Cirugía mínimamente invasiva versus radioterapia/quimiorradioterapia para el carcinoma orofaríngeo primario de volumen pequeño
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010963.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
James Howard (JH): protocol draft, search strategy development, acquiring trial copies, trial selection, data extraction, data analysis, data interpretation, review draft and future review update.
Liam Masterson (LM): protocol draft, search strategy development, trial selection, data interpretation and review draft.
Raghav C Dwivedi (RCD): protocol draft, search strategy development, trial selection, data interpretation and review draft.
Faruque Riffat (FR): protocol draft, data interpretation and review draft.
James Tysome (JT): protocol draft, data interpretation and review draft.
Richard Benson (RB): protocol draft, data interpretation and review draft.
Sarah Jefferies (SJ): protocol draft, data interpretation and review draft.
Piyush Jani (PJ): protocol draft, data interpretation and review draft.
Christopher Nutting (CN): protocol draft, data interpretation and review draft.
Sources of support
Internal sources
-
NIHR Cambridge Biomedical Research Centre, UK.
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
James EF Howard: none known.
Liam Masterson: none known.
Raghav C Dwivedi: none known.
Faruque Riffat: none known.
Richard Benson: none known.
Sarah Jefferies: none known.
Piyush Jani: none known.
James R Tysome: none known.
Chris Nutting: none known.
Acknowledgements
We acknowledge the very helpful contributions made by the editorial team of Cochrane ENT and in particular the guidance and input of Ms Jenny Bellorini.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Dec 11 | Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma | Review | James Howard, Liam Masterson, Raghav C Dwivedi, Faruque Riffat, Richard Benson, Sarah Jefferies, Piyush Jani, James R Tysome, Chris Nutting | |
| 2014 Feb 20 | Minimally invasive surgery versus radiotherapy/chemoradiotherapy for early‐stage oropharyngeal carcinoma | Protocol | James Howard, Liam Masterson, Raghav C Dwivedi, Faruque Riffat, Richard Benson, Sarah Jefferies, Piyush Jani, James R Tysome, Chris Nutting | |
Differences between protocol and review
Title:
-
'Early‐stage' changed to 'small‐volume, primary'. The same clarification was carried through the entire review.
Background:
-
Addition of work by Schache 2016 and new HPV demographic data.
Methods section:
-
Types of studies clarified to exclude quasi‐ and cluster‐randomised trials.
-
Types of interventions: clarification of planned comparisons.
-
Types of outcome measures: addition of statement to meet MECIR standards whereby studies are not included or excluded based on outcome measures; addition of further details of anticipated/allowable measurement instruments.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
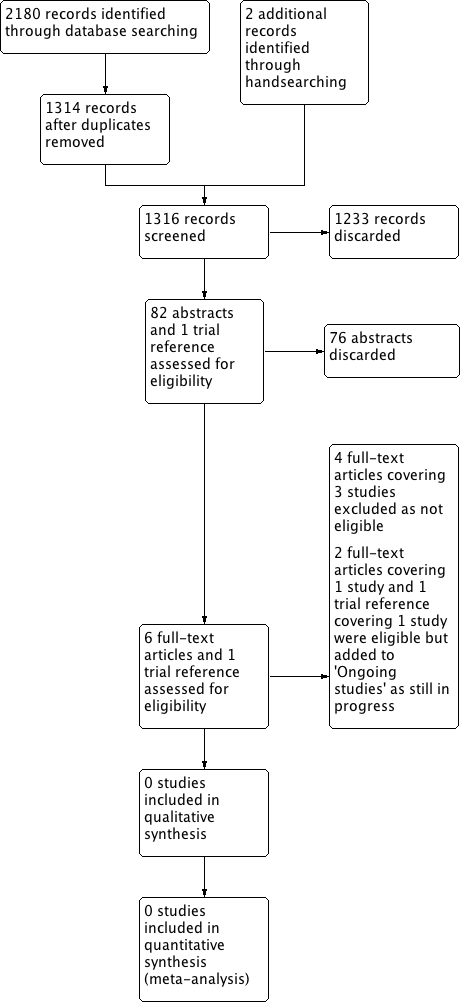
Process for sifting search results and selecting studies for inclusion.
| Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma | ||||||
| Patient or population: patients with small‐volume primary oropharyngeal carcinoma Settings: inpatient Intervention: transoral, minimally invasive surgery (transoral robotic surgery/transoral laser microsurgery) with or without adjuvant radiotherapy or adjuvant chemoradiotherapy Comparison: primary radiotherapy with or without induction or concurrent chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Primary radiotherapy ± induction or concurrent chemotherapy | Transoral, minimally invasive surgery ± adjuvant radiotherapy or adjuvant chemoradiotherapy | |||||
| Overall survival | No data | No data | No data | No data | — | — |
| Locoregional control | No data | No data | No data | No data | — | — |
| Progression‐free survival | No data | No data | No data | No data | — | — |
| Gastrostomy rate (at 1 year) | No data | No data | No data | No data | — | — |
| Tracheostomy rate | No data | No data | No data | No data | — | — |
| Swallowing function (MDADI) | No data | No data | No data | No data | — | — |
| Quality of life (EORTC QLQ‐C30 and H&N35) | No data | No data | No data | No data | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||

