Antidepresivos para el tratamiento de la depresión en pacientes con cáncer
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 8‐week, randomised study | |
| Participants | Female participants, age 18 years and over, affected by cancer (mixed sites, including breast, ovary, uterine cervix and others) at any stage, diagnosed with depression, according to the criteria proposed by Stewart 1965 for medically ill patients, with slight additional inclusion criteria suggested by Kathol and Petty (7): (i) low mood and loss of interest for at least 3 weeks; (ii) at least 4 of the following: difficulty in concentration or memory problems, irritability, feelings of worthlessness or hopelessness, fear of losing one's mind, lack of initiative, frequent crying or wanting to die, suicide attempt; (iii) social impairment at work, home etc; (iv) anorexia, sleep disturbance, fatigue, motor retardation. Further inclusion criteria were depression succeeding or paralleling development of cancer; Zung Self‐Rating Depression Scale (ZSRDS) score greater than 41; Hamilton Depression Rating Scale (HDRS) items 1 to 17 score greater than 16; and informed consent of the patient. Participants were mostly inpatients, but rates of in‐ and outpatients are not reported. | |
| Interventions | Mianserin: 36 participants. The dose was flexible starting from 10 mg, 1 tablet per day in the first week and 2 tablets per day from the second week (range not reported; mean dose between weeks 1 and 4 was 44.5 mg/day) Placebo: 37 participants | |
| Outcomes | Efficacy and tolerability of mianserin versus placebo, assessed with Zung Self‐Rating Depression Scale (ZSRDS); Hamilton Depression Rating Scale (HDRS‐17); Clinical Global Impression Scale for Severity of Illness (CGI‐S); Clinical Global Impression Scale for Severity of Illness (CGI‐I); Efficacy Index (EI) and a checklist for somatic findings and side effects | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated"; no further details on the sequence generation process. However, quote: "Treatment groups were well matched for social data (education, occupation and marital status) [not reported in tables]. Treatment groups were also well matched for main cancer localizations, clinical stages of cancer, and baseline Karnofsky scores [reported in tables]." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patient compliance and physician blindness were good throughout the trial. Thus, the number of psychiatrist's correct guesses as to which treatment the patients were receiving (22, mianserin; 16, placebo) were not significantly higher than expected by chance". Procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Efficacy was evaluated using double‐blind assessment...". No further clarifications on which procedure was used. |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout rates: in the mianserin group 7/36 (19.4%), in the placebo group 15/37 (40.5%). The imbalance in total rates and possible different reason for losses between groups is not discussed. All randomised participants were included in the analysis, which is consistent with an 'intention‐to‐treat' analysis (but this term is not reported). Quote: "[...] the only treatment comparison known to be unbiased is that based on the analysis of all randomised patients". Missing data were imputed according to the LOCF, quote: "Data used in the statistical analysis of efficacy were based on the 'last assessment carried forward approach' in which missing scores for those patients who dropped out before day 21 had their last observed score assigned to the missing assessment". Even if there was a high dropout rate in the placebo group, the risk of bias was rated as 'unclear' rather than 'high', since the ITT analysis and LOCF imputation were properly performed. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly pre‐specified in the methods (quote: "[...] compare the efficacy and safety of mianserin in women with cancer [...]"). However, outcomes of interest are properly reported in the results. Scores for HDRS, ZSRDS, CGI‐S, EI and the number of participants with each side effect on the checklist were reported for every week. The number of responders is reported, but only according to the CGI‐I endpoint scores. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out since a 'financial disclosure' or possible conflicts of interest are not reported. |
| Methods | 12 weeks, randomised, double‐blind, placebo‐controlled study | |
| Participants | People with (a) cancer of the upper aerodigestive tract (buccal cavity, larynx, oropharynx, hypopharynx), solitary or multiple synchronous localisations, stage I to IVb, to be treated by surgery and/or radiotherapy and/or chemotherapy (first‐line curative treatment); (b) HADS more than 11 (excluded those with a diagnosis of major depressive episode with severity criteria and/or suicidal thoughts); (c) aged between 18 and 75 years, having signed an informed consent | |
| Interventions | Escitalopram: 20 participants Placebo: 18 participants | |
| Outcomes | Primary outcome: subscore depression of the HADS, W12 Secondary outcomes: CES‐D; MADRS; CGI; SCL‐90‐R; health‐related quality of life (EORTC QLQC‐30, H‐N 35), alcohol or tobacco consumption (CO, CDT) | |
| Notes | Data were partially provided by the authors before the publication of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported (unpublished study) |
| Allocation concealment (selection bias) | Unclear risk | Not reported (unpublished study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported (unpublished study) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported (unpublished study) |
| Incomplete outcome data (attrition bias) | High risk | Dropout rate: escitalopram arm 4/20 (20%); placebo arm 3/18 (16.7%). Only participants who completed the assessment at each time point were analysed and missing data were not imputed ('per protocol' analysis). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported for the endpoint assessment (week 12) and for week 4. |
| Other bias | Low risk | The baseline features of the population of the study are not reported. The Gustave Roussy, which is a private non‐profit hospital, was the sponsor of the trial. Lundbeck funded only the costs of drugs and did not play any role in planning, conducting and writing the study. |
| Methods | Randomised, placebo‐controlled, multicentre (15 centres) study | |
| Participants | Ambulatory people of either sexes with advanced cancer (mixed sites) and depressive symptoms, as assessed with a score of 2 or greater on the Two‐Question Screening Survey (TQSS), excluding people with major depression diagnosed by a psychiatrist in the past 6 months. All participants gave informed consent | |
| Interventions | Fluoxetine: 83 participants. The dose was 20 mg/day, fixed Placebo: 80 participants | |
| Outcomes | The primary outcome was the quality of life (QoL) assessed with the Functional Assessment of Cancer Therapy–General (FACT‐G, version 3). The secondary outcome was the depressive symptoms assessed with the 11‐item BZSDS. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[...] randomly assigned in a double‐blind manner to receive either fluoxetine (20‐mg tablets) or an identical placebo tablet. The randomisation was performed centrally through a preprinted randomisation table, and the study drug was sent by overnight mail directly to the patient" and "Patients in each study arm were comparable at baseline with respect to age, sex, performance status, symptom status regarding pain and depression, disease distribution, and current treatment with chemotherapy." |
| Allocation concealment (selection bias) | Low risk | Quote: "[...] The randomisation was performed centrally through a preprinted randomisation table, and the study drug was sent by overnight mail directly to the patient." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients were then randomly assigned in a double‐blind manner to receive either fluoxetine (20‐mg tablets) or an identical placebo tablet". This should ensure patient blinding. The study is described as 'double‐blind', however procedures for ensuring the blinding of who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) | High risk | Only participants who completed the assessment at each time point were analysed and missing data were not imputed ('per protocol' analysis). At the 'primary endpoint' (second visit, mean of 4.6 (fluoxetine group) versus 4.7 (placebo group) weeks from baseline) 64 versus 65 participants were assessed (over 83 versus 80 participants randomised). Only dropout rates due to side effects at the end of the study are reported, and whether there was imbalance between groups in term of reasons for leaving the study early is not discussed. |
| Selective reporting (reporting bias) | Low risk | Relevant data for the pre‐specified (methods) outcomes are reported (results). |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out since a 'financial disclosure' or possible conflicts of interest are not reported. |
| Methods | 6‐week, prospective, randomised, double‐blind, multicentric (6 investigative sites) study | |
| Participants | Women affected by cancer (mostly breast cancer at stage II, II, IV) and major depressive disorder (for at least 30 days before entering the study) or adjustment disorder with depressed mood (for at least 60 days before entering the study), according to the criteria of DSM‐III‐R and a score of more than 14 on the first 17 items of the HAM‐D. Participants gave signed informed consent. | |
| Interventions | Fluoxetine: 17 participants. The dose was 20 mg/day for the first month, thereafter the dose was flexible. However, the maximum dose allowed is not reported Desipramine: 21 participants, starting with a dose of 25 mg/day and titrated in 25 mg/week increments to a dose of 100 mg/day at week 4. Thereafter the dose was flexible to a maximum of 150 mg/day. There was not a placebo arm, but all participants received placebo + active drug (alternated during the day) in order to maintain the blindness ('double‐dummy' approach). | |
| Outcomes | Safety and efficacy of fluoxetine versus desipramine. Depression and anxiety were assessed with the 17‐item Hamilton Rating Scale for Depression (HAM‐D‐17), the Hamilton Anxiety Rating Scale (HAM‐A), the Clinical and Patient's Global Impression (CGI and PGI) scales. Quality of life was assessed with the Functional Living Index for Cancer (FLIC), the Memorical Pain Assessment Card (MPAC), and the SF‐36 Health Survey. Adverse events were self reported and evaluated weekly through clinical assessment | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[...] a 6‐week, double‐blind (randomisation of placebo non‐responders) phase [...]. Treatment groups [...] had comparable demographics and baseline psychiatric assessment scores". No further details on the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Fluoxetine‐treated patients received 20 mg of active drug in the morning and placebo in the evening. Desipramine‐treated participants received 25 mg of active drug in the evening and placebo in the morning". The study is described as double‐blind, however procedures for ensuring the blinding of who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The assessment was performed by the clinician, whose blindness is not discussed. |
| Incomplete outcome data (attrition bias) | High risk | Dropout rate: 6 participants in the fluoxetine group (6/17, 35.3%) and 7 participants in the desipramine group (7/21, 33.3%). Number of participants and reasons for discontinuation are apparently balanced between the 2 groups. According to the text missing data were imputed, quote: "The endpoint analysis calculated changes from baseline [...] to the last observation carried forward...", however whether a proper ITT analysis was applied is unclear, since the number of analysed participants is not reported in the text or in the graphs. |
| Selective reporting (reporting bias) | High risk | Outcomes are not clearly pre‐specified (quote: "[...] our study prospectively examined the safety and efficacy of fluoxetine and desipramine in 40 depressed women [...]"). Outcomes of interest are poorly reported: neither mean scores on scales nor rates of remission are reported at any time point. The baseline‐to‐endpoint mean changes are represented in graphs, but not clearly reported in the text. |
| Other bias | High risk | Quote: "This work was sponsored by Eli Lilly and Company". The role of funders in planning, conducting and writing the study is not discussed. |
| Methods | 6‐week, randomised, double‐blind, placebo‐controlled, multicentric (2 centres), parallel‐group study | |
| Participants | Female outpatients aged 18 to 75 years with a current diagnosis of breast carcinoma (stage I‐IV); DSM‐III‐R criteria for major depression or adjustment disorder with depressed mood for at least 2 months; score of at least 14 on the first 17 items of the 21‐items HAM‐D; last cancer treatment within the last 5 years | |
| Interventions | Paroxetine: 13 participants. The dose was flexible, starting with 20 mg/day for the first 4 weeks, thereafter it could be increased at 40 mg/day. Desipramine: 11 participants. The dose was flexible, starting with 25 mg/day and gradually titrated to 125 mg/day within the fourth week; thereafter it could be increased by 25 mg/day every 3 days up to 200 mg/day as the maximum dose. Placebo: 11 participants | |
| Outcomes | Efficacy and tolerability of paroxetine versus desipramine versus placebo in women with breast cancer, assessed with 21‐item observer‐rated Hamilton Rating Scale for Depression (HAM‐D), 14‐item observer‐rated Hamilton Rating Scale for Anxiety (HAM‐A), Clinical Global Impression Scale for Severity of Illness (CGI‐S), routine adverse event monitoring and vital assessment for exploring tolerability. Quote: "The primary efficacy parameter was the mean change from baseline in the total score of the 21‐item HAM‐D. The secondary outcome measure was the mean change from baseline in the CGI‐S score." | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were then randomly assigned to one of the three double‐blind treatment groups"; no further details on the sequence generation process. The 3 groups were similar for demographic and clinical features (with the exception of stage, being less advanced in the placebo‐treated group, and previous chemotherapy, being less frequent in the placebo‐treated group). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study is described as "double‐blind", however procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout rates: 5/13 (38.5%) participants in paroxetine group; 4/11 (36.4%) participants in desipramine group; 5/11 (45.4%) in placebo group. Reason for leaving the study are apparently balanced between groups, however dropout rates are relevant. Moreover, a relevant portion of missing data are possibly related to the true outcome (2 versus 2 versus 0 participants dropped due to inefficacy). Missing data were imputed. Quote: "Data are presented from the intention‐to‐treat population" and "the last‐observation‐carried‐forward approach was applied for the missing data due to early dropout in the study." |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported for the endpoint assessment (week 6). |
| Other bias | Unclear risk | 3 authors report having received research support from several drug companies. Sponsorship bias cannot be ruled out since the funders of the study and their role in planning, conducting and writing it are not reported. |
| Methods | 24‐week, randomised, double‐blind, placebo‐controlled study | |
| Participants | Women with early‐stage breast cancer (stages I, II) who were candidates for adjuvant hormonal therapy, local radiation and/or adjuvant chemotherapy treatment and had depressive symptoms, as indicated by a score of 2 or greater on the Two Question Screening Survey (TQSS). Participants who were "clinically depressed" were excluded. | |
| Interventions | Fluoxetine: number of participants not reported. The dose was 20 mg/day (not clearly reported if it was a fixed dose) Placebo: number of participants not reported | |
| Outcomes | Efficacy of fluoxetine versus placebo on depressive symptoms (assessed with the 11‐item Brief Zung Self‐Rating Depression Scale ‐ BZSDS), quality of life (assessed with the Functional Assessment of Cancer Therapy–General ‐ FACT‐G, version 3) and completion of adjuvant treatment. Quote: "The primary end points of the study were depressive symptoms, quality of life, and completion of adjuvant treatment." | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients with depressive symptoms were randomised to a daily oral antidepressant or a placebo"; no further details on the sequence generation process. Quote: "The groups were comparable at baseline in terms of age, disease distribution, performance status, and level of depressive symptoms". However, only the total number of randomised participants is reported, not the number of participants in each arm. Tables report results for 90 participants per arm |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study is described as 'double‐blind', however procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) | High risk | 193 people were randomly assigned, but the number of participants for each arm is not reported. 180/193 (93%) participants completed the study. Dropout rates among the 2 groups and reasons for leaving the study early are not clearly reported. Missing data were not imputed and only participants who completed the study were analysed ('per protocol' analysis). |
| Selective reporting (reporting bias) | High risk | Results are reported only for subgroups (according to the type of adjuvant therapy assumed) not pre‐specified. For relevant outcomes only results for "relevant improvement in depressive symptoms at 6 months" are reported, however how "significant improvement" is assessed is not clearly discussed. |
| Other bias | Unclear risk | The Reich Family Endowment provided financial support for this investigation (not clearly reported if it is a private funder). The role of funders in planning, conducting and writing the study is not discussed. |
| Methods | Interventional, randomised, cross‐over, 8‐week, double‐blind study. The randomisation was stratified according to stage of disease (stage IIIB with effusions vs stage IV) and current treatment (radiation vs chemotherapy vs novel agent). | |
| Participants | Patients diagnosed with advanced lung or gastrointestinal cancer and major depressive disorder (according to DSM‐IV and Endicott criteria). Age: 35 to 85 years. | |
| Interventions | The study had a cross‐over design. Patients were randomised into three arms: placebo‐escitalopram (the switch from one to the other took place after 4 weeks), escitalopram‐placebo, and placebo‐placebo. In the first phase of the trial 11 patients received escitalopram 10 mg/day and 13 patients received placebo. | |
| Outcomes | Primary outcomes: response rate, defined as a 50% reduction in the Hamilton Depression Rating Scale (HAM‐D) scores over 4 weeks; change in Hamilton Depression Rating Scale (HAM‐D) scores at week 4. Seconday outcome: side effect burden, defined as the total score of the UKU Side Effects Rating Scale. | |
| Notes | According to the protocol the study started in March 2006 and was supposed to be completed in April 2011. Results for primary and secondary outcomes for the first 4 weeks of treatment were made available at clinicaltrials.gov. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however details on the sequence generation were not provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Masking: Triple (Participant, Care Provider, Investigator)" and "[...] one placebo pill identical in appearance to the escitalopram pill [...]". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | Apparently an ITT analysis was performed, considering that all randomised patients were analysed in the majority of analyses, including therefore also patients who left the study early. However, the methodology employed to impute missing data is not discussed (note that only the protocol of the study is available). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were clearly prespecified in the protocol, and were reported. |
| Other bias | Low risk | The study was supported by the Massachusetts General Hospital and the National Cancer Institute (NCI). |
| Methods | 8‐week, multicentric (25 centres), double‐blind, parallel‐group, randomised study | |
| Participants | Women, aged 18 to 65 years (according to data reported in tables, older participants were also analysed), with a diagnosis of breast cancer (at any stage, but without cerebral metastases), with a rating of less than 2 on the World Health Organization (WHO) performance status scale and a life expectancy greater than 3 months; who had received chemotherapy and were scheduled to receive further cycles during the study period, and had received tamoxifen or paclitaxel and were scheduled to receive further treatment during the study. Participants had to be diagnosed with a mild, moderate or severe depressive episode, according to International Classification of Disease‐10 (ICD‐10) and have a score of greater than 16 on the Montgomery Åsberg Depression Rating Scale (MADRS). All participants gave written informed consent | |
| Interventions | Paroxetine: 88 participants. Flexible dose, starting with 20 mg/day for the first 3 weeks. Thereafter the dose could be increased to 30 mg/day (after week 3) and to 40 mg/day (after week 5) if clinically indicated Amitriptyline: 87 participants. Flexible dose, titrating up to 75 mg/day within the first 3 weeks. Thereafter the dose could be increased to 100 mg/day (after week 3) and to 150 mg/day (after week 5) if clinically indicated. Placebo capsules were administered in order to maintain blindness. | |
| Outcomes | Quote: "[...] primary aim of comparing the efficacy and tolerability of paroxetine and amitriptyline in the treatment of depression in women with breast cancer". Efficacy was assessed with MADRS, CGI‐S, Functional Living Index Cancer (FLIC) and patient's global evaluation (PGE) at endpoint. Tolerability was assessed by recording adverse events and evaluating vital signs and laboratory parameters. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a multicenter, double‐blind, parallel‐group, randomised study" and "...study participants [...] were randomly assigned in a ratio of 1:1 to 8‐weeks treatment with either paroxetine [...] or amitriptyline [...]"; no further details on the sequence generation process. However, according to the tables, clinical and demographic features are similar between the 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "...a multicenter, double‐blind, parallel‐group, randomised study" and "a double‐dummy technique was used to ensure blinding". Procedures for ensuring the blinding of who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) | Low risk | Dropout rates: 16/88 (18.2%) in the paroxetine group; 19/87 (21.8%) in the amitriptyline group. Side effects represent the most frequent reason for withdrawal (9 versus 10 participants). Other reasons are not discussed, however rates and reasons for losses are apparently balanced between groups. Imputations for missing data were performed. Quote: "Visitwise and endpoint statistical analyses were performed on the intent‐to‐treat (ITT) population (i.e. all participants who had taken at least one dose of study medication and who had at least one on‐dose efficacy assessment). Endpoint analyses were constructed from week 8 observations, where available, and on a ‘last observation carried forward' basis for participants who had discontinued study medication prematurely." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly prespecified (quote: "[...] primary aim of comparing the efficacy and tolerability of paroxetine and amitriptyline [...]"), however key outcomes are reported as mean change scale scores at different time points. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out since a 'financial disclosure' is not reported. |
| Methods | 5‐week, double‐blind, placebo‐controlled, randomised, multicentric trial (14 centres) | |
| Participants | People (mostly females), aged over 18 years, diagnosed with an adjustment disorder (with a depressive mood or with mixed features) or from a major depressive disorder (excluding MDD with melancholic features) as defined by the DSM‐III‐R "in relation to" a cancer disease that had been diagnosed for a period of between 6 weeks and 7 years. Participants had to have a score of 13 or higher on the Hospital Anxiety and Depression Scale (HADS) before and after the 1‐week period of placebo treatment, a rating of 60 or higher on the Karnofsky Performance Scale, and had to provide written informed consent | |
| Interventions | Fluoxetine: 45 participants. The dose was 20 mg 1 tablet per day Placebo: 46 participants | |
| Outcomes | Effectiveness and tolerance of fluoxetine versus placebo, assessed with the Hospital Anxiety and Depression Scale (HADS), Montgomery and Åsberg Depression Rating Scale (MADRS), Hamilton Anxiety Scale (HAS), Revised Symptom Checklist (SCL90‐R) and the Spitzer Quality of Life Index (SQOLI). The main assessment criterion was the success rate defined by a HADS score lower than 8 after 5 weeks of treatment. Treatment tolerance was assessed with AMDP5, weight, blood pressure, pulse, biochemical and haematological tests and spontaneous side effect reports. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a double‐blind, placebo‐controlled, randomised, multicenter trial"; no further details on the sequence generation process. "The descriptive statistics for the baseline characteristics (demographic data and clinical variables) are comparable in the two treatment arms, except for delay since diagnosis, which was longer in the PA [placebo] group than in the FA [fluoxetine] group for randomised participants (P value = 0.03)." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study is described as "double‐blind", however procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) | High risk | Dropout rates: 15/45 (33.3%) participants in the fluoxetine group, 7/46 (15.2%) participants in the placebo group. Relevant rate particularly for the intervention group. There is imbalance between groups, however reasons for leaving the study early are described as apparently balanced between group. Quote: "Data analyses were performed [...] on an intent‐to‐treat basis on all randomised patients for the success rate, response rate and spontaneous side‐effect reports. For evolution of assessment scales, analyses were performed on an intent‐to‐treat basis on patients who completed the study". However, only data for participants who completed the study have been analysed (according to a 'per protocol' analysis), and actually missing data were not imputed. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly pre‐specified (quote: "[...] evaluate, in a double‐blind placebo‐controlled design, the effectiveness of fluoxetine to treat and/or to control anxiety and depression [...]"). For relevant outcomes mean scores on rating scales are reported for 'visit 1' (but it is not clearly explained if it matches with the baseline point) and for 'visit 5'. |
| Other bias | High risk | Quote: "This study was supported by grants from Lilly France and Lilly Benelux". The role of funders in planning, conducting and writing the study is not discussed. |
| Methods | 6‐week, randomised, double‐blind, placebo‐controlled, single‐centre study | |
| Participants | Women over 18 years with breast cancer at stage I or II, without metastases, not qualifying for primary surgical treatment, treated with radiotherapy, and depression, diagnosed according to DSM‐III criteria, and a score of at least 16 on the 21‐item HDRS | |
| Interventions | Mianserin: 28 participants. The dose was fixed at 30 mg/day for the first week and 60 mg/day thereafter. Placebo: 27 participants | |
| Outcomes | Efficacy and safety of mianserin versus placebo. Depression was assessed with the 21‐item HRDS after 2, 4 and 6 weeks. Tolerability was assessed with the ROSE (Record of Symptoms Emerging) and clinical evaluation of vital signs and laboratory measurements | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After baseline assessment [...] patients still satisfying entrance criteria were randomised to treatment with mianserin (M; n = 28) or placebo (P; n = 27)..." and "Both treatment groups were well matched regarding baseline characteristics...". No further details on the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "...a randomised, double‐blind, placebo‐controlled study" and "...mianserin (M; n = 28) or placebo (P; n = 27), which had been prepared as indistinguishable capsules and given as a single night‐time dose". Not reported who was blinded (clinician, statistician, outcome assessor) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Dropout rates: mianserin group 6/28 (21.4%); placebo group 15/27 (55.5%); 2 versus 11 due to inefficacy, 2 versus 4 due to side effects.The imbalance in total rates and in reasons for losses between groups is not discussed. This might have introduced bias, since dropouts in the placebo group mostly referred to inefficacy, which is likely related to the true outcome. Quote: "Efficacy analyses were performed on an intention to‐treat basis, thus including the patients who received at least one dose of study medication and had at least one post‐baseline efficacy assessment. Last observation carried forward (LOCF) analysis was performed at each assessment point, substituting missing values at all subsequent assessments by the last available value". Actually not all the randomised participants were analysed, but only those who received at least one dose of medication and had at least one assessment, which is closer to an 'as treated' analysis. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly prespecified (quote: "The aim of our study was to evaluate the efficacy and safety of mianserin in patients with breast cancer [...]"). However, mean change scores on HDRS, response rates and rates of relevant adverse events are reported. |
| Other bias | High risk | Quote: "This study was supported by a grant from NV Organon, Oss, The Netherlands". The role of funders in planning, conducting and writing the study is not discussed. |
BZSDS: Brief Zung Self‐Rating Depression Scale
CDT: Carbohydrate‐deficient transferrin
CGI: Clinical Global Impression scale
CGI‐I/CGI‐S: Clinical Global Impression Scale for Severity of Illness
CO: Test for diffusing capacity for carbon monoxide
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders ‐ III ‐ Revision
EI: Efficacy Index
EORTC: European Organisation for Research and Treatment of Cancer
HADS: Hospital Anxiety and Depression Scale
HAM‐D: Hamilton Depression Rating Scale
HRSD: Hamilton Rating Scale for Depression
ITT: Intention‐to‐treat
LOCF: Last observation carried forward
MADRS: Montgomery Åsberg Depression Rating Scale
MDD: major depressive disorder
ZSRDS: Zung Self‐Rating Depression Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong comparison: participants in the 2 arms received the same drug at different doses | |
| Wrong design: not randomised | |
| Wrong comparison: control group without placebo | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong design: not randomised | |
| Wrong condition: participants with panic disorder and generalised anxious disorder were also enrolled | |
| Wrong condition: participants not depressed at enrollment. | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong design. This is a review and it refers to 3 studies, none of which are eligible | |
| Wrong design: not randomised | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong comparison: control group without placebo | |
| Study eligible according to the protocol, however no published or unpublished data were retrieved. We contacted the authors and they stated that the study never started due to concerns around drug interactions and cancer symptoms. No further clarifications were provided | |
| Wrong design: not randomised | |
| Wrong condition: participants not depressed at enrollment | |
| Only the abstract of the study was available. Study eligible according to the abstract, but the author's feedback was negative: the study has been concluded due to recruitment issues | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong design: not randomised | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| According to information provided by the author (Prof. EG Shaw) the study closed due to the low number of patients enrolled (only 8) | |
| Wrong comparison: the experimental arm received methylphenidate plus SSRI, the control arm received placebo plus SSRI | |
| Wrong design: not randomised | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong comparison: no placebo or antidepressant in the control group | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| The study is eligible according to the protocol. We contacted the authors and they provided negative feedback; the design of the study has been changed and the antidepressant arm has been removed | |
| Wrong condition: participants not depressed at enrollment | |
| According to information provided by the author (Dr Yi Ba) the study was withdrawn before enrollment. | |
| Wrong design: not randomised | |
| Wrong design: not randomised | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong intervention: ketamine not included among antidepressants according to WHO/DDD | |
| Wrong comparison: control group without placebo | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: not only participants affected by cancer recruited | |
| Wrong comparison: control group without placebo | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: mixed population was enrolled, also including participants with fatigue and anxious symptoms | |
| Wrong condition: participants not affected by cancer | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong design: not randomised | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong condition: participants not depressed at enrollment | |
| Wrong population: patients not depressed at enrollment. | |
| Wrong design: the study described as "randomised", but the treatment received by the comparison arm is not clearly reported | |
| Wrong comparison: control group without placebo | |
| Wrong population: patients not depressed at enrollment | |
| Wrong condition: participants with thyroid cancer and benign thyroid tumours were recruited, and not only depressed participants were recruited |
SSRI: selective serotonin reuptake inhibitor
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | People with lung cancer |
| Interventions | Venlafaxine versus placebo |
| Outcomes | Effects on symptom profiles after 12 weeks (not clearly specified) |
| Notes | According to the protocol the study has been completed, but no published or unpublished data have been retrieved. Not clear if the study is eligible. Authors did not reply to our request for clarification and for data. |
| Methods | Parallel, randomised, open‐label study |
| Participants | Male and females with cancer, diagnosed with major depression; age greater than 20 years |
| Interventions | Mirtazapine versus duloxetine hydrochloride |
| Outcomes | Primary outcome: change in HAM‐D scores between pretreatment baseline and 6‐week treatment |
| Notes | The study is eligible according to the abstract, but results are not available. Authors did not reply to our request for data. |
HAM‐D: Hamilton Depression Rating Scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.01, 0.11] |
| Analysis 1.1  Comparison 1 Depression: efficacy as a continuous outcome at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 4 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.50, 0.08] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.95, 1.04] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| Analysis 1.2  Comparison 1 Depression: efficacy as a continuous outcome at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Paroxetine versus desipramine | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.73, 0.88] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.46, 0.14] |
| 2.3 Fluoxetine versus desipramine | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.45, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.72, 0.13] |
| Analysis 2.1 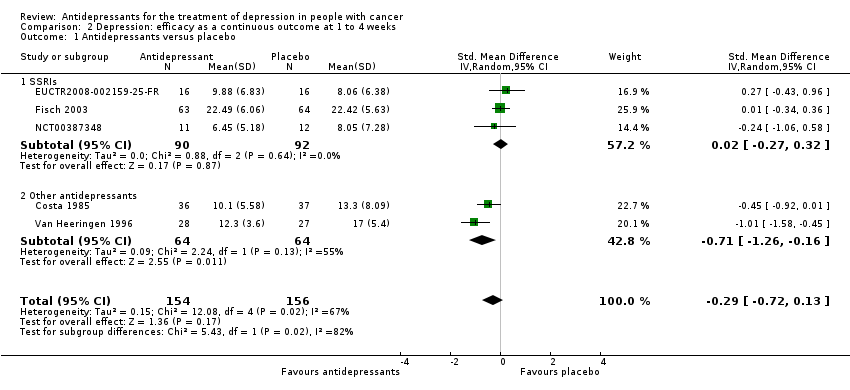 Comparison 2 Depression: efficacy as a continuous outcome at 1 to 4 weeks, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 3 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.27, 0.32] |
| 1.2 Other antidepressants | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.26, ‐0.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.62, 1.08] |
| Analysis 3.1  Comparison 3 Depression: efficacy as a dichotomous outcome at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.11] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.42, 2.86] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.75] |
| 2 Antidepressants versus antidepressants Show forest plot | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.53] |
| Analysis 3.2 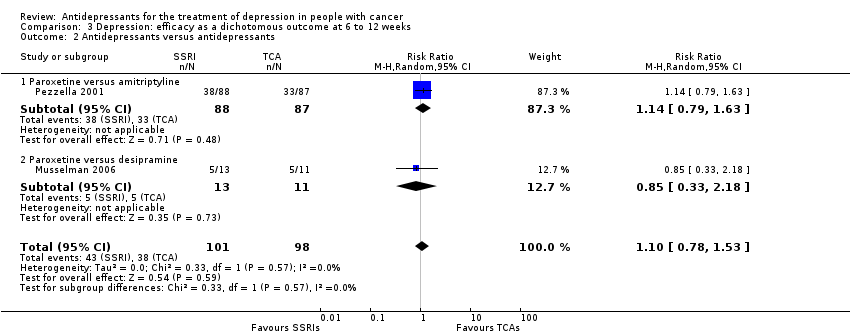 Comparison 3 Depression: efficacy as a dichotomous outcome at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.79, 1.63] |
| 2.2 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.33, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus antidepressants Show forest plot | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
| Analysis 4.1  Comparison 4 Social adjustment at 6 to 12 weeks, Outcome 1 Antidepressants versus antidepressants. | ||||
| 1.1 Paroxetine versus amitriptyline | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.27, 0.37] |
| Analysis 5.1  Comparison 5 Quality of life at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2 Antidepressants versus antidepressants Show forest plot | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.21, 12.79] |
| Analysis 5.2  Comparison 5 Quality of life at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Paroxetine versus amitriptyline | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.21, 12.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 6 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.32] |
| Analysis 6.1  Comparison 6 Dropouts due to inefficacy, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 4 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.10, 7.31] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [0.16, 52.47] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.65] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.14, 5.06] |
| Analysis 6.2  Comparison 6 Dropouts due to inefficacy, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.14, 5.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 7 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.54, 2.62] |
| Analysis 7.1 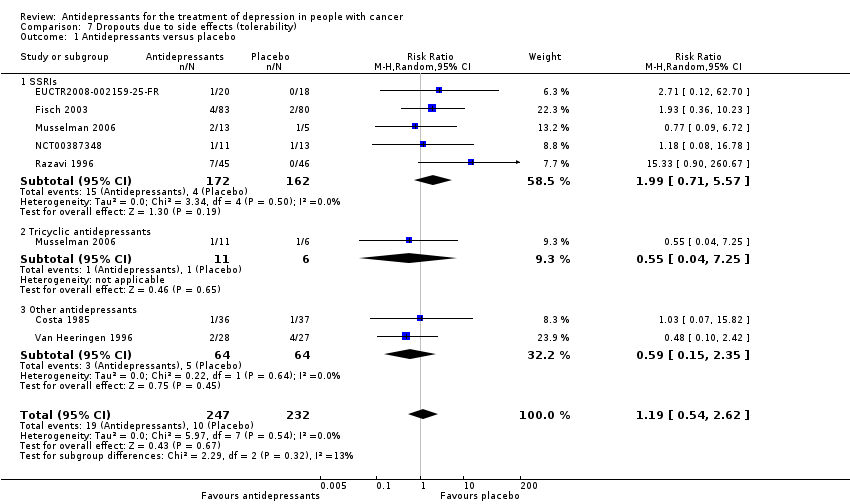 Comparison 7 Dropouts due to side effects (tolerability), Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 5 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.71, 5.57] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.04, 7.25] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.35] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.55, 1.99] |
| Analysis 7.2 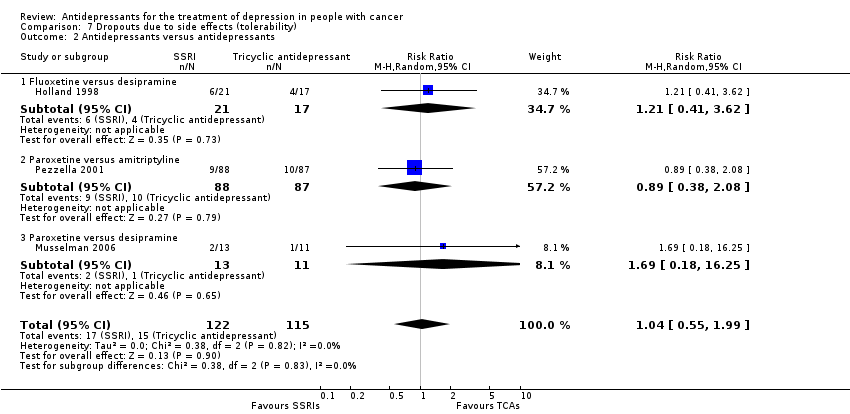 Comparison 7 Dropouts due to side effects (tolerability), Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.41, 3.62] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.08] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.18, 16.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 7 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.38] |
| Analysis 8.1  Comparison 8 Dropouts due to any cause (acceptability), Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 5 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.84, 2.24] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.24, 2.23] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.25, 0.75] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.30] |
| Analysis 8.2 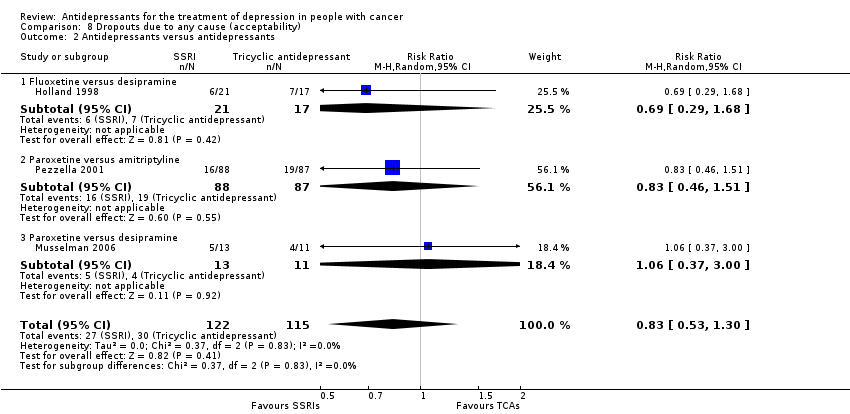 Comparison 8 Dropouts due to any cause (acceptability), Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.68] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.37, 3.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 4 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.23, 0.21] |
| Analysis 9.1  Comparison 9 Subgroup analysis: psychiatric diagnosis, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 Patients with major depressive disorder | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.94, 0.78] |
| 1.2 Patients with adjustment disorder, dysthymic disorder, depressive symptoms | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.67, 0.10] |
| 2 Antidepressants versus antidepressants Show forest plot | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| Analysis 9.2  Comparison 9 Subgroup analysis: psychiatric diagnosis, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Patients with major depressive disorder | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.2 Patients with adjustment disorder, dysthymic disorder, depressive symptoms | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.01, 0.11] |
| Analysis 10.1  Comparison 10 Subgroup analysis: cancer site, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 Patients with breast cancer | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.94, 0.78] |
| 1.2 Patients with other cancer types | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| Analysis 10.2 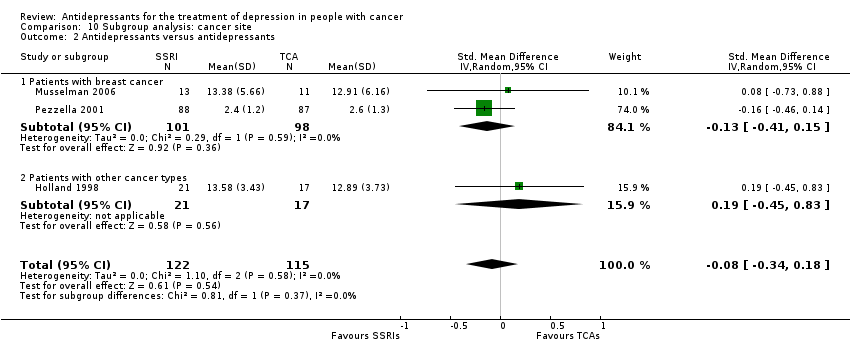 Comparison 10 Subgroup analysis: cancer site, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Patients with breast cancer | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.2 Patients with other cancer types | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.45, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.66, 0.16] |
| Analysis 11.1  Comparison 11 Subgroup analysis: cancer stage, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 Patients with an early stage cancer | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.65, 0.31] |
| 1.2 Patients with a late stage cancer | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.30, 0.33] |
| 2 Antidepressants versus antidepressants Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.61, 2.99] |
| Analysis 11.2  Comparison 11 Subgroup analysis: cancer stage, Outcome 2 Antidepressants versus antidepressants. | ||||
| 2.1 Patients with an early stage cancer | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.61, 2.99] |
| 2.2 Patients with a late stage cancer | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 4 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.23, 0.25] |
| Analysis 12.1  Comparison 12 Sensitivity analysis: excluding trials that did not employ depressive symptoms as their primary outcome, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.58, 0.18] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.95, 1.04] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 4 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.35, 0.06] |
| Analysis 13.1  Comparison 13 Sensitivity analysis: excluding trials with imputed data, Outcome 1 Antidepressants versus placebo. | ||||
| 1.1 SSRIs | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 1.2 Tricyclic antidepressants | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
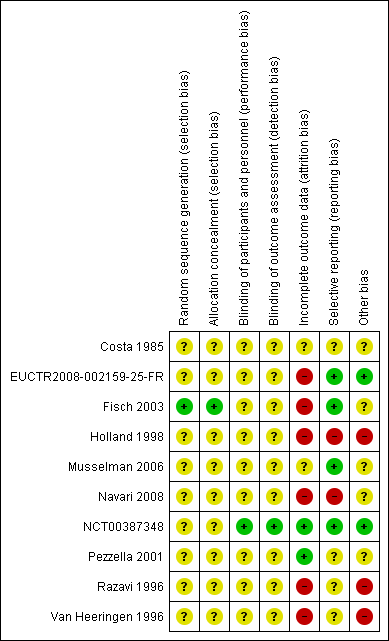
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
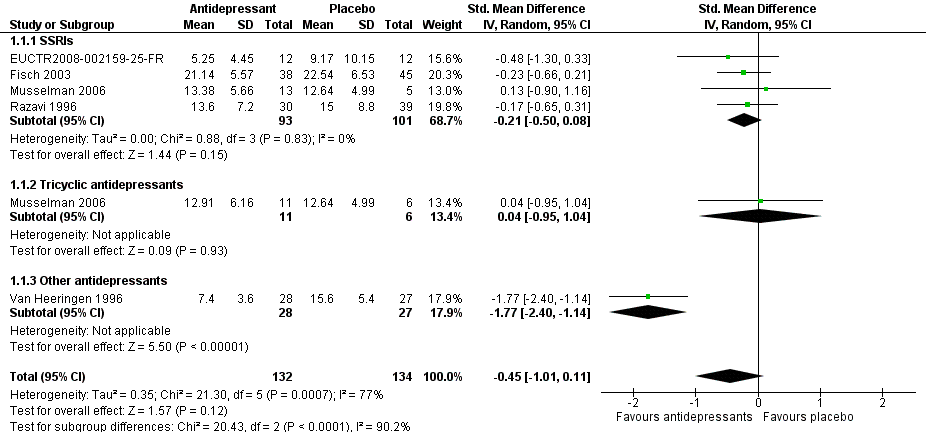
Forest plot of comparison: 1 Depression: efficacy at 6‐12 weeks (continuous outcome), outcome: 1.1 Antidepressants versus placebo.
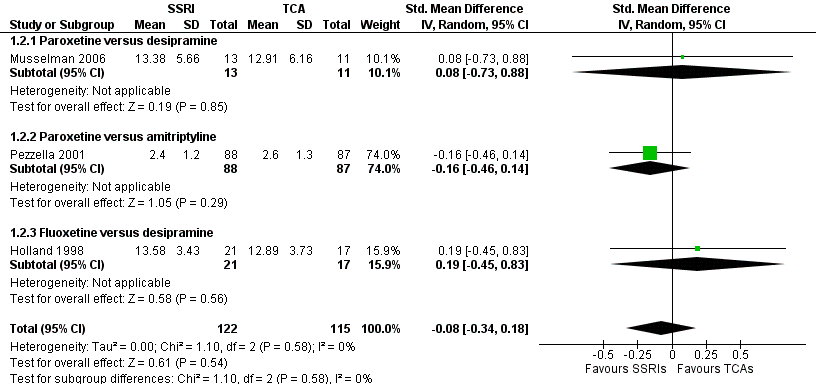
Forest plot of comparison: 1 Depression: efficacy at 6‐12 weeks (continuous outcome), outcome: 1.2 Antidepressants versus Antidepressants.

Comparison 1 Depression: efficacy as a continuous outcome at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo.

Comparison 1 Depression: efficacy as a continuous outcome at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants.

Comparison 2 Depression: efficacy as a continuous outcome at 1 to 4 weeks, Outcome 1 Antidepressants versus placebo.
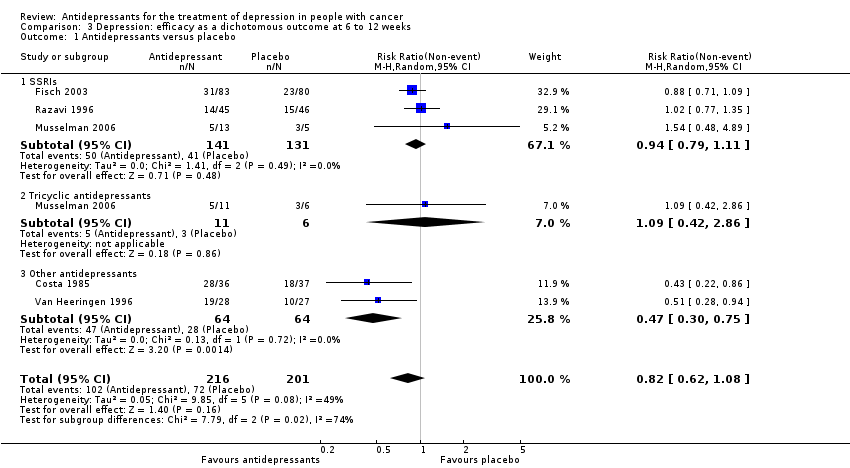
Comparison 3 Depression: efficacy as a dichotomous outcome at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo.

Comparison 3 Depression: efficacy as a dichotomous outcome at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants.

Comparison 4 Social adjustment at 6 to 12 weeks, Outcome 1 Antidepressants versus antidepressants.

Comparison 5 Quality of life at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo.

Comparison 5 Quality of life at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants.

Comparison 6 Dropouts due to inefficacy, Outcome 1 Antidepressants versus placebo.

Comparison 6 Dropouts due to inefficacy, Outcome 2 Antidepressants versus antidepressants.

Comparison 7 Dropouts due to side effects (tolerability), Outcome 1 Antidepressants versus placebo.

Comparison 7 Dropouts due to side effects (tolerability), Outcome 2 Antidepressants versus antidepressants.

Comparison 8 Dropouts due to any cause (acceptability), Outcome 1 Antidepressants versus placebo.
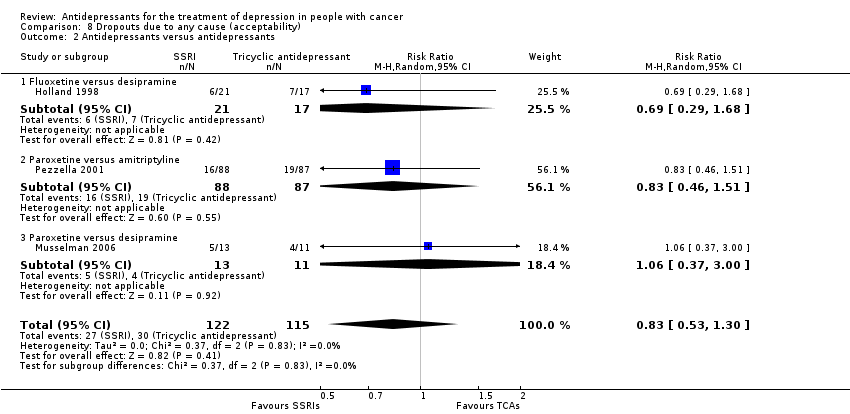
Comparison 8 Dropouts due to any cause (acceptability), Outcome 2 Antidepressants versus antidepressants.

Comparison 9 Subgroup analysis: psychiatric diagnosis, Outcome 1 Antidepressants versus placebo.
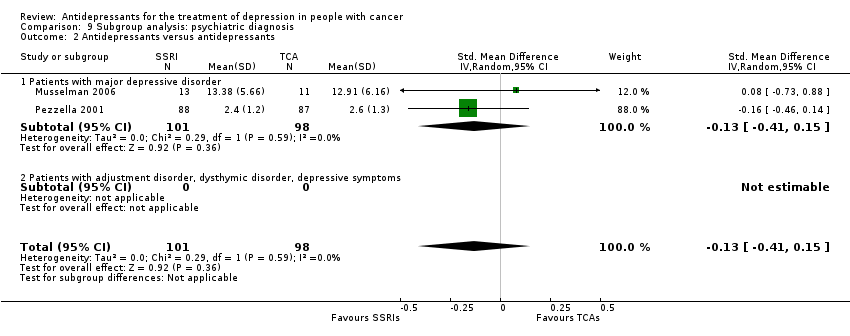
Comparison 9 Subgroup analysis: psychiatric diagnosis, Outcome 2 Antidepressants versus antidepressants.

Comparison 10 Subgroup analysis: cancer site, Outcome 1 Antidepressants versus placebo.

Comparison 10 Subgroup analysis: cancer site, Outcome 2 Antidepressants versus antidepressants.

Comparison 11 Subgroup analysis: cancer stage, Outcome 1 Antidepressants versus placebo.

Comparison 11 Subgroup analysis: cancer stage, Outcome 2 Antidepressants versus antidepressants.

Comparison 12 Sensitivity analysis: excluding trials that did not employ depressive symptoms as their primary outcome, Outcome 1 Antidepressants versus placebo.

Comparison 13 Sensitivity analysis: excluding trials with imputed data, Outcome 1 Antidepressants versus placebo.
| Antidepressants compared to placebo for patients with cancer and depression | ||||||
| Patient or population: adults with cancer and depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainity (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antidepressants | |||||
| Efficacy as a continuous outcome | The mean efficacy as a continuous outcome (SMD) in the intervention groups was | 266 | ⊕⊝⊝⊝ | |||
| Efficacy as a dichotomous outcome | 358 per 1000 | 294 per 1000 | RR 0.82 | 417 | ⊕⊝⊝⊝ | |
| Dropouts due to any cause (acceptability) | 215 per 1000 | 187 per 1000 | RR 0.85 | 479 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded as no studies described the outcome assessment as masked. This should be considered a major limitation, which is likely to result in a biased assessment of the intervention effect. | ||||||
| SSRIs compared to TCAs for patients with cancer and depression | ||||||
| Patient or population: patients with cancer and depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty (Quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| TCAs | SSRIs | |||||
| Efficacy as a continuous outcome | The mean efficacy as a continuous outcome (SMD) in the intervention groups was | 237 | ⊕⊝⊝⊝ | |||
| Efficacy as a dichotomous outcome | Study population | RR 1.10 (0.78 to 1.53 | 199 | ⊕⊝⊝⊝ | ||
| 388 per 1000 | 454 per 1000 | |||||
| Dropouts due to any cause (acceptability) | Study population | RR 0.83 | 237 | ⊕⊝⊝⊝ | ||
| 261 per 1000 | 217 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded as no studies described the outcome assessment as masked. This should be considered a major limitation, which is likely to result in a biased assessment of the intervention effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.01, 0.11] |
| 1.1 SSRIs | 4 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.50, 0.08] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.95, 1.04] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| 2.1 Paroxetine versus desipramine | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.73, 0.88] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.46, 0.14] |
| 2.3 Fluoxetine versus desipramine | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.45, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.72, 0.13] |
| 1.1 SSRIs | 3 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.27, 0.32] |
| 1.2 Other antidepressants | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.26, ‐0.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.62, 1.08] |
| 1.1 SSRIs | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.11] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.42, 2.86] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.75] |
| 2 Antidepressants versus antidepressants Show forest plot | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.53] |
| 2.1 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.79, 1.63] |
| 2.2 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.33, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus antidepressants Show forest plot | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
| 1.1 Paroxetine versus amitriptyline | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.27, 0.37] |
| 1.1 SSRIs | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2 Antidepressants versus antidepressants Show forest plot | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.21, 12.79] |
| 2.1 Paroxetine versus amitriptyline | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.21, 12.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 6 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.32] |
| 1.1 SSRIs | 4 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.10, 7.31] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [0.16, 52.47] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.65] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.14, 5.06] |
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.14, 5.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 7 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.54, 2.62] |
| 1.1 SSRIs | 5 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.71, 5.57] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.04, 7.25] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.35] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.55, 1.99] |
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.41, 3.62] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.08] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.18, 16.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 7 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.38] |
| 1.1 SSRIs | 5 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.84, 2.24] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.24, 2.23] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.25, 0.75] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.30] |
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.68] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.37, 3.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 4 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.23, 0.21] |
| 1.1 Patients with major depressive disorder | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.94, 0.78] |
| 1.2 Patients with adjustment disorder, dysthymic disorder, depressive symptoms | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.67, 0.10] |
| 2 Antidepressants versus antidepressants Show forest plot | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.1 Patients with major depressive disorder | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.2 Patients with adjustment disorder, dysthymic disorder, depressive symptoms | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.01, 0.11] |
| 1.1 Patients with breast cancer | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.94, 0.78] |
| 1.2 Patients with other cancer types | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 2 Antidepressants versus antidepressants Show forest plot | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| 2.1 Patients with breast cancer | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.2 Patients with other cancer types | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.45, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.66, 0.16] |
| 1.1 Patients with an early stage cancer | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.65, 0.31] |
| 1.2 Patients with a late stage cancer | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.30, 0.33] |
| 2 Antidepressants versus antidepressants Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.61, 2.99] |
| 2.1 Patients with an early stage cancer | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.61, 2.99] |
| 2.2 Patients with a late stage cancer | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 4 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.23, 0.25] |
| 1.1 SSRIs | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.58, 0.18] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.95, 1.04] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressants versus placebo Show forest plot | 4 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.35, 0.06] |
| 1.1 SSRIs | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 1.2 Tricyclic antidepressants | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |


