1000 (95% CI from 51 more to 191 more). This finding was based on low‐certainty evidence from 5 studies that enrolled a total of 945 participants and reported 420 outcome events. An assessment of low‐certainty evidence means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Data on adverse events or other outcomes produced very low‐certainty evidence, due to the limited number of included studies and their small sizes in terms of participants or events, or both.
Pooled data from non‐dressing non‐antimicrobial topical treatments compared with non‐dressing antimicrobial topical treatments produced low‐ and very low‐certainty evidence (based on the results from 4 trials with a total of 132 participants) for the proportion of wounds healed, resolution of infection, surgical resection, and adverse events.
Eight studies with a total of 250 participants compared different topical antimicrobial treatments (the comparisons varied, so these data were not pooled). Reported outcome data were limited, leading us to conclude that the evidence for the relative effects of any one antimicrobial topical treatment versus another was of very low certainty for all review outcomes, including wound healing and adverse events.
Four studies (937 participants) compared systemic antibiotics with topical antimicrobial treatments. No wound‐healing data were reported, and there was very low‐certainty evidence for the relative effects of the various agents on resolution of wound infection and need for surgical resection. Using an average treatment effect, it is possible that there is no difference in adverse events between the systemic and topical antimicrobial treatments evaluated here.
One included study (40 participants) compared the use of growth factors to topical antimicrobials. For the only outcome reported that was relevant to this review, that is the effect on the number of ulcers, the data were of very low certainty.
Overall completeness and applicability of evidence
Overall, the evidence for this review question was limited. Whilst we included 22 studies, these studies evaluated a wide range of treatment options, leading to a lack of homogeneity and limited data for specific comparisons of interest. It was also not clear how the evaluations undertaken in these studies relate to current practice, which is also likely to be varied. This variation is reflected in our analytical approach of often viewing non‐antimicrobial topical treatments and antimicrobial topical treatments as a 'class', despite the obvious variations within these treatments. For example, our 'class' of antimicrobial dressings contains trials of agents as varied as those using silver, iodine, and honey; readers should bear this in mind when interpreting our findings. We have frequently used random‐effects models, which allow for the treatment effects to vary from study to study following a normal distribution. We have thus assumed that there might be real variation between the relative effects of these classes of treatment, as well as random error.
In addition to the variation in treatments and types of wounds evaluated, the generally poor reporting of the included studies means that we have limited wound‐related baseline information in terms of the infection status and the severity of wounds. In studies in which authors reported an ulcer 'grade', they used different (and sometimes ill‐defined) measures. Specifically, 10 of the 22 studies (45%) did not report data on baseline ulcer severity, and 13 (59%) of the studies did not report baseline infection status. Nevertheless, we have summarised based on the general pattern that increasing wound grade denoted increased disease severity.
We also note that we have located details of eight trials from the trials registry that may be eligible for the review but that seem to be unpublished. We are continuing to try to obtain details of these studies.
Our review of this topic allowed us to identify several key issues that we think investigators should consider when planning future trials of topical antimicrobial agents for treating clinically infected or uninfected foot ulcers in people with diabetes. Firstly, it is essential to use a standard, and preferably validated, method of classifying the wounds, especially insofar as their infection status (for which the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (IDSA/IWGDF) classification seems most appropriate). While clinical definitions are imperfect, microbiological, imaging, and biomarker definitions of infection are less well validated. Secondly, investigators should be clear on how they define both the presence, and the resolution, of infection in the wounds. Thirdly, they should use consistent, and preferably validated, methods of measuring the healing of wounds (preferably using complete epithelialisation). Finally, the protocols used should clarify the primary and any secondary outcomes to be used for each type of wound (Clarke 2007). Following this approach may reduce the risk of reporting bias (Kirkham 2010). In addition, all trials should follow these key recommendations for good practice: include the robust generation of a randomisation sequence (e.g. by a computer‐generated randomisation schedule); use a robust method of allocation concealment (e.g. through the use of a telephone randomisation service); and ensure blinded outcome assessment where possible. Blinded outcome assessment is crucial for outcomes such as healing and infection, which are inherently subjective, thus introducing the risk of detection or observer bias (Hróbjartsson 2012).
Quality of the evidence
The quality of reporting in the included studies was limited. Not all trials reported the same outcomes, and many did not report key outcomes on infection prevention or resolution, or on wound healing. Over 75% of included studies were at unclear risk for selection bias and for detection bias, which are key domains. Many of the studies were also at risk of performance bias (which is avoided by blinding participants and healthcare professionals to treatments). While the risk of performance bias is not yet clear in wound care studies, the importance of detection bias (avoided by employing a blinded outcome assessment) is well recognised (Hróbjartsson 2012). The reporting of adverse events was poor or absent in the large majority of studies. It was thus difficult or impossible for us to make accurate assessments of the risk of adverse events that were specifically associated with the tested topical agents and their comparators.
In addition to the 'Risk of bias' issues, the included studies were also often small in terms of numbers of participants and numbers of documented outcome events. These factors are reflected in our assessment of the certainty of evidence, which was often low or very low.
Potential biases in the review process
Three of the included trials were led by one of the authors of this review (Lipsky 2008a; Lipsky 2008b; Lipsky 2012a); to overcome this potential bias, three other review authors (MC, MF, JD) conducted the data extractions, 'Risk of bias' assessments, and analyses.
We conducted a comprehensive search that included trial registries, and obtained translations to English as required, so we do not believe that language bias is an issue. We do not know the risk publication bias, as we were unable to explore this with the available studies. As we did not deviate from our original (and previously published) protocol for this review, with the exception of those changes highlighted in Differences between protocol and review, we do not believe we introduced bias in terms of selective outcome reporting.
It is noteworthy that we found 15 studies reported on a trial register that we are unable to link to published data. We emailed all available trial contacts to try to obtain additional data but were successful in only four cases. The risk of publication bias is increased if unpublished data exist that were not available for inclusion in the review.
Agreements and disagreements with other studies or reviews
There are few other reviews specifically examining the role of topical antimicrobial therapy for diabetic foot wounds or infections. One Cochrane review examined the role of systemic (but not topical) antibiotics for treating diabetic foot infections (Selva Olid 2015). Another Cochrane review examined data on silver‐based wound dressings and topical agents for treating diabetic foot ulcers (Bergin 2006), and concluded (as we did) that there are no randomised trials or controlled clinical trials evaluating their clinical effectiveness. Similarly, another Cochrane review concluded that there was insufficient evidence to establish whether or not silver‐containing dressings or topical agents promote wound healing or prevent wound infection (Vermeulen 2007). A review of the evidence for the use of topical antimicrobial agents in wound care concluded that despite limited data, judicious prophylactic use of antiseptics may prevent the development of infections while minimising antibiotic use, as well as promote faster healing (Cooper 2004). This review also noted that it was important to avoid misuse and abuse of topical antiseptics. In a review of the use of topical antimicrobial agents for treating various kinds of chronic wounds, the authors concluded that there are few proven indications for these agents (Lipsky 2009). Guidelines on management of diabetic foot infections from both the Infectious Diseases Society of America and the International Working Group on the Diabetic Foot (Lipsky 2012a; Lipsky 2016), recognising the scarce data, offered similar recommendations to ours on the current limited role of topical antimicrobial agents. A recent systematic review of the effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes included in their search papers on wound bed preparation using antiseptics, applications, and dressing products (Game 2016). They concluded that there is little published evidence to justify the use of any of these therapies.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
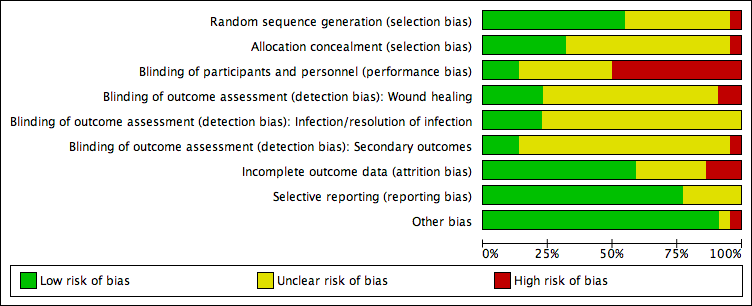
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 1 Proportion of wounds healed.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 2 Incidence of infection: medium term follow‐up.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 3 Surgical resection: medium term follow‐up.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 4 Adverse events.

Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 1 Proportion of wounds healed: medium term follow‐up.
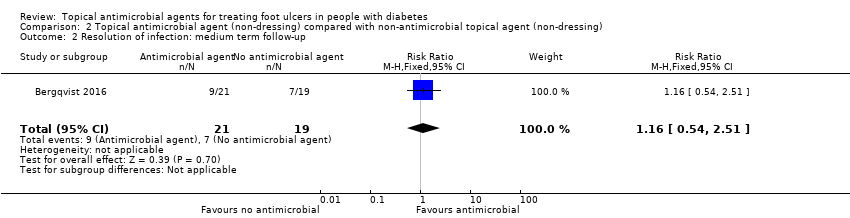
Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 2 Resolution of infection: medium term follow‐up.

Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 3 Surgical resection: medium term follow‐up.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 1 Proportion of wounds healed.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 2 Resolution of infection: medium term follow‐up.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 3 Surgical resection: medium term follow‐up.

Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 1 Resolution of infection.
Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 2 Surgical resection: medium term follow‐up.

Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 3 Adverse events.
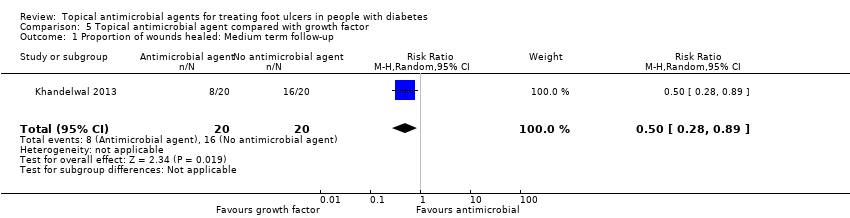
Comparison 5 Topical antimicrobial agent compared with growth factor, Outcome 1 Proportion of wounds healed: Medium term follow‐up.
| Antimicrobial dressings compared with non‐antimicrobial dressings | ||||||
| Patient or population: Foot ulcers in people with diabetes Settings: Mixed Intervention: Antimicrobial dressings Comparison: Standard dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard dressings | Risk with antimicrobial dressings | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 425 per 1000 | 544 per 1000 | RR 1.28 (1.12 to 1.45) | 945 participants (5 studies) | ⊕⊕⊝⊝ | On average, use of an antimicrobial dressing compared with a non‐antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period. |
| Risk difference: 119 more healed wounds per 1000 (51 more to 191 more) | ||||||
| Incidence of infection Up to 24 weeks' follow‐up | 183 per 1000 | 62 per 100 (7 to 567) | RR 0.34 (0.04 to 3.10) | 173 participants (2 studies) | ⊕⊝⊝⊝ | On average, it is unclear whether or not use of an antimicrobial dressing compared with a non‐antimicrobial dressing reduces the incidence of ulcer infection over a medium‐term follow‐up period. |
| Risk difference: 121 fewer infections per 1000 (176 fewer to 384 more) | ||||||
| Resolution infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Adverse events Up to 24 weeks' follow‐up | 388 per 1000 | 373 per 1000 (241 to 574) | RR 0.96 (0.62 to 1.48) | 134 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether use of an antimicrobial dressing affects the risk of adverse events compared with use of a non‐antimicrobial dressing over a medium‐term follow‐up period. |
| Risk difference: 16 fewer adverse events per 1000 (147 fewer to 186 more) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias due to one study (with the highest weighting in the meta‐analysis) being at unclear risk of selection bias and three studies being at high risk of performance bias (36% weighting in analysis), although the studies were at unclear or low risk of detection bias for this outcome. | ||||||
| Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with non‐antimicrobial treatment | Risk with topical antimicrobial treatment | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 241 per 1000 | 679 per 1000 (135 to 1000) | RR 2.82 (0.56 to 14.23) | 112 participants (3 studies) | ⊕⊝⊝⊝ | The average effect of antimicrobial agents compared with non‐antimicrobial treatment is uncertain over a medium‐term follow‐up period. |
| Risk difference: 438 more healed wounds per 1000 (106 fewer to 1000 more) | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 368 per 1000 | 427 per 1000 (199 to 925) | RR 1.16 (0.54 to 2.51) | 40 participants (1 study) | ⊕⊕⊝⊝ | It is unclear whether use of an antimicrobial topical agent has an effect on risk of infection over a medium‐term follow‐up period. |
| Risk difference: 59 more resolved infections per 1000 (169 fewer to 556 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 81 participants (2 studies) | ⊕⊝⊝⊝ | 2 studies reported adverse event data. We were unable to extract per‐participant data for 1 study. The second study stated that no adverse events were reported in each arm. We judged this as very low‐certainty evidence. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias with two studies at high risk of detection bias, which is of particular concern when healing is being assessed, and one study not accounting for a small number of participants with multiple ulcers in their trial. Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for inconsistency: one small study reported all wounds healed in one arm and few wounds healed in the other. These data are adding heterogeneity to the analysis. | ||||||
| One topical antimicrobial agent compared with another topical antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical antimicrobial agent | Risk with alternative topical antimicrobial agent | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | Data were not pooled due to the 3 studies evaluating different interventions. | N/A | 85 participants (3 studies) | ⊕⊝⊝⊝ | It is generally uncertain whether 1 topical treatment has an increased likelihood of healing compared with the alternative treatment. We judged this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias. | |
| Incidence of infection Up to 24 weeks' follow‐up | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 625 per 1000 | 906 per 1000 (606 to 1000) | RR 1.45 (0.97 to 2.17) | 37 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether 1 specific type of topical antimicrobial agent has a different effect on resolution of infection than another over a medium‐term follow‐up period. |
| Risk difference: 281 more resolved infections per 1000 (19 fewer to 731 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 41 participants (1 study) | ⊕⊝⊝⊝ | The 1 study noted that no events were reported in either group. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded for risk of performance and detection bias. | ||||||
| Topical antimicrobial agent compared with systemic antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic antibiotic agent | Risk with topical antimicrobial agent | |||||
| Proportion of wounds healed | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | 333 per 1000 | 503 per 1000 (303 to 830) | RR 1.51 (0.91 to 2.49) | 102 participants (2 studies) | ⊕⊝⊝⊝ | It is uncertain whether the effects of topical antimicrobial treatment on resolution of infection differ from those of systemic antibiotics. |
| Risk difference: 170 more resolved infections per 1000 (30 fewer to 497 more) | ||||||
| Adverse events | 450 per 1000 | 409 per 1000 (351 to 477) | RR 0.91 (0.78 to 1.06) | 937 participants (4 studies) | ⊕⊕⊕⊝ moderate2 | On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments compared here. |
| Risk difference: 40 fewer adverse events per 1000 (99 fewer to 27 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for risk of performance bias. | ||||||
| Topical antimicrobial agent compared with growth factor | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with growth factor | Risk with topical antimicrobial | |||||
| Proportion of wounds healed | 800 per 1000 | 400 per 1000 (224 to 712) | RR 0.50 (0.28 to 0.89) | 40 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether treatment with growth factor affects the risk of healing when compared with antiseptic dressing. |
| Risk difference: 400 fewer resolved infections 576 fewer to 88 fewer | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Adverse events | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision: small sample size and small number of events ‐ optimal information size not met and results are fragile. Downgraded twice for risk of performance and attrition bias. | ||||||
| Clinical manifestation of infection | PEDIS grade | IDSA infection |
| No symptoms or signs of infection | 1 | Uninfected |
| Infection present, as defined by the presence of at least 2 of the following items:
| ||
| Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). If erythema, must be > 0.5 cm to ≤ 2 cm around the ulcer. | 2 | Mild |
| Local infection (as described above) with erythema > 2 cm, or involving structures deeper than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic arthritis, fasciitis), and no systemic inflammatory response signs (as described below) | 3 | Moderate |
| Local infection (as described above) with the signs of SIRS, as manifested by ≥ 2 of the following:
| 4 | Severe* |
| Abbreviations: IDSA, Infectious Diseases Society of America; PaCO2, partial pressure of arterial carbon dioxide; PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation; SIRS, systemic inflammatory response syndrome *Ischaemia may increase the severity of any infection, and the presence of critical ischaemia often makes the infection severe. Systemic infection may sometimes manifest with other clinical findings, such as hypotension, confusion, vomiting, or evidence of metabolic disturbances, such as acidosis, severe hyperglycaemia, and new‐onset azotaemia. | ||
| Product and formulations | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Acetic acid | 0.25%, 0.5%, and 1% solutions | Bactericidal against most gram‐positive and gram‐negative organisms, including Pseudomonas aeruginosa | Inexpensive; shown to eliminate P aeruginosa colonisation from burn | Cytotoxic in vitro although maybe not in vivo; limited activity against biofilm | $ | No longer as widely used as in the past |
| Cadexomer iodine | Gel,c ointment, and dressing | Polysaccharide starch lattice; active agent is slowly released free iodine; broad spectrum of activity (same as iodine) | Reduced local toxicity compared to iodine; elemental iodine released on exposure to exudate | Application may cause stinging and erythema, but less tissue damage than other iodine products; effect may not persist, and efficacy may be reduced in body fluids. | $$ | Indicated for use in cleaning wet ulcers and wounds and reducing microbial load in the wound environment |
| Cetrimide | Solution, 40% | Active against bacteria and fungi; not active against P aeruginosa | May be less toxic to wound tissues than other antiseptics | May be corrosive and is potentially harmful if swallowed | $ | Not available in the USA |
| Chlorhexidine gluconate | Solution, 2% and 4%; liquid, 2% and 4%; hand rinse, 0.5%; wipes, 0.5%; sponge/brush, 4%; and foam, 4% | Active against gram‐positive bacteria (e.g. Staphylococcus aureus) and gram‐negative bacteria, including P aeruginosa | Persistent activity up to 6 h after application; few adverse effects | Hypersensitivity, including anaphylaxis, generalised urticaria, bronchospasm, cough, dyspnoea, wheezing, and malaise; may cause serious injury to the eye and middle ear; avoid contact with face or head; some resistance reported | $ | 2% chlorhexidine indicated as surgical hand scrub, hand wash, skin and wound cleanser; polyhexanide is a similar, newer biguanide. |
| Hexachlorophene | Liquid, 3%; foam, 0.23% with 56% alcohol | Biguanide that is bacteriostatic against Staphylococcus species and other gram‐positive bacteria | May retain residual effect on skin for several days | Rapidly absorbed and may result in toxic blood levels; application to burns has resulted in neurotoxicity and death; may cause central nervous system stimulation and convulsions, dermatitis, and photosensitivity reactions | $$$ | Not recommended for routine use on wounds due to potential toxicity |
| Iodine compounds and iodine tincturec | Solution (aqueous) 2% and 2.4%; and tincture (44% to 50% alcohol) 2% and 2.4% | Microbicidal against bacteria, fungi, viruses, spores, protozoa, and yeasts | Broad spectrum | Highly toxic if ingested or significantly absorbed; do not use with occlusive dressings; causes pain and stains skin and clothing; use cautiously in people with thyroid disorders | $ | Iodine compounds are now rarely used for wound management; cadexomer iodine and povidone iodine products are less toxic. |
| Povidone iodinec | Ointment, 1%, 4.7%, 10%; solution, 1% and 10%; also wash, scrub, cleanser, gel, aerosol, gauze pad, swab, and other forms | Broad spectrum includes S aureus and enterococci; active ingredient is liberated free iodine; shares spectrum but is less potent than iodine | Less irritating to skin and allergenic than iodine. Can be covered with dressings. Clinically significant resistance very rare | Antibacterial action requires at least 2 min contact; may cause stinging and erythema; effect may not persist, and efficacy may be reduced in body fluids; prolonged use may cause metabolic acidosis; stains skin and clothing; possible interaction with starches in dressings | $ | Indicated for perioperative skin cleansing and for cleansing and prevention of infection in superficial burns, incisions, and other superficial wounds |
| Sodium hypochlorite (Dakin’s solution and EUSOL) | Solution, 0.0125%, 0.125%, 0.25%, and 0.5% | Vegetative bacteria, viruses, and some spores and fungi | Inexpensive | No known systemic toxicity. May require prolonged contact for antibacterial action; inactivated by pus; toxic to fibroblasts and keratinocytes, and may cause pain or lyse blood clots | $ | A concentration of 0.025% is both bactericidal and non‐toxic to tissues (Heggers 1991). |
| Hydrogen peroxidec | Solution, 1% and 3%; and cream, 1% | Oxidizing agent active against many gram‐positive and gram‐negative bacteria | Broad‐spectrum, bactericidal, inexpensive; no known 1q11 | May cause some discomfort | $ | Commonly used, but few clinical studies |
| Silver nitrate | Solution 0.5%, 10%, 25%, and 50%; ointment, 10%; and swabs, 25% to 50% | Silver ions are bactericidal against a broad spectrum of gram‐positive and gram‐negative bacteria. | Low cost; easily applied | Painful on application; stains tissues; may delay healing; concentrations 10.5% cause cauterisation; inactivated by wound exudates and chlorine | $ | Previously widely used, but now largely replaced by other compounds, including newer silver dressings |
| Silver dressings | At least 6 approved products with different properties | Slowly released silver ions have broad spectrum, including MRSA and VRE. | Provide sustained levels of active silver ions; microbial resistance is rare; less painful and few adverse effects than silver nitrate; variety of products adaptable to different types of wounds; infrequent application required | Levels of silver ions at wound interface not well defined; may cause silver staining of tissues; may delay epithelialisation; relatively expensive; few published comparative trials | $$ | Should not substitute for non‐medicated dressings for uninfected wounds; may be useful for subclinically infected, highly colonised wounds or for wounds being prepared for skin grafting |
| Abbreviations: EUSOL, Edinburgh University Solution of Lime; MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococci. aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. | ||||||
| Product and | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Bacitracin c | Ointment, 500 units/g; and powder combinations with neomycin, polymyxin B, and zinc | Many gram‐positive organisms, including aerobic staphylococci and streptococci, corynebacteria, anaerobic cocci, and clostridia; inactive against most gram‐negative organisms | Activity not impaired by blood, pus, necrotic tissue, or large bacterial inocula; resistance is rare but increasing among staphylococci; no cross‐resistance with other antibiotics; minimal absorption | May cause allergic reactions, contact dermatitis, and (rarely) anaphylactic reactions; may lead to overgrowth of drug‐resistant organisms, including fungi | $ | Widely used for many years; indicated for prevention of infection in minor skin wounds |
| Fusidic acid | Cream, 2%; ointment, 2%; and gel, 2% | Staphylococcus aureus, streptococci (in topical concentrations), corynebacteria, and clostridia | Penetrates intact and damaged skin as well as crust and cellular debris | Occasional hypersensitive reactions; resistance among staphylococci is emerging; must apply 3 times daily | $$ | Not available in the USA |
| Gentamicin | Cream, 0.1%; and ointment, 0.1% | Streptococci, staphylococci, Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae | Broad spectrum; inexpensive | Must be applied 3 to 4 times daily; may drive resistance to an agent used systemically | $ | Indicated for primary skin infections (pyodermas) and secondary skin infections, including infected excoriations, and for bacterial superinfections |
| Mafenide acetate | Solution, 5%; and cream, 85 mg/g | A sulfonamide that is bacteriostatic against many gram‐negative organisms, including P aeruginosa, and some gram‐positive organisms, but minimal activity against staphylococci and some obligate anaerobes | Remains active in the presence of pus and serum, and its activity is not affected by acidity of environment | Systemic absorption may occur; drug and metabolites may inhibit carbonic anhydrase, potentially causing metabolic acidosis; use cautiously in patients with renal impairment; pain on application; hypersensitive reactions. | $$$ | Indicated as adjunctive therapy in second‐ and third‐degree burns; may be used in rapidly progressing bacterial necrotising fasciitis; limited use in other wounds |
| Metronidazole | Cream, 0.75%; gel, 1%; lotion, 0.75% | Many clinically important anaerobic bacteria | May reduce odour associated with anaerobic infections; application only 1 to 2 times daily | Relatively expensive; systemic formulations available; could drive resistance to these | $–$$ | Indicated for inflammatory papules and pustules of rosacea |
| Mupirocin and mupirocin calcium | Ointment, 2%; for mupirocin calcium, cream, 2.15%; and nasal ointment, | Gram‐positive aerobes, including S aureus (most MRSA), Staphylococcus epidermidis, Staphylococcus saprophyticus, and streptococci (groups A, B, C, and G) but not enterococci, some gram‐negative aerobes (not P aeruginosa), corynebacteria, and obligate anaerobes | Minimal potential for allergic reactions | Rare local burning and irritation; applying ointment to large wounds in azotaemic patients can cause accumulation of polyethylene glycol; long‐term use can lead to resistance among staphylococci, which is increasing | $$ | Indicated for topical treatment of impetigo and eradication of nasal colonisation with S aureus |
| Neomycin sulfatec | Powder; cream, 0.5%; combinations with polymyxin B and pramoxine, and ointment, 0.5%; combinations with bacitracin, polymyxin B, lidocaine, and pramoxine | Good for gram‐negative organisms but not P aeruginosa; active against some gram‐positive | Low cost; applied only 1 to 3 times daily; may | Topical powder in wound irrigating solution | $ | Use of topical powder alone or in solution is not recommended; cream and ointment, in combination with other agents, are indicated for prevention of infection in minor skin injuries. |
| Nitrofurazone | Solution, 0.2%; ointment, 0.2%; and cream, 0.2% | Broad gram‐positive and gram‐negative activity, | Used mainly for burn wounds | Hypersensitive reactions; polyethylene glycols (in some formulations) may be absorbed and can cause problems in azotaemic patients | $$ | Indicated as adjunctive to prevent infections in people with second‐ and third‐degree |
| Polymyxin Bc | Cream, 5000 units/g or | Bactericidal against many gram‐negative organisms, | Inexpensive | Some hypersensitive and neurological or | $ | Only available in combination with other agents, including bacitracin and neomycin; |
| Retapamulin | Ointment, 1% | Active against staphylococci (but uncertain | May be active against some mupirocin‐resistant S aureus strains; broader activity than mupirocin | Not evaluated for use on mucosal surfaces; may cause local irritation | $$$ | Indicated for impetigo due to S aureus (methicillin‐susceptible only) or Streptococcus pyogenes |
| Silver sulphadiazine | Cream, 1% | A sulfonamide; the released silver ions are the primary active ingredient; active against many gram‐positive and gram‐negative organisms, including P aeruginosa | Applied only once or twice daily; soothing | Potential cross‐reaction with other sulphonamides; may rarely cause skin staining | $ | Indicated as adjunctive treatment to prevent |
| Sulfacetamide Na+ | Lotion, 10% | Bacteriostatic against many gram‐positive and gram‐negative pathogens | Broad spectrum; can be combined with sulphur | Systemic absorption and rarely severe side | $$$ | Indicated for secondary bacterial skin infections |
| There are no published studies supporting the use of topical erythromycin, clindamycin, aminoglycosides other than neomycin, gramicidin, or tetracyclines for treating chronically infected wounds. Abbreviations: FDA, US Food and Drug Administration; MRSA, methicillin‐resistant Staphylococcus aureus. aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. | ||||||
| Title (comparator) | Current status | Relevant outcomes listed | Database | Results (# enrolled) | Listed contact | Company and any further information received |
| Phase IIa Randomised, Placebo Controlled Trial to Investigate Antimicrobial Photodynamic Therapy in Chronic Leg Ulcers and Diabetic Foot Ulcers (placebo = “cream”) | Prematurely ended (date unclear) | Photodynamic therapy using the combined effect of 3,7‐bis(N,N‐dibutylamino) phenothiazin‐5‐ium bromide (PPA904) and light; measure reduction of bacterial content of diabetic foot ulcers | ClincialTrialsRegister.eu EudraCT number: 2005‐001363‐58 | None (not listed) | None listed. | Photopharmacia |
| Pexiganan Versus Placebo Control for the Treatment of Mild Infections of Diabetic Foot Ulcers (OneStep‐1 and 2) | Completed (August 2016) | 1°: clinical response (resolution of infection); 2°: microbiological response; safety | ClinicalTrials.gov; NCT01594762 | No results (200 for each of the 2 trials) reported on website. | Robert Deluccia, Dipexium | Dipexium Pharmaceuticals, Inc. Multicentre study; all sites outpatient centre in USA |
| Comparison of Resin Salve and Octenidine in Patients with Neuropathic Diabetic Foot Ulcers (comparator: octenidine dihydrochloride‐impregnated gauze) | Completed (May 2015) | Investigate healing rate and healing time of neuropathic diabetic foot ulcer in people suffering from infected fore‐ or mid‐foot ulceration. 2°: eradication of bacteria; wound healing and infection | ClinicalTrials.gov; NCT02169167 | No results on website (n = 35) (see addendum in “comments”) | Janne J Jokinen | Salve prepared from Norway spruce (Repolar Ltd.) |
| Clinical Outcomes for Diabetic Foot Ulcers Treated With Clostridial Collagenase (SANTYL®) Ointment or With a Comparator Product Containing Silver (investigator choice of silver) | Running until January 2017 (last updated November 2016) | Randomly assigned to apply SANTYL or a topical treatment containing silver to their to foot ulcer. 1°: mean change in ulcer area at end of treatment; 2°: target ulcer infection rate | ClinicalTrials.gov; NCT02581488 | No results (102) | Jaime E Dickerson, PhD (Smith & Nephew) | (Smith & Nephew) Information from the sponsor received end of December 2016 stated that the trial is not yet complete but last participant out will be achieved in the next week. The trial enrolled its target number of participants, with the last participant completed December 2016. The evaluability will be carried out prior to the scheduled database lock in January 2017. As intention‐to‐treat is the analysis set for primary inference, it is anticipated that all participants will be included. Final study report is timed for April 2017 (15 December 2016). Waiting for further information to assess eligibility for review |
| Randomized, Controlled Study to Investigate the Efficacy and Safety of a Topical Gentamicin‐Collagen Sponge in Combination with Systemic Antibiotic Therapy in Diabetic Patients With a Moderate or Severe Foot Ulcer Infection | Recruiting (as of September 2013) | 1°: "clinical cure" at the test of cure; 2°: clinical response; time to clinical cure; eradication of baseline pathogen | ClinicalTrials.gov; NCT01951768 | No results (estimate 144) | Ilker Uckay, MD; Hospital of the University of Geneva | Innocoll, Inc. |
| Comparison of the Efficacy of Standard Treatment Associated with Phage Therapy Versus Standard Treatment Plus Placebo for Diabetic Foot Ulcers Monoinfected by Staphylococcus aureus: a Randomized, Multi‐centre, Controlled, 2‐parallel‐group, Double‐blind, Superiority Trial | Starting January 2017 | 1°: reduction in wound surface area; 2°: safety; changes in resistance and virulence of S aureus isolates; production of anti‐phage antibodies | ClinicalTrials.gov; NCT026647401 | No results (estimate 60) | Albert Sotto, MD, PhD +33.(0)6.09.56.66.55 | Centre Hospitalier Universitaire de Nīmes; Pherecydes Pharma. Per correspondence from Prof Sotto on 8 January 2017, National Agency for the Safety of Medicines and Health Products requested “pre‐clinical phase complements”, causing a postponement of the start of the clinical trial. |
| A Phase I/IIa, Randomized Double Blind, Placebo‐Controlled, Dose Escalating Study to Evaluate the Safety and Tolerability of Topically Applied Bisphosphocin Nu‐3 on Infected Diabetic Ulcers of Subjects With Type I or II Diabetes Mellitus (placebo) | Enrolling by invitation only (last verified April 2016) | Diabetic foot ulcers; infection localised to area of ulcer and mild. 1° outcome: treatment‐related adverse events, safety 2°: microbiological activity evaluated by wound assessments, presence of pathogenic bacteria | ClinicalTrials.gov; NCT02737722 | No results (estimate 30) | Paul DiTullio, MSc | Lakewood‐Amedex, Inc. |
| A Phase II, Randomized, Parallel, Double‐blind, Placebo‐controlled Study to Assess Prevention of Infection Using a Topical Gentamicin‐Collagen Sponge in Diabetic Patients With Uninfected Lower Extremity Skin Ulcers (placebo sponge) | Terminated (last verified March 2012) | 1° outcome: uninfected diabetic foot ulcers that remain free of signs/symptoms of infection to end of study 2°: days to wound closure; time to any signs/symptoms of infection; decrease in wound area; pathogen burden in infected wounds | ClinicalTrials.gov; NCT00658957 | No results (49) | David Prior, PhD; Chesapeake Foot and Ankle Center, Pasadena (MD), USA | Innocoll Pharmaceuticals |
| A Phase 3 Randomized, Placebo‐Controlled, Blinded Study to Investigate the Safety and Efficacy of a Topical Gentamicin‐Collagen Sponge in Combination With Systemic Antibiotic Therapy in Diabetic Patients With an Infected Foot Ulcer (COACT 1 and 2) (placebo is no sponge) | Last updated June 2016 | Sponge is adjunctive treatment to systemic antibiotic therapy. 1° outcome: per cent of participants with a clinical outcome of clinical cure (resolution of all clinical signs and symptoms of infection) ˜10 days after end of treatment; 2° outcomes: baseline pathogen eradication; re‐infection; time to clinical cure; amputation; ulcer closure | ClinicalTrials.gov: NCT02447172 | No results posted. | Nigel Jones, VP, Global Clinical Operations, Innocoll Pharmaceuticals | Innocoll Pharmaceuticals |
| Study of the Efficacy of Topical Application of Royal Jelly and Panthenol (PedyPhar® Ointment) on the Diabetic Foot Ulcers, an Open Label, Randomized, Non‐placebo‐controlled Study (active comparator panthenol ointment) | Terminated; (last updated February 2015) | Diabetic foot ulcers at any stage after proper surgical treatment (if needed) 1° outcome: healing of ulcer; 2°: reduction of infection in ulcer site; local reaction possibly related to study drug | ClinicalTrials.gov; NCT01531517 | No results (estimate 120; 47 enrolled) | (?) | European Egyptian Pharmaceutical Industries |
| Platelet Rich Fibrin in Combination With Topical Antibiotics or Antiseptics in the Treatment of Chronic Wounds ‐ a Prospective, Randomized, Active Controlled, Double Blind Pilot Trial With an Observer‐blinded Control Group (3 platelet rich fibrin arms & 1 active comparator (Acticoat)) | Recruiting (last verified January 2016) | People with infected chronic wounds (unclear if diabetic foot) 1° outcome: reduction of wound area; 2°: number requiring systemic antimicrobial therapy; C‐reactive protein level; wound volume; occurrence of drug‐resistant bacteria | ClinicalTrials.gov; NCT02652169 | No results (estimate 120) | Florian Thalhammer, Medical University of Vienna; 0043140400 ext 44400; [email protected] | Medical University of Vienna |
| Double Blind, Randomized, Placebo Controlled Clinical Trial for the Treatment of Diabetic Foot Ulcers, Using a Nitric Oxide Releasing Patch: PATHON | Completed (last verified November 2012) | 1° outcome: per cent reduction in ulcer size; 2°: complete cure of any infection; development of infection during treatment; adverse events | ClinicalTrials.gov; NCT00428727 | No results (?) | Fundación Cardiovascular de Colombia | (?) |
| A Phase I/II, Open Label, Controlled Study to Evaluate the Safety and Efficacy of AppliGel‐G (Gentamicin Sulfate Topical Gel) for Treatment of Mild to Moderately Infected Diabetic Foot Ulcers in Patients With Type 1 and Type 2 Diabetes (comparator oral ciprofloxacin and doxycycline alone) | Terminated (last verified May 2015) | For mild to moderately infected diabetic foot ulcers 1°: complete wound clearing of infection 2°: incidence infection cleared; wound volume and area change | ClinicalTrials.gov; NCT02036528 | No results | Royer Biomedical, Inc. | Royer Biomedical, Inc. |
| A Randomised, Double‐blind, Dose‐response, Placebo‐controlled, Multicenter, Phase IIA Clinical Study to Evaluate the Efficacy and Safety of Topical Application of G.68.y/EtOH in Patients with Type 1 or Type 2 Diabetes With Infected Foot Ulcers (placebo topical gel) | Completed | Enrolling patients with infected “grade 2 PEDIS” diabetic foot ulcers 1°: reduction of bacterial load 2°: maintenance of efficacy; tolerability and safety | EudraCT number: 2010‐019598‐13 | No results (plan for 60) | Molteni | |
| Trial to Assess Safety and Efficacy of Topical MBN‐101 (BisEDT ) in Patients With Moderate/ Severe Diabetic Foot Infections (placebo – vehicle‐controlled) | Not yet open for participant recruitment (last update March 2016) | Part I, participants will be enrolled into 1 of 3 escalating dose cohorts at a ratio of 3:1 (active to placebo). In Part II, participants will be randomised in a 1:1 ratio (active to placebo) based on the optimal dose demonstrated in Part I. People with infected foot ulcer | ClinicalTrials.gov; NCT02723539 | No results (plan for 88) | Department of Vascular Surgery, Rigshospitalet Copenhagen, Denmark, 2100 | Microbion Corporation |
| Abbreviations: PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation | ||||||
| Intervention 1 | Intervention 2 | Foot ulcer grade | Infection status at baseline | Follow‐up | Review‐relevant outcomes with reportable data | |
| Group 1: (n = 30) Pyodine bath and saline and vaseline gauze dressing | Group 2: (n = 30) Phenytoin powder | Grade I or II | Not reported | 8 weeks | None reported | |
| Group 1: (n = 19) Gentamicin solution | Group 2: (n = 22) Cadexomer iodine ointment | Grade I or II | Not reported | 12 weeks |
| |
| Group 1: (n = 19) Standard care | Group 2: (n = 21) Chloramine plus standard care | Not reported | Infected | 24 weeks |
| |
| Group 1: (n = 10) Saline solution | Group 2: (n = 10) Super‐oxidised aqueous solution | Grade I or II | Not infected | 4 weeks |
| |
| Group 1: (n = 15) Foam dressing | Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing | Grade II or III | Not infected | 14 weeks |
| |
| Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improve microcirculation | Group 2: (n = 40) Silver ion dressing plus standard care | Not reported | Not reported | 4 weeks |
| |
| Group 1: (n = not reported) Iodine gauze | Group 2: (n = not reported) Hydrofiber dressing with silver | Ulcers with bone and tendon exposure | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Saline dressing | Group 2: (n = 195) Honey dressing | Grade I or II | Not reported | 17 weeks |
| |
| Group 1: (n = 20) Silver sulphadiazine cream | Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream | Grade I or II | Not reported | 6 weeks |
| |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing | Group 3: (n = 106) Iodine‐containing dressing | Not reported | Not reported | 24 weeks |
| |
| Group 1: (n = 67) Calcium‐alginate dressing | Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver | Grade I or II | Mixed infected and not infected | 8 weeks |
| |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered further) Group 2: (n = 20) Recombinant human platelet‐derived growth factor | Group 3: (n = 20) Antiseptic treatments (EUSOL, hydrogen peroxide, and povidone iodine) | Grade III or IV | Not reported | More than 8 weeks |
| |
| Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day Group 2: (n = 21) Super‐oxidised aqueous solution (topical Microcyn) alone (not considered) | Group 3: (n = 21) super‐oxidised aqueous solution (topical Microcyn) therapy plus 750 mg levofloxacin once per day | Eligible foot ulcers involved skin and deeper soft tissue | Infected | 4 weeks |
| |
| Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets | Full‐thickness wounds | Infected | Up to 42 days |
| |
| Group 1: (n = 38) Systemic antibiotic therapy alone | Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 16) Povidone iodine and saline | Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution | Not reported | Infected | 20 weeks |
| |
| Group 1: (n = 25) Conventional treatment (no further details translated) | Group 2: (n = 25) Zinc hyaluronate | Not reported | Unclear | 20 weeks |
| |
| (30 participants randomised, but number in each group not specified) | Group 1: Standard‐dressing group (povidone iodine solution 10%) (n not reported) | Group 2: Honey dressing group (n not reported) | Grade II | Not reported | Not reported | No useable data |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) | Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | 16 weeks |
| |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing | Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Grade I and II | Not reported | Not reported |
| |
| Group 1: (n = 19) Polyherbal formulation | Group 2: (n = 19) silver sulphadiazine cream | Grade I, II, and III | Unclear | 20 weeks | No useable data | |
| Abbreviations: EUSOL, Edinburgh University Solution of Lime | ||||||
| Resolution of infection | Incidence of wound infection | Time to healing | Proportion of wounds healed | Microbial counts | Health‐related quality of life | Need for surgical resection, including partial or complete lower limb amputation | Safety (adverse events) | |
| Group 1: (n = 30) Povidone iodine bath and saline Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder plus povidone iodine bath and saline Vaseline gauze dressing Not infected at baseline | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Gentamicin solution Group 2: (n = 22) Cadexomer iodine ointment Baseline infection status not reported. | Not reported | Not reported | Not reported | Group 1: 2/18 Group 2: 5/17 | Not reported | Not reported | Surgical resection was reported: Group 1: 5/19 Group 2: 3/22 | Study reports that no adverse reactions related to the topical treatment were documented. |
| Group 1: (n = 19) Standard care alone Group 2: (n = 21) Chloramine plus standard care Infected at baseline | Group 1: 7/15 Group 2: 9/13 | Not reported | Time‐to‐event data presented with no reported hazard ratio. Given the small number of participants and events, no further attempts were made to calculate time‐to‐event values. | Healed at 24 weeks Group 1: 9/17 Group 2: 10/17 | Not reported | Not reported | Vascular procedure or amputation Group 1: 3/17 Group 2: 5/17 | Adverse event data reported but unable to get a per‐participant value, as it is noted that some participants had more than 1 event. |
| Group 1: (n = 10) Saline solution Group 2: (n = 10) Super‐oxidised aqueous solution Not infected at baseline | Not reported | Not reported | Not reported | Study notes that 15% of the study ulcers were healed, but this information not reported by group. | The bacterial load in the wound bed was defined as scattered (0/+), light (+), medium (++), or heavy (+++). At week 4 there was a reduction of 33% in the bacterial load versus baseline. Figure presented but difficult to interpret data by group. | Not reported | Not reported | No safety concerns were reported in either the super‐oxidised aqueous solution group or the saline group; no adverse reactions were recorded. |
| Group 1: (n = 15) Foam dressing Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing Not infected at baseline | Not reported | Wound infection Group 1: 4/13 Group 2: 0/23 | Not reported | Healed by week 14 Group 1: 4/13 Group 2: 12/23 | Not reported | Not reported | Not reported | Limited details of adverse events (in addition to infection data already recorded). There were no reported adverse events related to the use of collagen/oxidised regenerated cellulose/silver dressing, and 5 cases of adverse events (no further details) related to foam dressing. |
| Group 1: (n = 40) Routine debridement plus standard care Group 2: (n = 40) Silver ion dressing plus standard care Baseline infection status not reported. | Not reported | Not reported | Mean wound healing time in days: Group 1: 47.4 ± 11.5 Group 2: 31.3 ± 8.2 Mean granulation tissue occurrence time in days: Group 1: 10.8 ± 1.9 Group 2: 6.4 ± 0.72 | Group 1: 15/40 Group 2: 24/40 | Not reported | Not reported | Not reported | Not reported |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Treated with normal saline dressing Group 2: (n = 195) Treated with honey dressing | Not reported | Not reported | Median healing time in honey group is 18 days (IQR is 6 to 120), and in the saline group is 29 days (IQR 7 to 120). Data do not seem to have been calculated using correct time‐to‐event approaches and were not considered further. | Group 1: 97/169 Group 2: 136/179 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 20) Silver sulphadiazine Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream Baseline infection status not reported. | Not reported | Not reported | Not reported | Healed by week 6 Group 1: 6/20 Group 2: 8/20 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Baseline infection status not reported. | Not reported | Number of infected ulcers at 24 weeks: not reported by group Study reports the number of episodes of infection listed as serious adverse events, but it is unclear if foot infections, and not clear how many people had how many infection events. | Mean time to healing in days (SD) (fixed at max of 168 days) Group 2: 125.8 (55.9) Group 3: 127.8 (54.2) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed at 24 weeks: Group 2: 46/103 Group 3: 48/106 | Not reported | Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group 1: Physical functioning: 68.9 (19.1). Social functioning: 69.8 (23.5). Well‐being: 50.2 (21.1) Group 2: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well‐being: 53.1 (19.9) Other | Minor amputations (below ankle): Group 2: 1/103 n not clear; assumed to be all participants | Non‐serious adverse events Group 2: 227/103 Group 3: 239/106 Serious adverse events Group 2: 28/103 Group 3: 37/106 Not clear how many participants had how many events, but seems to be more than 1 per person; data not analysed further |
| Group 1: (n = 67) Calcium‐alginate dressing Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver Mixed wound infection status at baseline | Not reported | Group 1: 11/67 Group 2: 8/67 | Time to 100% healing also reported, but this is only for a subset of those that healed, so not a useful pan‐study measure. Not reported Mean time to healing in days Group 1: 52.6 ± 1.8 Group 2: 57.7 ± 1.7 | Number of ulcers healed in 8 weeks | Not reported | Not reported | Not reported | Group 1: 26/67 participants experienced adverse event. Death = 1; Infection = 8. 13 participants discontinued treatment due to adverse event. Group 2: 25/67 participants experienced 1 or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to adverse event. |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered in review) Group 2: (n = 20) Recombinant human platelet‐derived Group 3: (n = 20) Antiseptic dressings | Not reported | Not reported | Mean time to healing in weeks (standard error) Group 1: 6.83 (2.5) Group 2: 7.6 (2.5) Group 3: 6.75 (2.7) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed Group 1: 12/20 Group 2: 16/20 Group 3: 8/20 Review authors calculated figures from graph. | Not reported | Not reported | Not reported | Not recorded |
| Group 1: (n = 21) Levofloxacin plus saline Group 2: (n = 21) Super‐oxidised aqueous solution alone (not considered) Group 3: (n = 25) Levofloxacin plus super‐oxidised aqueous solution Ulcers infected at baseline. | Group 1: 6/21 Group 2: 11/21 Group 3: 11/25 | Not reported | Not reported | Mentioned, but data not presented. | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more event) Group 1: 7/21 Group 2: 7/21 Group 3: 9/25 |
| Group 1: (n = 246) Ofloxacin Group 2: (n = 247) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | See below ‐ results presented by study authors cumulatively for these 2 studies only. | Adverse events (number of participants with > 1 adverse event) Group 1: 109/246 Group 2: 98/247 |
| Group 1: (n = 171) Ofloxacin Group 2: (n = 171) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | Group 1: 9/417 Group 2: 11/418 (cumulative of two RCTs reported in single paper) | Adverse events (number of participants with > 1 adverse event) Group 1: 84/171 Group 2: 76/171 |
| Group 1: (n = 18) Systemic antibiotic therapy alone Group 2: (n = 38) Topical application of the gentamicin‐collagen sponge + systemic antibiotic therapy Ulcers infected at baseline. | Resolution of infection by 7 days Group 1: 7/18 Group 2: 22/38 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more events) Group 1: 5/18 Group 2: 11/38 |
| Group 1: (n = 16) Standard management with chemical antiseptics such as soap or povidone iodine Group 2: (n = 21) Super‐oxidised aqueous solution | Advances from infection to granulating tissue: Group 1: 10/16 Group 2: 19/21 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 25) Conventional treatment (no further details translated) Group 2: (n = 25) Zinc hyaluronate | Not reported/translated | Not reported/translated | Mean time to healing in weeks (not clear if standard deviation or standard error presented) Group 1: Only 2 ulcers healed; no time‐to‐event data reported Group 2: 7.80 (3.49) with all ulcers healing | Group 1: 2/25 Group 2: 25/25 | Not reported/translated | Not reported/translated | Not reported/translated | Not reported/translated |
| Group 1: Standard‐dressing group (povidone iodine solution 10%) Group 2: Honey dressing group 30 participants randomised, but number in each group not specified. | Not reported | Not reported | Time to healing in days Group 1: 15.4 days (range 9 to 36 days) Group 2: 14.4 days (range 7 to 26 days) Comment: mean and range, but no measure of variation provided. Unclear how many participants in each group and how many ulcers healed, thus if this measure is a valid time‐to‐healing measure | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | Data presented as time‐to‐event figure with no further data. Given the small number of participants and events, we have not tried to analyse further. | 16 weeks Group 1: 2/10 Group 2: 6/12 Unclear if ulcers were healed in the same or different participants; for the analysis we have assumed in different participants | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Not reported | Not reported | Not reported | Group 1: 0/2 Group 2: 2/2 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Polyherbal formulation Group 2: (n = 19) Silver sulphadiazine cream | Not reported | Not reported | "Number of days taken for healing of the wound: Group 1: 43.1 ± 26.8 Group 2: 43.6 ± 30.7" Not clear what sort of analysis was conducted | Healing was defined as complete epithelialisation either by secondary intention or by split skin graft. However, figures are not reported. | "the microbiological investigations were not done" | Not reported | Not reported | "There were no adverse events reported in both the groups." |
| Abbreviations: IQR, interquartile range; SD, standard deviation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 945 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.45] |
| 1.1 Short term follow up | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [1.00, 2.57] |
| 1.2 Medium term follow‐up | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.44] |
| 2 Incidence of infection: medium term follow‐up Show forest plot | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.10] |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.72] |
| 4 Adverse events Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: medium term follow‐up Show forest plot | 3 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.56, 14.23] |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.51] |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.47, 5.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Medium term follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Unknown follow‐up period | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.53, 7.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of infection Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.91, 2.49] |
| 1.1 Short‐term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.69, 3.45] |
| 1.2 Medium term follow‐up | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 2 Surgical resection: medium term follow‐up Show forest plot | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.51, 2.91] |
| 3 Adverse events Show forest plot | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| 3.1 Short‐term follow‐up | 3 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 3.2 Medium term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: Medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.28, 0.89] |

