Exámenes genéticos para la prevención de la erupción cutánea grave inducida por fármacos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010891.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 julio 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
AA was the contact person with the editorial base.
AA co‐ordinated contributions from the co‐authors and wrote the final draft of the review.
AJ and AA screened papers against eligibility criteria.
AJ and AA obtained data on ongoing and unpublished studies.
AJ and AA appraised the quality of papers.
AJ and AA extracted data for the review.
AA sought additional information about papers.
AA entered data into Review Manager 5.
AA, AJ, MP and BM analysed and interpreted data.
AA, AJ, MP and BM worked on the methods sections.
AA and MP drafted the clinical sections of the background and responded to the clinical comments of the referees.
AJ responded to the methodological and statistical comments of the referees.
LHS was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
AA is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, or NHS.
Sources of support
Internal sources
-
No financial support, Other.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
AA: none known.
MP: none known.
BM: none known.
LHS: none known.
ALJ: none known.
TC: none known.
Acknowledgements
The Cochrane Skin editorial base would like to thank the following people who commented on this review: the clinical referee, Neil H Shear; and the consumer referee, Jack Tweed. We would also like to thank Jessica Sharp for copy‐editing the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Jul 17 | Genetic testing for prevention of severe drug‐induced skin rash | Review | Ana Alfirevic, Munir Pirmohamed, Branka Marinovic, Linda Harcourt‐Smith, Andrea L Jorgensen, Tess E Cooper | |
| 2013 Dec 19 | Genetic testing for prevention of severe drug‐induced skin rash | Protocol | Ana Alfirevic, Munir Pirmohamed, Branka Marinovic, Andrea L Jorgensen, Linda Harcourt‐Smith | |
Differences between protocol and review
Our objectives and eligibility criteria remained the same between the protocol and the review. After the protocol stage, two new outcomes were added to our secondary outcomes: safety of abacavir and cost‐effectiveness. However, these were not in any way conditional to our eligibility criteria, nor were they influenced by the availability of the data from included studies.
We were unable to implement several methodologies from the protocol, due to there only being one included study. The following methods are not applicable with one study: addressing unit of analysis issues; dealing with missing data (contact was made but we did not receive a response); assessment of heterogeneity; assessment of reporting bias; data synthesis; subgroup analysis and investigation of heterogeneity (not applicable with one study, and outcomes not addressed); and sensitivity analysis.
Types of participants: we clarified how we would handle a study with only a subset of relevant participants; however, we did not encounter such studies.
Types of interventions: in the review, we additionally stated that we excluded any participants who had previously been administered the study drugs because of accurate causality assessment. Furthermore, we clarified the comparator in the review and included the following statement "and compared to standard clinical practice which does not include a genetic test".
Types of outcome measures: we added a definition of ‘severe drug‐induced skin rash’ to our primary outcome because phenotype definitions vary widely in the literature and some study authors report hypersensitivity reactions and SJS/TEN as severe or serious drug‐induced skin rash. We also added 'death' as a secondary outcome.
Searching other resources: although not planned in the protocol, we additionally checked the bibliographies of published reviews for relevant references and we requested relevant information from the authors of the included study.
Data extraction and management: we had planned to consult MP if consensus was not reached, but instead discussed disagreements with TC because authorship changed due to availability.
Assessment of risk of bias in included studies: we had planned in the protocol for all authors to assess the risk of bias of three studies as a pilot but did not do this because only one study fulfilled our inclusion criteria.
Unit of analysis issues: we did not encounter cluster‐randomised trials, so did not have to deal with the issues we had planned for in the protocol.
Dealing with missing data: we planned to report the limited results from studies with missing summary data in narrative form in the results section and consider whether they are consistent with the results of the meta‐analysis for that outcome. Also, where appropriate, we had planned to make assumptions about the missing data (e.g. assuming all missing values to have a particular value, e.g. an adverse event) and conduct sensitivity analyses to test how sensitive the analyses are to our assumptions. We had planned to address the potential implications of the missing data in the Discussion section. These plans were not implemented because only one study was included in this review which only reported data on two of our secondary outcomes.
Assessment of heterogeneity, Assessment of reporting biases, Subgroup analysis and investigation of heterogeneity, and Sensitivity analysis: we could not implement our planned methods because only one studies was included in this review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Flowchart of interventions (genetic testing) and outcomes (skin rash) in a patient population prescribed drug X
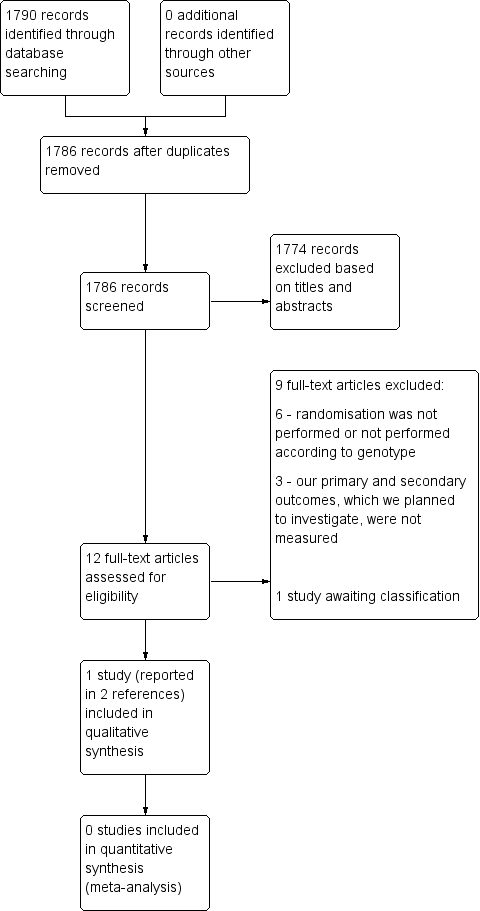
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
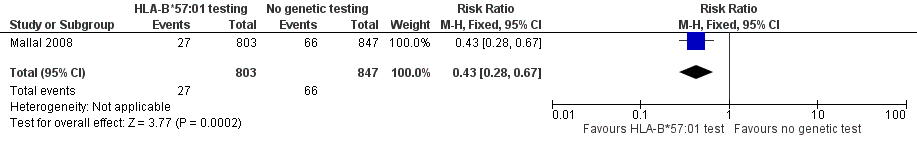
Forest plot of comparison: 1 Genetic testing vs no testing, outcome: 1.1 Hypersensitivity (HSS).

Forest plot of comparison: 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, outcome: 1.1 Hypersensitivity (HSS) immunologically confirmed.

Drug‐induced skin rash: top panel = maculopapular exanthema, bottom panel = Steven Johnson Syndrome (blistering skin rash with skin detachment)
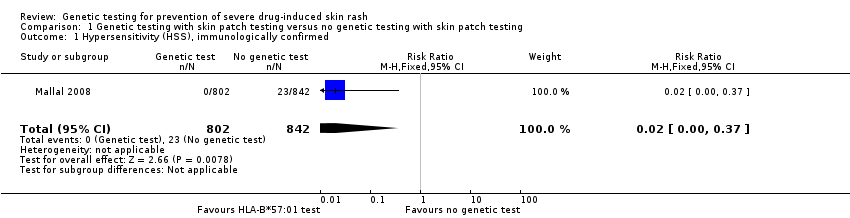
Comparison 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, Outcome 1 Hypersensitivity (HSS), immunologically confirmed.
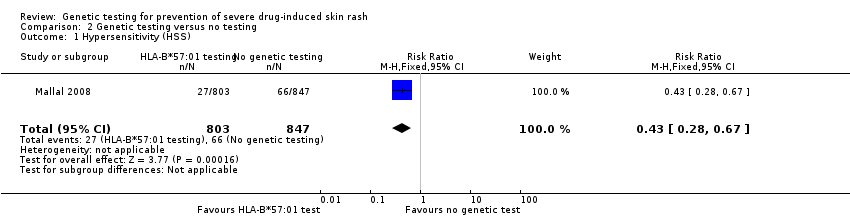
Comparison 2 Genetic testing versus no testing, Outcome 1 Hypersensitivity (HSS).
| Prospective genetic HLA‐B*57:01 screening compared with standard care for drug‐induced skin rash | ||||||
| Patient or population: patients with HIV‐1 infection and a pre‐established clinical need for treatment with an antiretroviral drug regimen containing abacavir but with an unknown HLA‐B*57:01 status. Settings: secondary care clinics Intervention: prospective genetic screening for the HLA‐B*57:01 allele Comparison: no prospective genotyping, standard‐of‐care treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard Carea | Prospective genetic HLA‐B*57:01 screening | |||||
| Severe skin drug‐induced rash | No data | ‐ | ‐ | ‐ | Not assessed | |
| Long‐term sequelae | No data | ‐ | ‐ | ‐ | Not assessed | |
| Hospitalisation for drug‐induced skin reaction | No data | ‐ | ‐ | ‐ | Not assessed | |
| SJS/TEN (Stevens‐Johnson syndrome/toxic epidermal necrolysis) | See hypersensitivity | ‐ | ‐ | ‐ | Not assessable | |
| AGEP (acute generalised exanthematous pustulosis) | No data | ‐ | ‐ | ‐ | Not assessed | |
| HSS (hypersensitivity) reaction including SJS/TEN (clinically diagnosed) (6 weeks clinical assessment) | 78 per 1000 | 34 per 1000 | RR 0.43 (0.28 to 0.67) | 1650 participants | ⊕⊕⊕⊝ | ‐ |
| HSS reaction including SJS/TEN (immunologically confirmed) (6 weeks clinical assessment) | 27 per 1000 | 0 per 1000 | RR 0.02 (0.00 to 0.37) | 1644 participants (1 study) | ⊕⊕⊕⊝ moderateb | ‐ |
| Death | No data | ‐ | ‐ | ‐ | Not assessed | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a The assumed risk is estimated from the event rate (confirmed clinically diagnosed occurrences of HSS including SJS/TEN or immunologically confirmed) in the control arm of the included study. b We downgraded the evidence to moderate quality, due to study limitations (high risk of detection and attrition bias). | ||||||
| Term | Explanation |
| Allele | One of two or more alternative forms of a gene at corresponding sites (loci) on homologous chromosomes |
| Antiretroviral | A class of drugs that inhibit the activity of retroviruses that cause HIV infection |
| Hardy‐Weinberg equilibrium | This states that allele and genotype frequencies in a population will remain constant from generation to generation in the absence of other evolutionary influences |
| HLA | Human leukocyte antigen: a group of protein molecules located on bone marrow and other cells that can provoke an immune response |
| Hypersensitivity | A state of altered reactivity in which the body reacts with an exaggerated immune response to a foreign substance, such as a drug |
| Immunologically confirmed | Patch testing is done to see whether a particular drug is causing allergic skin reaction. Patch test can detect delayed allergic or immunological reaction and confirm the diagnosis of hypersensitivity. |
| Phenotypes | The set of observable characteristics of an individual resulting from the interaction of its genotype with the environment |
| Polymorphic | A variation in the DNA that is too common to be due merely to new mutation. A polymorphism must have a frequency of at least 1% in a population |
| Maculopapular rash | A rash with both macules (flat and coloured like a freckle) and papules (a small raised spot) |
| Sequelae | A condition that is a consequence of a previous disease or injury |
| T‐cells | Another term for T‐lymphocyte, a type of cell that participates in immune response |
| Drugs associated with skin injury | Class of drug | HLA allele | Population | Reference |
| Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) | ||||
| Allopurinol | Antiuric acid | B*5801 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| ‐ | ‐ | ‐ | Japanese | |
| ‐ | ‐ | ‐ | Malay | |
| Carbamazepine | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | Kulkantrakorn 2012; Locharernkul 2008; Tassaneeyakul 2010; Tangamornsuksan 2013 |
| ‐ | ‐ | ‐ | Malay | |
| ‐ | ‐ | ‐ | Indian | |
| ‐ | ‐ | A*3101 | White | |
| ‐ | ‐ | A*3101 | Japanese | |
| Phenytoin | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| Oxicam | Non‐steroidal anti‐inflammatory drug (NSAID) | A2, B12 | White | |
| Sulphamethoxazole | Antibiotic | A29, B12, DR7 | White | |
| Hypersensitivity syndrome (drug‐induced hypersensitivity syndrome (DIHS) or drug reaction with eosinophilia and systemic symptoms (DRESS)) | ||||
| Abacavir | Antiretroviral | B*57:01 | White | Hetherington 2002; Hughes 2004a; Mallal 2002; Mallal 2008; Martin 2004 |
| ‐ | ‐ | ‐ | African American | |
| Aminopenicillins | Antibiotic | A2, Drw52 | White | |
| Nevirapine | Antiretroviral | DRB1*01 | White ‐ Australian | |
| ‐ | ‐ | DRB1*01 | White ‐ French | |
| ‐ | ‐ | Cw8, B14 | White ‐ Italian | |
| ‐ | ‐ | Cw8 | Japanese | |
| ‐ | ‐ | B*3505 | Thai | |
| ‐ | ‐ | Cw4 | Thai | |
| ‐ | ‐ | C*0404 | Black African | |
| ‐ | ‐ | Cw*04 | Chinese | |
| Aspirin | NSAIDs | DRB1*1302, DQB1*0609 | ‐ | |
| ‐ | NSAIDs | DR11 | ‐ | |
| Iodine contrast media | ‐ | DR | White ‐ Spanish | |
| Paraphenylenediamine | Hair dye | DP | White ‐ German | |
| Gold sodium thiomalate | Treatment of rheumatoid arthritis | DR5 | White ‐ Spanish | |
| Lamotrigine | Antiepileptic | B*5801, A*6801 | White | |
| Trichloroethylene | Industrial solvent, dry cleaning | B*1301 | Japanese | |
| Fixed drug eruptions | ||||
| Co‐trimoxazole | Antibiotic | A30, B13, Cw6 | White ‐ Turkish | |
| Feprazone | Analgesic | B22 | ‐ | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypersensitivity (HSS), immunologically confirmed Show forest plot | 1 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypersensitivity (HSS) Show forest plot | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.67] |

