Evaluación del riesgo para talasemia, anemia de células falciformes, fibrosis quística y enfermedad de Tay‐Sachs antes de la concepción
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010849.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Writing the protocol: all authors
Developing the search strategy: NH, NQ, JK and other (not review authors)
Searching for trials: NH and NQ
Selection of trials: NH, NQ, SW and other (not review authors)
Data entry: NH, SW
Analysis: NH, NQ, SW and JK
Interpret analysis: all authors
Draft final review: all authors
Update the review: NH and NQ
Sources of support
Internal sources
-
University of Nottingham, UK.
The university provided computer and internet access.
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Nadeem Qureshi and Dr Joe Kai are investigators on a UK National Institute of Health research project evaluating preconception screening in primary care and plan to pursue further research in this area. Dr Qureshi is also collaborating on a project to evaluate the evidence base relevant to NICE guidelines behind primary care.
Professor Jos Kleijnen declares Kleijnen Systematic Reviews Ltd has received project funding from various pharmaceutical companies for work in unrelated areas.
For the remaining authors there are no known declarations of interest.
Acknowledgements
The authors would like to Luke Robles and Richard Birnie for their help in screening and identifying full‐text articles for this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Oct 11 | Preconception risk assessment for thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease | Review | Norita Hussein, Lidewij Henneman, Joe Kai, Nadeem Qureshi | |
| 2018 Mar 14 | Preconception risk assessment for thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease | Review | Norita Hussein, Stephen F Weng, Joe Kai, Jos Kleijnen, Nadeem Qureshi | |
| 2015 Aug 12 | Preconception risk assessment for thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease | Review | Norita Hussein, Stephen F Weng, Joe Kai, Jos Kleijnen, Nadeem Qureshi | |
| 2013 Dec 23 | Preconception risk assessment for thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease | Protocol | Norita Hussein, Nadeem Qureshi, Stephen F Weng, Jos Kleijnen, Joe Kai | |
Differences between protocol and review
Hand searching of key journals was originally specified from 1984 to date in the protocol. In the review, hand searching was conducted from 1998 to date for the European Journal of Human Genetics and Genetics in Medicine and from 2010 to date for the Journal of Community Genetics. This change was made because these were the earliest dates that the online table of contents were accessible for these key journals.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans;
PICO
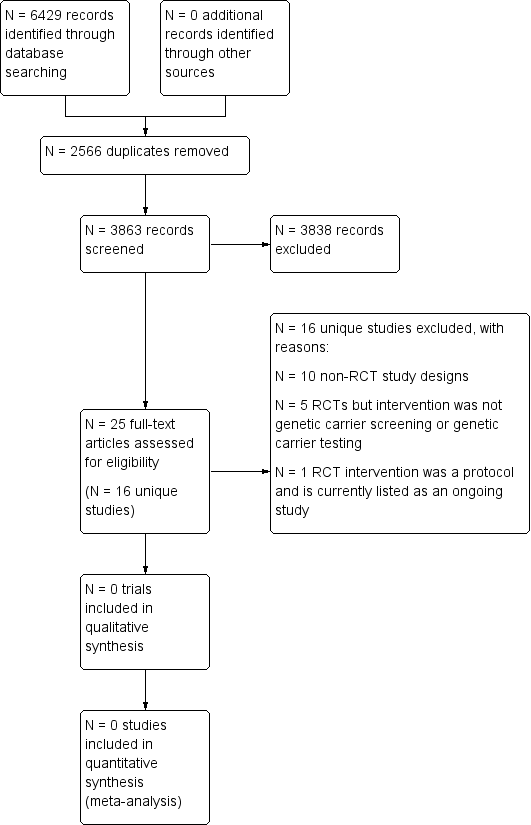
Study flow diagram

