Exámenes genéticos para la prevención de la erupción cutánea grave inducida por fármacos
Appendices
Appendix 1. Outcomes adapted from Pirmohamed 2011
Primary outcome:
-
Drug‐induced skin reaction (yes, no) Figure 7

Drug‐induced skin rash: top panel = maculopapular exanthema, bottom panel = Steven Johnson Syndrome (blistering skin rash with skin detachment)
Secondary outcomes:
-
SJS/TEN (Stevens‐Johnson syndrome, toxic epidermal necrolysis)
-
-
Skin detachment 1% to 10% (SJS), 10% to 30% (overlap syndrome), and > 30% (TEN)
-
Severe, often hemorrhagic, erosions of mucous membranes
-
Other manifestations indicating systemic involvement (e.g. fever, liver chemistry elevations, intestinal and pulmonary manifestations, or the presence of lymphopenia)
-
Severe pain and tenderness in the skin
-
Target lesions, representing the degree of epidermal necrosis
-
-
AGEP (acute generalised exanthematous pustulosis)
-
-
Acute widespread edematous erythema followed by a sterile pustular eruption. Often the pustules are first localised in the neck, groin, and axillae, and later become widely disseminated
-
Fever (temperature > 38 °C)
-
Neutrophilia with or without a mild eosinophilia
-
-
HSS (Hypersensitivity syndrome)
-
-
Additional terminology includes Drug‐induced hypersensitivity syndrome (DIHS), Drug reaction with eosinophilia and systemic symptoms (DRESS), Drug‐induced delayed multiorgan hypersensitivity syndrome
-
Variable skin manifestations; exanthema are most common
-
Increased liver function tests, hepatitis, cholestasis
-
Colitis
-
Nephritis
-
Pneumonitis
-
Aseptic meningitis, encephalitis, inappropriate antidiuretic hormone syndrome
-
Myocarditis
-
Myositis
-
Lymphocytic thyroiditis
-
Eosinophilia, atypical lymphocytes, agranulocytosis, thrombocytopenia, haemolytic anaemia, aplastic anaemia
-
Lymphadenopathy, pseudolymphoma
-
Appendix 2. CRSW/Skin Specialised Register search strategy
(Exanthema or rash or rashes or “drug induced skin injur*” or “drug hypersensitiv*” or “hypersensitiv* reaction*” or “hypersensitiv* syndrome*” or “drug eruption*” or “drug toxicity” or “adverse drug reaction*” or “toxic epidermal necrolysis” or ten or “stevens johnson syndrome” or “Acute Generalized Exanthematous Pustulosis” or “erythema multiforme” or “dress syndrome” or “Drug Reaction with Eosinophilia and Systemic Symptoms” or “Drug Rash with Eosinophilia and Systemic Symptoms”) and (“genetic test*” or pharmacogenomic* or pharmacogenetic* or screening or “patch test*” or “HLA Antigen*” or “hla allele*” or “genetic polymorphism*” or “genetic variation*” or “genetic variant*" or "genetic variabilit*”)
Appendix 3. CENTRAL (Cochrane Library) search strategy
#1 exanthema:ti,ab
#2 MeSH descriptor: [Exanthema] explode all trees
#3 (rash or rashes):ti,ab
#4 drug induced skin injur*:ti,ab
#5 MeSH descriptor: [Drug Hypersensitivity] explode all trees and with qualifier(s): [Genetics ‐ GE, Prevention & control ‐ PC]
#6 drug near/2 hypersensitiv*:ti,ab
#7 hypersensitiv* reaction*:ti,ab
#8 hypersensitivity syndrome*:ti,ab
#9 drug eruption*:ti,ab
#10 MeSH descriptor: [Drug‐Related Side Effects and Adverse Reactions] explode all trees and with qualifier(s): [Genetics ‐ GE, Prevention & control ‐ PC]
#11 drug toxic*:ti,ab
#12 adverse drug reaction*:ti,ab
#13 toxic epidermal necrolysis:ti,ab
#14 MeSH descriptor: [Stevens‐Johnson Syndrome] explode all trees
#15 stevens johnson syndrome:ti,ab
#16 Acute Generalized Exanthematous Pustulosis:ti,ab
#17 MeSH descriptor: [Acute Generalized Exanthematous Pustulosis] explode all trees
#18 erythema multiforme:ti,ab
#19 MeSH descriptor: [Erythema Multiforme] explode all trees
#20 dress syndrome:ti,ab
#21 Drug Reaction with Eosinophilia and Systemic Symptoms:ti,ab
#22 Drug Rash with Eosinophilia and Systemic Symptoms:ti,ab
#23 {or #1‐#22}
#24 MeSH descriptor: [Genetic Testing] explode all trees
#25 genetic test*:ti,ab
#26 MeSH descriptor: [Pharmacogenetics] explode all trees
#27 (pharmacogenomic* or pharmacogenetic*):ti,ab
#28 screening:ti,ab
#29 patch test*:ti,ab
#30 MeSH descriptor: [Patch Tests] explode all trees
#31 MeSH descriptor: [HLA Antigens] explode all trees
#32 hla allele*:ti,ab
#33 MeSH descriptor: [Polymorphism, Genetic] explode all trees
#34 genetic polymorphism*:ti,ab
#35 (genetic variant* or genetic variation* or genetic variabilit*):ti,ab
#36 MeSH descriptor: [Genetic Variation] explode all trees
#37 {or #24‐#36}
#38 #23 and #37
Appendix 4. MEDLINE (Ovid) search strategy
1. exp Exanthema/
2. exanthema.ti,ab.
3. (rash or rashes).ti,ab.
4. drug induced skin injur$.ti,ab.
5. exp Drug Hypersensitivity/ge, pc [Genetics, Prevention & Control]
6. (drug adj2 hypersensitiv$).ti,ab.
7. hypersensitiv$ reaction$.ti,ab.
8. hypersensitivity syndrome$.ti,ab.
9. drug eruption$.ti,ab.
10. exp Drug Toxicity/ge, pc [Genetics, Prevention & Control]
11. drug toxic$.ti,ab.
12. adverse drug reaction$.ti,ab.
13. toxic epidermal necrolysis.ti,ab. or exp Epidermal Necrolysis, Toxic/
14. stevens johnson syndrome.ti,ab. or exp Stevens‐Johnson Syndrome/
15. exp Acute Generalized Exanthematous Pustulosis/
16. Acute Generalized Exanthematous Pustulosis.ti,ab.
17. erythema multiforme.ti,ab. or exp Erythema Multiforme/
18. dress syndrome.ti,ab.
19. "Drug Reaction with Eosinophilia and Systemic Symptoms".ti,ab.
20. "Drug Rash with Eosinophilia and Systemic Symptoms".ti,ab.
21. or/1‐20
22. exp Genetic Testing/
23. genetic test$.ti,ab.
24. exp Pharmacogenetics/
25. (pharmacogenomic$ or pharmacogenetic$).ti,ab.
26. screening.ti,ab.
27. patch test$.ti,ab.
28. exp Patch Tests/
29. exp HLA Antigens/
30. hla allele$.ti,ab.
31. exp Polymorphism, Genetic/
32. genetic polymorphism$.ti,ab.
33. exp Genetic Variation/
34. (genetic variant$ or genetic variation$ or genetic variabilit$).ti,ab.
35. or/22‐34
36. randomized controlled trial.pt.
37. controlled clinical trial.pt.
38. randomized.ab.
39. placebo.ab.
40. clinical trials as topic.sh.
41. randomly.ab.
42. trial.ti.
43. 36 or 37 or 38 or 39 or 40 or 41 or 42
44. exp animals/ not humans.sh.
45. 43 not 44
46. 21 and 35 and 45
[Lines 36‐45: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 5. Embase (Ovid) search strategy
1. exp rash/
2. exanthema.ti,ab.
3. (rash or rashes).ti,ab.
4. drug induced skin injur$.ti,ab.
5. exp drug hypersensitivity/pc [Prevention]
6. (drug adj2 hypersensitiv$).ti,ab.
7. hypersensitiv$ reaction$.ti,ab.
8. hypersensitivity syndrome$.ti,ab.
9. drug eruption$.ti,ab.
10. exp drug toxicity/pc [Prevention]
11. drug toxic$.ti,ab.
12. adverse drug reaction$.ti,ab.
13. toxic epidermal necrolysis.ti,ab.
14. toxic epidermal necrolysis/
15. Stevens Johnson syndrome/
16. stevens johnson syndrome.ti,ab.
17. acute generalized exanthematous pustulosis/
18. Acute Generalized Exanthematous Pustulosis.ti,ab.
19. erythema multiforme/
20. erythema multiforme.ti,ab.
21. DRESS syndrome/
22. dress syndrome.ti,ab.
23. "Drug Reaction with Eosinophilia and Systemic Symptoms".ti,ab.
24. "Drug Rash with Eosinophilia and Systemic Symptoms".ti,ab.
25. or/1‐24
26. genetic screening/
27. genetic test$.ti,ab.
28. exp pharmacogenetics/
29. (pharmacogenomic$ or pharmacogenetic$).ti,ab.
30. screening.ti,ab.
31. patch test$.ti,ab.
32. patch test/
33. exp HLA antigen/
34. hla allele$.ti,ab.
35. exp genetic polymorphism/
36. genetic polymorphism$.ti,ab.
37. genetic variability/
38. (genetic variant$ or genetic variation$ or genetic variabilit$).ti,ab.
39. or/26‐38
40. crossover procedure.sh.
41. double‐blind procedure.sh.
42. single‐blind procedure.sh.
43. (crossover$ or cross over$).tw.
44. placebo$.tw.
45. (doubl$ adj blind$).tw.
46. allocat$.tw.
47. trial.ti.
48. randomized controlled trial.sh.
49. random$.tw.
50. or/40‐49
51. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
52. human/ or normal human/
53. 51 and 52
54. 51 not 53
55. 50 not 54
56. 25 and 39 and 55
Appendix 6. LILACS search strategy
tw:((exanthema OR exantema OR rash OR rashes OR "toxic epidermal necrolysis" OR "stevens johnson syndrome" OR "erythema multiforme" OR "dress syndrome") AND ("genetic test" OR "genetic testing" OR screening OR "patch test" OR "patch testing"))
In LILACS we searched using the above terms and the Controlled clinical trials topic‐specific query filter.
Appendix 7. Contacted authors
| Contacted authors | Study | Reason for the contact | Response |
| Simon Mallal | To ask for details on long term outcomes and patient exclusion | No response |

Flowchart of interventions (genetic testing) and outcomes (skin rash) in a patient population prescribed drug X
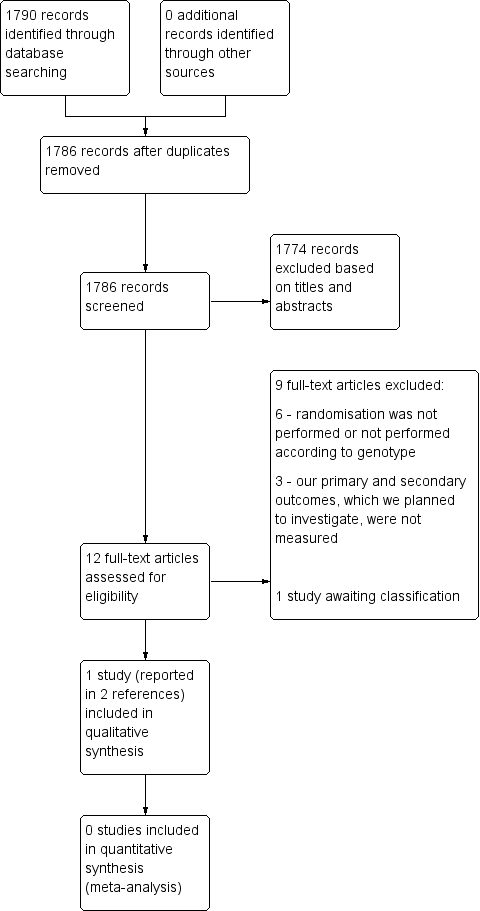
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
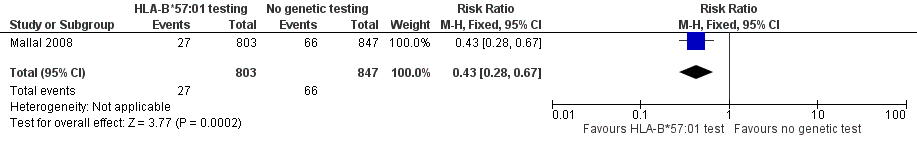
Forest plot of comparison: 1 Genetic testing vs no testing, outcome: 1.1 Hypersensitivity (HSS).

Forest plot of comparison: 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, outcome: 1.1 Hypersensitivity (HSS) immunologically confirmed.

Drug‐induced skin rash: top panel = maculopapular exanthema, bottom panel = Steven Johnson Syndrome (blistering skin rash with skin detachment)
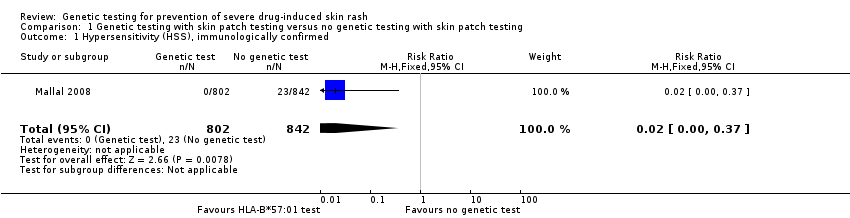
Comparison 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, Outcome 1 Hypersensitivity (HSS), immunologically confirmed.
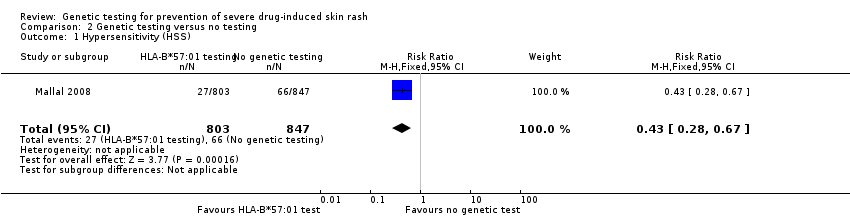
Comparison 2 Genetic testing versus no testing, Outcome 1 Hypersensitivity (HSS).
| Prospective genetic HLA‐B*57:01 screening compared with standard care for drug‐induced skin rash | ||||||
| Patient or population: patients with HIV‐1 infection and a pre‐established clinical need for treatment with an antiretroviral drug regimen containing abacavir but with an unknown HLA‐B*57:01 status. Settings: secondary care clinics Intervention: prospective genetic screening for the HLA‐B*57:01 allele Comparison: no prospective genotyping, standard‐of‐care treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard Carea | Prospective genetic HLA‐B*57:01 screening | |||||
| Severe skin drug‐induced rash | No data | ‐ | ‐ | ‐ | Not assessed | |
| Long‐term sequelae | No data | ‐ | ‐ | ‐ | Not assessed | |
| Hospitalisation for drug‐induced skin reaction | No data | ‐ | ‐ | ‐ | Not assessed | |
| SJS/TEN (Stevens‐Johnson syndrome/toxic epidermal necrolysis) | See hypersensitivity | ‐ | ‐ | ‐ | Not assessable | |
| AGEP (acute generalised exanthematous pustulosis) | No data | ‐ | ‐ | ‐ | Not assessed | |
| HSS (hypersensitivity) reaction including SJS/TEN (clinically diagnosed) (6 weeks clinical assessment) | 78 per 1000 | 34 per 1000 | RR 0.43 (0.28 to 0.67) | 1650 participants | ⊕⊕⊕⊝ | ‐ |
| HSS reaction including SJS/TEN (immunologically confirmed) (6 weeks clinical assessment) | 27 per 1000 | 0 per 1000 | RR 0.02 (0.00 to 0.37) | 1644 participants (1 study) | ⊕⊕⊕⊝ moderateb | ‐ |
| Death | No data | ‐ | ‐ | ‐ | Not assessed | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a The assumed risk is estimated from the event rate (confirmed clinically diagnosed occurrences of HSS including SJS/TEN or immunologically confirmed) in the control arm of the included study. b We downgraded the evidence to moderate quality, due to study limitations (high risk of detection and attrition bias). | ||||||
| Term | Explanation |
| Allele | One of two or more alternative forms of a gene at corresponding sites (loci) on homologous chromosomes |
| Antiretroviral | A class of drugs that inhibit the activity of retroviruses that cause HIV infection |
| Hardy‐Weinberg equilibrium | This states that allele and genotype frequencies in a population will remain constant from generation to generation in the absence of other evolutionary influences |
| HLA | Human leukocyte antigen: a group of protein molecules located on bone marrow and other cells that can provoke an immune response |
| Hypersensitivity | A state of altered reactivity in which the body reacts with an exaggerated immune response to a foreign substance, such as a drug |
| Immunologically confirmed | Patch testing is done to see whether a particular drug is causing allergic skin reaction. Patch test can detect delayed allergic or immunological reaction and confirm the diagnosis of hypersensitivity. |
| Phenotypes | The set of observable characteristics of an individual resulting from the interaction of its genotype with the environment |
| Polymorphic | A variation in the DNA that is too common to be due merely to new mutation. A polymorphism must have a frequency of at least 1% in a population |
| Maculopapular rash | A rash with both macules (flat and coloured like a freckle) and papules (a small raised spot) |
| Sequelae | A condition that is a consequence of a previous disease or injury |
| T‐cells | Another term for T‐lymphocyte, a type of cell that participates in immune response |
| Drugs associated with skin injury | Class of drug | HLA allele | Population | Reference |
| Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) | ||||
| Allopurinol | Antiuric acid | B*5801 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| ‐ | ‐ | ‐ | Japanese | |
| ‐ | ‐ | ‐ | Malay | |
| Carbamazepine | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | Kulkantrakorn 2012; Locharernkul 2008; Tassaneeyakul 2010; Tangamornsuksan 2013 |
| ‐ | ‐ | ‐ | Malay | |
| ‐ | ‐ | ‐ | Indian | |
| ‐ | ‐ | A*3101 | White | |
| ‐ | ‐ | A*3101 | Japanese | |
| Phenytoin | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| Oxicam | Non‐steroidal anti‐inflammatory drug (NSAID) | A2, B12 | White | |
| Sulphamethoxazole | Antibiotic | A29, B12, DR7 | White | |
| Hypersensitivity syndrome (drug‐induced hypersensitivity syndrome (DIHS) or drug reaction with eosinophilia and systemic symptoms (DRESS)) | ||||
| Abacavir | Antiretroviral | B*57:01 | White | Hetherington 2002; Hughes 2004a; Mallal 2002; Mallal 2008; Martin 2004 |
| ‐ | ‐ | ‐ | African American | |
| Aminopenicillins | Antibiotic | A2, Drw52 | White | |
| Nevirapine | Antiretroviral | DRB1*01 | White ‐ Australian | |
| ‐ | ‐ | DRB1*01 | White ‐ French | |
| ‐ | ‐ | Cw8, B14 | White ‐ Italian | |
| ‐ | ‐ | Cw8 | Japanese | |
| ‐ | ‐ | B*3505 | Thai | |
| ‐ | ‐ | Cw4 | Thai | |
| ‐ | ‐ | C*0404 | Black African | |
| ‐ | ‐ | Cw*04 | Chinese | |
| Aspirin | NSAIDs | DRB1*1302, DQB1*0609 | ‐ | |
| ‐ | NSAIDs | DR11 | ‐ | |
| Iodine contrast media | ‐ | DR | White ‐ Spanish | |
| Paraphenylenediamine | Hair dye | DP | White ‐ German | |
| Gold sodium thiomalate | Treatment of rheumatoid arthritis | DR5 | White ‐ Spanish | |
| Lamotrigine | Antiepileptic | B*5801, A*6801 | White | |
| Trichloroethylene | Industrial solvent, dry cleaning | B*1301 | Japanese | |
| Fixed drug eruptions | ||||
| Co‐trimoxazole | Antibiotic | A30, B13, Cw6 | White ‐ Turkish | |
| Feprazone | Analgesic | B22 | ‐ | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypersensitivity (HSS), immunologically confirmed Show forest plot | 1 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypersensitivity (HSS) Show forest plot | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.67] |

