Tomografía computarizada de emisión de fotón único del flujo sanguíneo cerebral regional para la detección de la demencia frontotemporal en pacientes con demencia presunta
Appendices
Appendix 1. Classification of dementia
WHO ICD‐10
Dementia
-
G1. Evidence of each of the following:
-
-
(1) A decline in memory, which is most evident in the learning of new information, although in more severe cases the recall of previously learned information may be also affected. The impairment applies to both verbal and non‐verbal material. The decline should be objectively verified by obtaining a reliable history from an informant, supplemented, if possible, by neuropsychological tests or quantified cognitive assessments. The severity of the decline, with mild impairment as the threshold for diagnosis, should be assessed as follows:
-
Mild: a degree of memory loss sufficient to interfere with everyday activities, though not so severe as to be incompatible with independent living. The main function affected is the learning of new material. For example, the individual has difficulty in registering, storing and recalling elements in daily living, such as where belongings have been put, social arrangements, or information recently imparted by family members.
-
Moderate: a degree of memory loss which represents a serious handicap to independent living. Only highly learned or very familiar material is retained. New information is retained only occasionally and very briefly. The individual is unable to recall basic information about where he lives, what he has recently been doing, or the names of familiar persons.
-
Severe: a degree of memory loss characterised by the complete inability to retain new information. Only fragments of previously learned information remain. The subject fails to recognise even close relatives.
-
-
(2) A decline in other cognitive abilities characterised by deterioration in judgement and thinking, such as planning and organising, and in the general processing of information. Evidence for this should be obtained when possible from interviewing an informant, supplemented, if possible, by neuropsychological tests or quantified objective assessments. Deterioration from a previously higher level of performance should be established. The severity of the decline, with mild impairment as the threshold for diagnosis, should be assessed as follows:
-
Mild: the decline in cognitive abilities causes impaired performance in daily living, but not to a degree making the individual dependent on others. More complicated daily tasks or recreational activities cannot be undertaken.
-
Moderate: the decline in cognitive abilities makes the individual unable to function without the assistance of another in daily living, including shopping and handling money. Within the home, only simple chores are preserved. Activities are increasingly restricted and poorly sustained.
-
Severe: the decline is characterised by an absence, or virtual absence, of intelligible ideation. The overall severity of the dementia is best expressed as the level of decline in memory or other cognitive abilities, whichever is the more severe (e.g. mild decline in memory and moderate decline in cognitive abilities indicate a dementia of moderate severity).
-
-
-
G2. Preserved awareness of the environment during a period of time long enough to enable the unequivocal demonstration of G1. When there are superimposed episodes of delirium the diagnosis of dementia should be deferred.
-
G3. A decline in emotional control or motivation, or a change in social behaviour, manifest as at least one of the following:
-
-
emotional lability;
-
irritability;
-
apathy;
-
coarsening of social behaviour.
-
-
G4. For a confident clinical diagnosis, G1 should have been present for at least six months; if the period since the manifest onset is shorter, the diagnosis can only be tentative.
Comments: The diagnosis is further supported by evidence of damage to other higher cortical functions, such as aphasia, agnosia, apraxia.
Judgements about independent living or the development of dependence (upon others) need to take account of the cultural expectation and context.
Dementia is specified here as having a minimum duration of six months to avoid confusion with reversible states with identical behavioural syndromes, such as traumatic subdural haemorrhage (S06.5), normal pressure hydrocephalus (G91.2) and diffuse or focal brain injury (S06.2 and S06.3).
Neary criteria for behavioural variant of frontotemporal dementia
-
I. Core diagnostic features
-
A. Insidious onset and gradual progression
-
B. Early decline in social interpersonal conduct
-
C. Early impairment in regulation of personal conduct
-
D. Early emotional blunting
-
E. Early loss of insight
-
-
II. Supportive diagnostic features
-
A. Behavioural disorder
-
1. Decline in personal hygiene and grooming
-
2. Mental rigidity and inflexibility
-
3. Distractibility and impersistence
-
4. Hyperorality and dietary changes
-
5. Perseverative and stereotyped behaviour
-
6. Utilisation behaviour
-
-
B. Speech and language
-
1. Altered speech output
-
a. Aspontaneity and economy of speech
-
b. Press of speech
-
-
2. Stereotypy of speech
-
3. Echolalia
-
4. Perseveration
-
5. Mutism
-
-
C. Physical signs
-
1. Primitive reflexes
-
2. Incontinence
-
3. Akinesia, rigidity, and tremor
-
4. Low and labile blood pressure
-
-
D. Investigations
-
1. Neuropsychology: significant impairment on frontal lobe tests in the absence of severe amnesia, aphasia, or perceptuospatial disorder
-
-
-
-
-
2. Electroencephalography (EEG): normal on conventional EEG despite clinically evident dementia
-
-
-
-
-
3. Brain imaging (structural and/or functional): predominant frontal and/or anterior temporal abnormality
-
-
Appendix 2. Search strategies
| Source | Search strategy | Hits retrieved |
| 1. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. Tomography, Emission‐Computed, Single‐Photon/ or Tomography, Emission‐Computed/ 2. SPECT.ti,ab. 3. SPET.ti,ab. 4. single photon emission tomography.ti,ab. 5. single photon emission computed tomography.ti,ab. 6. "SPECT/CT".ti,ab. 7. or/1‐6 8. exp Dementia/ 9. Delirium/ 10. Delirium, Dementia, Amnestic, Cognitive Disorders/ 11. dement*.ti,ab. 12. alzheimer*.ti,ab. 13. (lewy* adj2 bod*).ti,ab. 14. (chronic adj2 cerebrovascular).ti,ab. 15. ("organic brain disease" or "organic brain syndrome").ti,ab. 16. "benign senescent forgetfulness".ti,ab. 17. (cerebr* adj2 deteriorat*).ti,ab. 18. (cerebral* adj2 insufficient*).ti,ab. 19. (pick* adj2 disease).ti,ab. 20. "Frontotemporal lobar degeneration".ti,ab. 21. "progressive non‐fluent aphasia".ti,ab. 22. "primary progressive aphasia".ti,ab. 23. (FTD or FTLD).ti,ab. 24. Frontotemporal Lobar Degeneration/ 25. Primary Progressive Nonfluent Aphasia/ 26. Aphasia, Primary Progressive/ 27. or/8‐26 28. 7 and 27 29. (animals not (humans and animals)).sh. 30. 28 not 29 31. (2012* or 2013*).ed. 32. 30 and 31 | July 2012: 2387 June 2013: 161 |
| 2. EMBASE 1980‐2013 June 21 (Ovid SP) | 1. single photon emission computer tomography/ 2. SPECT.ti,ab. 3. SPET.ti,ab. 4. single photon emission tomography.ti,ab. 5. single photon emission computed tomography.ti,ab. 6. ("99mTc‐SPECT" or "99mTc SPECT" or "99mTc SPECT/CT").ti,ab. 7. or/1‐6 8. dementia/ 9. delirium/ 10. dement*.ti,ab. 11. alzheimer*.ti,ab. 12. (lewy* adj2 bod*).ti,ab. 13. (chronic adj2 cerebrovascular).ti,ab. 14. ("organic brain disease" or "organic brain syndrome").ti,ab. 15. "benign senescent forgetfulness".ti,ab. 16. (cerebr* adj2 deteriorat*).ti,ab. 17. (cerebral* adj2 insufficient*).ti,ab. 18. (pick* adj2 disease).ti,ab. 19. "Frontotemporal lobar degeneration".ti,ab. 20. "progressive non‐fluent aphasia".ti,ab. 21. "primary progressive aphasia".ti,ab. 22. (FTD or FTLD).ti,ab. 23. frontotemporal dementia/ 24. progressive nonfluent aphasia/ 25. primary progressive aphasia/ 26. or/8‐25 27. 7 and 26 28. (2012* OR 2013*).em. 29. 27 and 28 | July 2012: 3439 June 2013: 426 |
| 3. PSYCINFO 1806‐July week 1 2012 (Ovid SP) | 1. SPECT.mp. 2. SPET.mp. 3. single photon emission tomography.ti,ab. 4. single photon emission computed tomography.ti,ab. 5. ("99mTc‐SPECT" or "99mTc SPECT" or "99mTc SPECT/CT").ti,ab. 6. or/1‐5 7. exp Dementia/ 8. Delirium/ 9. dement*.ti,ab. 10. alzheimer*.ti,ab. 11. (lewy* adj2 bod*).ti,ab. 12. (chronic adj2 cerebrovascular).ti,ab. 13. ("organic brain disease" or "organic brain syndrome").ti,ab. 14. "benign senescent forgetfulness".ti,ab. 15. (cerebr* adj2 deteriorat*).ti,ab. 16. (cerebral* adj2 insufficient*).ti,ab. 17. (pick* adj2 disease).ti,ab. 18. "Frontotemporal lobar degeneration".ti,ab. 19. "progressive non‐fluent aphasia".ti,ab. 20. "primary progressive aphasia".ti,ab. 21. (FTD or FTLD).ti,ab. 22. or/7‐21 23. 6 and 22 24. 2012*.up. or 2013*.up. 25. 23 and 24 | July 2012: 657 June 2013: 76 |
| 4. Biosis previews 1926 to present (ISI Web of Knowledge) | Topic=(SPECT OR SPET OR "single photon emission tomography" OR "single photon emission computed tomography" OR "99mTc‐SPECT" OR "99mTc SPECT" OR "99mTc SPECT/CT") AND Topic=(dement* OR alzheimer* OR FTLD OR FTD OR "primary progressive aphasia" OR "progressive non‐fluent aphasia" OR "frontotemporal lobar degeneration" OR "frontolobar degeneration" OR "frontal lobar degeneration" OR "pick* disease" OR "lewy bod*") Timespan=All Years. Databases=BIOSIS Previews. Lemmatization=On | July 2012: 1691 June 2013: 77 |
| 5. Web of Science and conference proceedings (1945‐present) | Topic=(SPECT OR SPET OR "single photon emission tomography" OR "single photon emission computed tomography" OR "99mTc‐SPECT" OR "99mTc SPECT" OR "99mTc SPECT/CT") AND Topic=(dement* OR alzheimer* OR FTLD OR FTD OR "primary progressive aphasia" OR "progressive non‐fluent aphasia" OR "frontotemporal lobar degeneration" OR "frontolobar degeneration" OR "frontal lobar degeneration" OR "pick* disease" OR "lewy bod*") Timespan=records processed from 2011‐07‐01 ‐ 2013‐06‐23. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH. Lemmatization=On | July 2012: 2556 June 2013: 276 |
| 6. LILACS (BIREME) | demências OR dementia OR dementias OR demência OR Alzheimer OR Alzheimers OR Alzheimer’s OR cognitive OR cognitive OR cognitive OR cognition OR "déficit cognitive" OR cognición OR cognição OR Memória OR memory OR Memoria OR "frontotemporal lobar degeneration" OR "degeneração lobar frontotemporal" OR FTLD OR FTD OR "pick’s disease" OR "primary progressive aphasia" [Words] and 99mTc‐HmPAO OR SPECT OR "Single photon emission computerized tomography" OR SPET OR "Single photon emission tomography" OR "tomografía computarizada de emisión de fotón único" [Words] and 2012 OR 2013 [Country, year publication] | July 2012: 98 June 2013: 2 |
| TOTAL before de‐duplication | July 2012: 10828 June 2013: 1018 | |
| TOTAL after de‐dupe and first‐assess | July 2012: 302 June 2013: 86 | |
Appendix 3. Information for extraction to proforma
Bibliographic details of primary paper:
-
Author, title of study, year and journal
Details of index test:
-
Method of [index test] administration, including who administered and interpreted the test, and their training
-
Thresholds used to define positive and negative tests
Reference standard:
-
Reference standard used
-
Method of [reference standard] administration, including who administered the test and their training
Study population:
-
Number of subjects
-
Age
-
Gender
-
Other characteristics, e.g. Apolipoprotein E (APOE) genotype
-
Settings: i) community; ii) primary care; iii) secondary care outpatients; iv) secondary care inpatients and residential care
-
Participant recruitment
-
Sampling procedures
-
Time between index test and reference standard
-
Proportion of people with dementia in sample
-
Subtype and stage of dementia if available
-
MCI (mild cognitive impairment) definition used (if applicable)
-
Duration of follow‐up in delayed verification studies
-
Attrition and missing data
Appendix 4. Assessment of methodological quality QUADAS‐2 tool
| DOMAIN | PATIENT SELECTION | INDEX TEST | REFERENCE STANDARD | FLOW AND TIMING |
| Description | Describe methods of patient selection; describe included patients (prior testing, presentation, intended use of index test and setting) | Describe the index test and how it was conducted and interpreted | Describe the reference standard and how it was conducted and interpreted | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 x 2 table (refer to flow diagram); describe the time interval and any interventions between index test(s) and reference standard |
| Signalling questions (yes/no/unclear) | Was a consecutive or random sample of patients enrolled? | Were the index test results interpreted without knowledge of the results of the reference standard? | Is the reference standard likely to correctly classify the target condition? | Was there an appropriate interval between index test(s) and reference standard? |
| Was a case‐control design avoided? | If a threshold was used, was it prespecified? | Were the reference standard results interpreted without knowledge of the results of the index test? | Did all patients receive a reference standard? | |
| Did the study avoid inappropriate exclusions? | Did all patients receive the same reference standard? | |||
| Were all patients included in the analysis? | ||||
| Risk of bias: high/low/unclear | Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the patient flow have introduced bias? |
| Concerns regarding applicability: high/low/unclear | Are there concerns that the included patients do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? | ‐ |
Appendix 5. Anchoring statements for methodological quality assessment using the QUADAS‐2 tool
| Question | Judgement | Explanation |
| Patient selection | ||
| Was the sampling method appropriate? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Where sampling was used, the designs least likely to cause bias are consecutive sampling or random sampling. Sampling based on volunteers or selecting subjects from a clinic or research resource is prone to bias. |
| Was a case‐control or similar design avoided? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Designs similar to a case‐control design the may introduce bias are those designs where the study team deliberately increase or decrease the proportion of subjects with the target condition, which may not be representative. Some case‐control methods may already be excluded if they mix subjects from various settings. |
| Are exclusion criteria described and appropriate? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Study will be automatically graded as unclear if exclusions are not detailed (pending contact with study authors). Where exclusions are detailed, the study will be graded as 'low risk' if exclusions are felt to be appropriate by the review authors. Certain exclusions common to many studies of dementia are: medical instability; terminal disease; alcohol/substance misuse; concomitant psychiatric diagnosis; other neurodegenerative condition. Exclusions are not felt to be appropriate if 'difficult to diagnose' patients are excluded. Post hoc and inappropriate exclusions will be labelled 'high risk' of bias. |
| Index test | ||
| Was rCBF SPECT assessment/interpretation performed without knowledge of reference standard? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Terms such as "blinded" or "independently and without knowledge of" are sufficient and full details of the blinding procedure are not required. Interpretation of the results of the index test may be influenced by knowledge of the results of reference standard. If the index test is always interpreted prior to the reference standard then the person interpreting the index test cannot be aware of the results of the reference standard and so this item could be rated as 'yes'. |
| Were rCBF SPECT thresholds prespecified? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | For scales and biomarkers there is often a reference point (in units or categories) above which subjects are classified as 'test positive'; this may be referred to as a threshold, clinical cut‐off or dichotomisation point. A study is classified as at high risk of bias if the authors define the optimal cut‐off post‐hoc based on their own study data because selecting the threshold to maximise sensitivity, specificity or both may lead to overoptimistic measures of test performance. Certain papers may use an alternative methodology for analysis that does not use thresholds and these papers should be classified as not applicable. |
| Reference standard | ||
| Is the assessment used for clinical diagnosis of FTD acceptable? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Ante‐mortem clinical diagnosis of FTD will be based on recognised diagnostic criteria, Lund and Manchester Groups 1994 or Neary 1998 or McKhann 2001 criteria (as previously outlined) or histopathological diagnosis and/or genetic mutation known to be causative of FTD (if available). For other types of dementia potentially used to define control groups in our review, clinical diagnoses of dementia will include all‐cause (unspecified) dementia, using any recognised diagnostic criteria, for example DSM‐IV and ICD‐10 (American Psychiatric Association 2013; WHO 2010). NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association) criteria are the most accepted ante‐mortem clinical consensus gold standard for Alzheimer's dementia (McKhann 1984), defining three ante‐mortem groups: 'probable', 'possible' and 'unlikely' Alzheimer's dementia. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) (Mirra 1991), ICD10 and DSM‐IV definitions of AD are also acceptable. National Institute of Neurological Disorders and Stroke‐Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) (Román 1993), Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) (Chui 1992), DSM‐IV, ICD‐10, and the Cambridge Mental Disorders of the Elderly Examination (CAMDEX) criteria (Hendrie 1988) are all acceptable for the diagnosis of vascular dementia (VD). Where the criteria used for assessment are not familiar to the review authors or to the Cochrane Dementia and Cognitive Improvement Group ('unclear') this item should be classified as at high risk of bias. |
| Was clinical assessment for FTD performed without knowledge of the rCBF SPECT biomarker? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Terms such as "blinded" or "independently and without knowledge of" are sufficient and full details of the blinding procedure are not required. Interpretation of the results of the reference standard may be influenced by knowledge of the results of index test. |
| Patient flow | ||
| Was there an appropriate interval between rCBF SPECT and clinical assessment? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | For cross‐sectional case‐control studies the index test and application of the reference standard are ideally administered on the same day, but a delay is unlikely to introduce bias as the condition of dementia is irreversible. For longitudinal studies, the time between reference standard and index test will influence the accuracy (Geslani 2005; Okello 2009; Visser 2006), and therefore we will note time as a separate variable (both within and between studies) and will test its influence on the diagnostic accuracy. We have set a minimum mean time to follow‐up assessment of one year for longitudinal cohort (delayed verification) studies. |
| Did all subjects get the same assessment for dementia regardless of rCBF SPECT result? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | There may be scenarios where subjects who score 'test positive' on the index test have a more detailed assessment. Where dementia assessment (i.e. reference standard) differs between groups of subjects this should be classified as at high risk of bias. |
| Were all patients who received rCBF SPECT assessment included in the final analysis? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | If the number of patients enrolled differs from the number of patients included in the 2 x 2 table then there is the potential for bias. If participant data missing due to drop‐out differ systematically from those for the remaining participants, then estimates of test performance may differ. If drop‐outs occur these should be accounted for; a maximum proportion of drop‐outs has been specified as 20% in order to be scored as low risk of bias, but this will depend upon length of follow up in longitudinal cohort studies. |
| Were missing or uninterpretable rCBF SPECT results reported? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Where missing or uninterpretable results are reported, and if there is substantial attrition (we have set an arbitrary value of 50% missing data), this should be scored as at high risk of bias. If these results are not reported, this should be scored as 'unclear' and authors will be contacted. |
| Anchoring statements to assist with assessment of applicability | ||
| Question | Explanation | |
| Were included patients representative of the general population of interest? | The included patients should match the intended population as described in the review question. The review authors should consider population in terms of symptoms; pre‐testing; potential disease prevalence; setting. If there are clear grounds for suspecting an unrepresentative spectrum the item should be rated 'poor applicability'. | |
| Index test | ||
| Were sufficient data on rCBF SPECT application given for the test to be repeated in an independent study? | Variation in technology, test execution, and test interpretation may affect estimate of accuracy. In addition, the background and training/expertise of the assessor should be reported and taken into consideration. If the rCBF SPECT biomarker was not performed consistently this item should be rated 'poor applicability'. | |
| Reference standard | ||
| Was clinical diagnosis of dementia made in a manner similar to current clinical practice? | For many reviews, clinical diagnosis of dementia will be made using standard clinical criteria. For certain reviews an applicability statement relating to reference standard may not be applicable. There is the possibility that a current reference standard, although valid, may diagnose a far smaller proportion of subjects with disease than in usual clinical practice. In this instance the item should be rated 'poor applicability'. | |
rCBF= regional cerebral blood flow; SPECT= single‐photon emission computerised tomography; FTD=frontotemporal dementia; DSM‐IV= DSM=Diagnostic and Statistical Manual of Mental Disorders‐IV; ICD‐10=International Classificatio of Diseases‐10
Review question and inclusion criteria
| Category | Review question | Inclusion criteria |
| Participants | Participants with suspected FTD (Primary Objective 1)
| Participants fulfilling the criteria for the clinical diagnosis of any forms of dementia in secondary and tertiary care setting |
| Index test | rCBF SPECT biomarker | rCBF SPECT biomarker |
| Target condition | Frontotemporal dementia (FTD)
| Initial diagnosis of FTD
Differential diagnosis of FTD from other dementia subtypes
|
| Reference standard | Diagnosis of FTD as determined by the Manchester‐Lund or NINDS criteria, histopathological confirmation of diagnosis and/or genetic mutation known to be causative of FTD (if available)
| Diagnosis of FTD as determined by the Manchester‐Lund or NINDS criteria, histopathological confirmation of diagnosis and/or genetic mutation known to be causative of FTD (if available)
NINCDS‐ADRDA criteria are the most accepted ante‐mortem clinical consensus gold standard for Alzheimer's dementia, defining three ante‐mortem groups: 'probable', 'possible' and 'unlikely' Alzheimer's dementia. CERAD, ADDTC, ICD‐10 and DSM‐IV definitions of ADD were also acceptable.
NINDS‐AIREN, ADDTC, DSM‐IV, ICD‐10, CAMDEX criteria were acceptable for VaD
|
| Outcome | N/A | Data to construct 2 x 2 table |
| Study design | N/A | Longitudinal cohort studies and nested case‐control studies if they incorporate a delayed‐verification design (case‐control nested in cohort studies) (Objectives) Cross‐sectional studies in which: i) rCBF SPECT results and the clinical diagnostic criteria were obtained within a narrow time‐frame, and ii) FTD patients were differentiated from patients with other dementia subtypes
|
In assessing individual items, the assessment of 'unclear' should be given only if there is genuine uncertainty. In these situations review authors will contact the relevant study teams for additional information.
FTD = frontotemporal dementia; rCBF=; SPECT=; NINDS = National Institute of Neurological Disorders and Stroke; NINCDS‐ADRDA=National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease; CERAD=; ADDTC=ICD‐10; DSM‐IV=; ADD=Alzheimer's disease dementia; NINDS‐AIREN=; CAMDEX=; VaD=vascular dementia;

'Risk of bias' and applicability concerns summary: review authors' judgements about each domain for each included study

'Risk of bias' and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Forest plot of single‐headed and multiple‐headed camera rCBF SPECT for differentiating frontotemporal dementia (FTD) from non‐FTD. The studies are ordered according to study design, reference standard (RS) and sensitivity. TP: true positive; FP: false positive; FN: false negative; TN: true negative; CI: confidence interval.
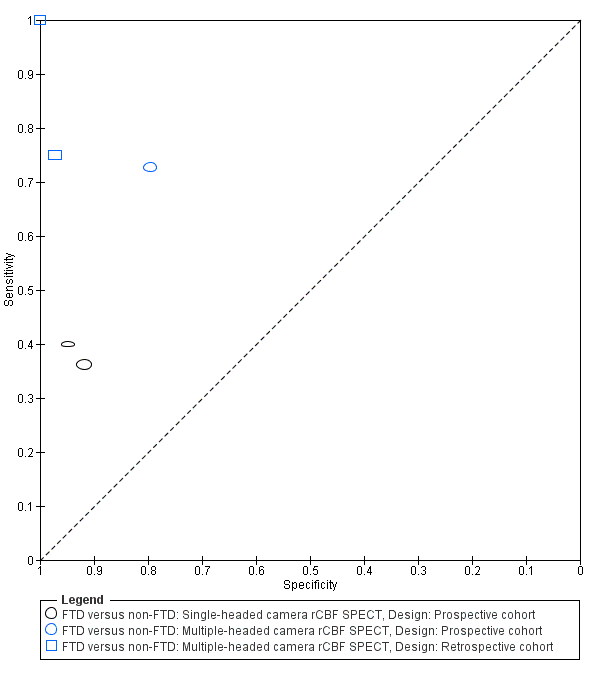
Summary ROC plot of single‐headed and multiple‐headed camera rCBF SPECT for differentiating frontotemporal dementia (FTD) from non‐FTD. Each symbol represents the sensitivity and specificity of a study. Different colours are used to indicate the two camera types and different symbols are used to indicate study design.

Forest plot of single‐headed and multiple‐headed camera rCBF SPECT for differentiating frontotemporal dementia (FTD) from Alzheimer's disease dementia (AD). The studies are ordered according to study design, reference standard (RS) and sensitivity. TP: true positive; FP: false positive; FN: false negative; TN: true negative; CI: confidence interval.
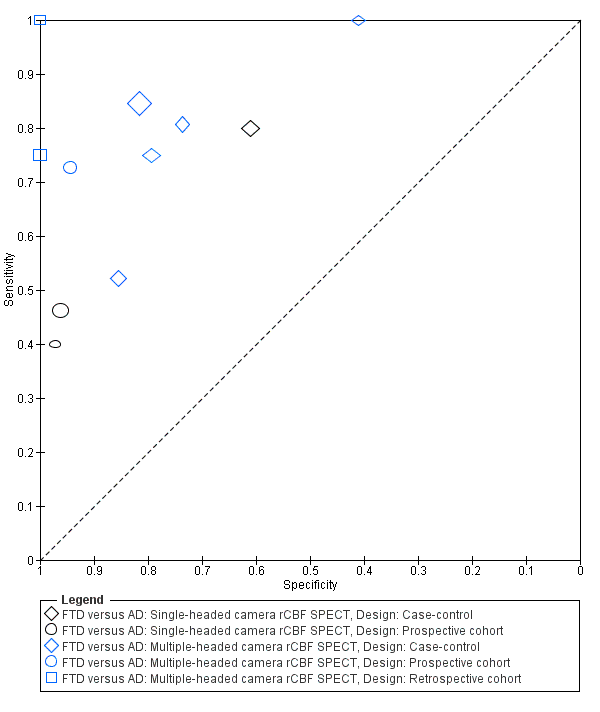
Summary ROC plot of single‐headed and multiple‐headed camera rCBF SPECT for differentiating frontotemporal dementia (FTD) from Alzheimer's disease dementia (AD). Each symbol represents the sensitivity and specificity of a study. Different colours are used to indicate the two camera types and different symbols are used to indicate study design.
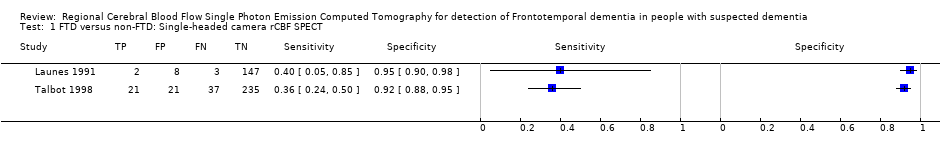
FTD versus non‐FTD: Single‐headed camera rCBF SPECT.

FTD versus non‐FTD: Multiple‐headed camera rCBF SPECT.

FTD versus AD: Single‐headed camera rCBF SPECT.

FTD versus AD: Multiple‐headed camera rCBF SPECT.
| Is the SPECT FTD pattern indicative of developing FTD over time in populations with suspected dementia? What is the diagnostic accuracy of rCBF SPECT biomarker for discriminating FTD from non‐FTD, and FTD from Alzheimer's disease dementia and other dementia subtypes? | |||||||
| Population | Participants with suspected dementia and rCBF SPECT administered at baseline (prospective cohort design) (n = 3) Participants with suspected dementia and rCBF SPECT administered at baseline with histopathological confirmation (retrospective cohort design) (n = 2) Participants clinically diagnosed with FTD or other dementia subtypes using standard clinical diagnostic criteria (case‐control design) (n = 6) | ||||||
| Setting | Outpatients from University centres or University memory clinics (n = 7) Outpatients from General Hospital memory clinics (n = 1) Tertiary referral centre (n = 1) Multicentre (different French hospitals) (n = 1) Not reported (n = 1) | ||||||
| Sampling procedure | Consecutive or random (n = 3) Not consecutive or random (n = 4) Not reported (n = 4) | ||||||
| Prior testing | Prior to performing rCBF SPECT scans, diagnostic criteria for identifying dementia subtypes were applied in studies that used a case‐control study design | ||||||
| Index tests | 99mTc‐HMPAO SPECT; 99mTc‐ECD SPECT; Xenon SPECT | ||||||
| Threshold prespecified at baseline | Yes (n = 6) No (n = 3) Unclear (n = 2) | ||||||
| Threshold | Included studies used a range of thresholds | ||||||
| SPECT scan interpretation | Combined visual and semiquantitative interpretation (n = 6) Visual interpretation only (n = 5) | ||||||
| rCBF hypoperfusion region | Authors used brain regions that were expected to be affected by FTD and so frontal and/or temporal lobes were involved in all studies. Two studies also included parietal and occipital lobes in their evaluations. One study used a range of Broadmann areas (BAs) | ||||||
| Target condition | Frontotemporal dementia (FTD): ante‐mortem clinical diagnosis of FTD (n = 7) or neuropathological diagnosis of FTD (n = 4) | ||||||
| Reference standard | For ante‐mortem clinical diagnosis: Neary 1998 criteria (n = 4); Brun 1994 criteria (n = 1); not specified (n = 2) For neuropathological diagnosis: Shi 2005 (n = 1); Cairns 2007 (n = 1); not specified (n = 2) | ||||||
| Diagnostic criteria for dementia subtypes | For AD dementia: NINCDS‐ADRDA criteria (n = 6); not specified (n = 1); histopathological criteria (n = 4) For vascular dementia: NINDS‐AIREN criteria (n = 2); histopathological (n = 1) | ||||||
| Included studies | Eleven studies (n = 1077 participants) assessed rCBF SPECT for differentiating between FTD and AD. Five of these studies (n = 609) also assessed rCBF SPECT for differentiating between FTD and non‐FTD | ||||||
| Reference standard: neuropathological diagnosis. Objective A: rCBF SPECT FTD type pattern (at baseline) indicative of FTD (at follow up) in participants with suspected FTD at baseline; Objective B: The accuracy of rCBF SPECT pattern in differentiating FTD from AD | |||||||
| Objective | Study | N | Confirmed FTD | Sensitivity (95% CI) | Specificity (95% CI) | Quality | Comment |
| A | Read 1995 | 27 | 7 | 100% (0.59, 1.00) | 100% (0.83, 1.00) | Unclear risk of bias was seen in the patient selection QUADAS‐2 domain. The remaining three domains considered to be at low risk of bias. There were no concerns about applicability (Read 1995; Rollin‐Sillaire 2012). High risk of bias was seen in patient selection, index test, and flow and timing QUADAS‐2 domains. The reference standard was strength of the study. There were no concerns about applicability (Lipton 2004) High risk of bias was seen in participant selection and index test domain. The reference standard was strength of the study. There were no concerns about applicability (McNeill 2007) | Objective A: These papers used the gold standard of histopathological diagnosis; however. the methods used in participant selection and image analysis have led to the introduction of a degree of bias Objective B: These papers used the gold standard of histopathological diagnosis. Although the diagnosis is robust, case‐control design, small study numbers, different methodologies with a wide range of sensitivities and specificities mean that it is difficult to make recommendations on the basis of these results |
| A | Rollin‐Sillaire 2012 | 48 | 9 | 75% (0.43, 0.95) | 97% (0.85. 1.00) | ||
| B | Read 1995 | 20 | 7 | 100% (0.59, 1.00) | 100% (0.75, 1.00) | ||
| B | Rollin‐Sillaire 2012 | 35 | 9 | 75% (0.43, 0.95) | 100% (0.85, 1.00) | ||
| B | Lipton 2004 | 23 | 6 | 100% (0.54, 1.00) | 41% (0.18, 0.67) | ||
| B | McNeill 2007 | 56 | 20 | 80% (0.59, 0.93) | 61 % (0.42, 0.78) | ||
| Investigation of heterogeneity | We were not able to formally assess the effect of potential sources of heterogeneity because meta‐analyses were not performed | ||||||
| Conclusion | Further research on the use of rCBF SPECT for differentiating FTD from other dementias is required. In particular, protocols should be standardised, study populations well described, threshold for 'abnormal' scans predefined and clear details given on how scans are analysed | ||||||
| rCBF=regional cerebral blood flow; SPECT=single‐photon emission computerised tomography; FTD=frontotemporal dementia; 99mTc‐HMPAO=Technetium exametazime hexamethylpropyleneamine oxime; 99mTc‐ECD=Technetium exametazime ethyl cysteinate diethylester; AD=Alzheimer's disease; NINCDS‐ADRDA=National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NINDS‐AIREN=National Institute of Neurological and Communicative Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences; QUADAS‐2=Quality Assessment of Diagnostic Accuracy Studies | |||||||
| rCBF SPECT | Reference standard (Lund‐Manchester; NINDS; histopathological criteria) | |
| FTD present (disease positive) | FTD absent (disease negative) | |
| 'FTD pattern' present (test positive) | True positive | False positive |
| 'FTD pattern' absent (test negative) | False negative | True negative |
| Author year (country) | Target population | Study size (number analysed in review) | Sampling procedure | Number of cases (FTD) identified by reference standard | Index test/camera/interpretation/brain hypoperfusion | Reference standard/target condition | Sensitivity | Specificity |
| Prospective cohort studies | ||||||||
| Boutoleau‐ Bretonniere 2012 (France) | Neurological Memory Center attendees with clinically ambiguous dementias | 69 (19, 29 or 60) | Not reported | 11/60 | 99mTc‐HMPAO SPECT/multiple camera visual/frontal ± temporal | Clinical diagnosis FTD vs non‐FTD | 8/11 0.73 [0.39.0.94] | 39/49 0.80 [0.66‐0.90] |
| FTD vs AD | 8/11 0.73 [0.39, 0.94] | 17/18 0.94 [0.73, 1.00] | ||||||
| FTD vs VD | 8/11 0.73 [0.39, 0.94] | 6/8 0.75 [0.35, 0.97] | ||||||
| Talbot 1998* (UK) | Memory clinic attendees with suspected dementia | 363 (158, 212 or 314) | Consecutive | 58 (FTD)/363 80 (FTD & PPA)/363 | 99mTc‐HMPAO SPECT*/single camera/visual/bilateral anterior+ and bilateral anterior & unilateral posterior++ | Clinical diagnosis FTD vs non‐FTD | 21/58 0.36+ [0.24,0.50] | 235/256 0.92+ [0.88, 0.95] |
| FTD vs AD | 37/80 0.46++ [0.35; 0.58] | 127/132 0.96++ [0.91; 0.99] | ||||||
| FTD vs VD | 37/80 0.46++ [0.35, 0.58] | 57/78 0.73++ [0.73, 0.62] | ||||||
| Launes 1991* (Finland) | Memory clinic attendees with suspected dementia | 160 (41 or 160) | Consecutive | 5/160 | 99mTc‐HMPAO* SPECT/single camera/visual/frontal bilateral or frontal‐temporal | Clinical diagnosis FTD vs non‐FTD | 2/5 0.40 [0.05,0.85] | 147/155 0.95 [0.90, 0.98] |
| FTD vs AD | 2/5 0.40 [0.05, 0.85] | 35/36 0.97 [0.85, 1.00] | ||||||
| FTD vs VD | 2/5 0.40 [0.05, 0.58] | 31/33 0.94 [0.80, 0.99] | ||||||
| Retrospective cohort studies with post‐mortem diagnosis | ||||||||
| Read 1995** (USA) | AD/FTD/JCD/MID/LBD/hydrocephalus recruited from a chart review of the University‐based speciality dementia clinic | 27 (20 or 27) | Not reported | 7/27 | 99mTc‐HMPAO SPECT/double camera/visual/bilateral frontal | Pathological diagnosis FTD vs non‐FTD | 7/7 1.0 [0.59, 1.00] | 20/20 1.0 [0.83, 1.00] |
| FTD vs AD | 7/7 1.0 [0.59, 1.00] | 13/13 1.0 [0.75, 1.00] | ||||||
| Rollin‐Sillaire 2012 (France) | AD/DLB/FTD/VD/FTLD/bvFTD/SD/PPA/PSP/ CBD recruited from the caseload database of the University memory clinic | 48 (35 or 48) | Selected from initially consecutive sample | 12/48 | 99mTc‐HMPAO SPECT/multiple camera combined visual and semi‐quantitative/frontal | Pathological diagnosis FTD vs non‐FTD | 9/12 0.75 0.43, 0.95] | 35/36 0.97 [0.85,1.00] |
| FTD vs AD | 9/12 0.75 [0.43, 0.95] | 23/23 1.0 [0.85, 1.00] | ||||||
| Case‐control studies | ||||||||
| Horn 2007 (France) | FTD/AD recruited from a number of hospitals | 173 (173) | Not consecutive or random | 91/173 | Tc‐99m ECD SPECT/multiple camera/visual/automatic classifier for whole brain | Clinical diagnosis FTD vs AD | 77/91 0.85 [0.76, 0.91] | 67/82 0.82 [0.72, 0.89] |
| Lipton 2004 (USA) | FTD/AD. Sources of recruitment not reported | 27 (23) | Not consecutive or random | 6/23 | Xenon or 99mTc‐HMPAO SPECT/multiple camera/combined visual and semiquantitative/global lateralisation | Pathological diagnosis FTD vs AD | 6/6 1.00 [0.54, 1.00] | 7/17 0.41 [0.18, 0.67] |
| McNeill 2007* (UK) | AD /FTD recruited from a tertiary care centre | 56 (56) | Not consecutive or random | 25/56 | 99mTc‐HMPAO SPECT*/single camera/combined visual and semiquantitative/bifrontal | Pathological diagnosis FTD vs AD | 20/25 0.80 [0.59, 0.93] | 19/31 0.61 [0.42, 0.78] |
| Nagao 2003 (Japan) | FTD/AD recruited from the Higher Brain Function Clinic for outpatients of the University Hospital + healthy controls (not included in the analysis) | 42 (42) | From data file of initially consecutive sample | 21/42 | 99mTc‐HMPAO SPECT multiple camera semiquantitative/Bifrontal+++ or bifrontal & posterior++++ | Clinical diagnosis FTD vs AD | 11/21 0.52+++ [0.30; 0.74] | 18/21 0.86+++ [0.64, 0.97] |
| FTD vs AD | 11/21 0.52++++ [0.30, 0.74] | 21/21 1.0++++ [0.62, 0.82] | ||||||
| Pickut 1996 (Belgium) | FTD/AD recruited from a memory clinic | 40 (40) | Not consecutive or random | 21/40 | 99mTc‐HMPAO SPECT multiple camera combined visual and semiquantitative/frontal and temporal | Clinical diagnosis FTD vs AD | 17/21 0.81 [0.58, 0.95] | 14/19 0.74 [0.49, 0.91] |
| Valotassiou 2012 (Greece) | FTLD (bvFTD; lvFTD; PNFA; CBD+PSP)/AD recruited from an outpatient memory clinic of the General Hospital 21 CBD+PSP participants not included in the analysis; they are not the patients with the target condition considered in the review | 112 (59 or 60 or 50) | Consecutive | 20 (bvFTLD)/59 21 (SD)/60 11 (PNFA)/50 | 99mTc‐HMPAO SPECT/multiple camera semiquantitative/brain Broadmann areas | Clinical diagnosis bvFTD vs AD | 15/20 0.75 [0.51, 0.91] | 31/39 0.79 0.64, 0.91] |
| SD vs AD | 17/21 0.81 [0.58, 0.95] | 20/39 0.51 [0.35, 0.68] | ||||||
| PNFA vs AD | 8/11 0.73 [0.39, 0.94] | 25/39 0.64 0.47, 0.79] | ||||||
| N = number of participants in the study; n = number of participants included in the analysis in the review; *Study used a single‐headed camera with no extended acquisition and did not use image analysis; **Study used two cameras but details of total counts can only realistically apply to the brain‐dedicated camera; + bilateral anterior brain hypoperfusion; ++ bilateral anterior & unilateral posterior brain hypoperfusion; +++bifrontal brain hypoperfusion; ++++ bifrontal & posterior brain hypoperfusion; bvFTD = behavioural variant frontotemporal degeneration; CBD = corticobasal degeneration; FTLD = frontotemporal degeneration; JCD = Jakob‐Creutzfeldt Disease; lvFTD = language variant frontotemporal degeneration; MID = mixed dementia; PM = post‐mortem; PPA = primary progressiva aphasia; PNFA = progressive non‐fluent aphasia, PSP = progressive supranuclear palsy; SD = semantic dementia; SPECT = Single photon emission computed tomography; 99mTc‐HMPAO = 99mTc‐hexamethylpropyleneamineoxime | ||||||||
| Test | No. of studies | No. of participants |
| 1 FTD versus non‐FTD: Single‐headed camera rCBF SPECT Show forest plot | 2 | 474 |
| 2 FTD versus non‐FTD: Multiple‐headed camera rCBF SPECT Show forest plot | 3 | 135 |
| 3 FTD versus AD: Single‐headed camera rCBF SPECT Show forest plot | 3 | 309 |
| 4 FTD versus AD: Multiple‐headed camera rCBF SPECT Show forest plot | 8 | 421 |


