Evaluación del riesgo para talasemia, anemia de células falciformes, fibrosis quística y enfermedad de Tay‐Sachs antes de la concepción
Resumen
Antecedentes
A nivel global, cerca del 5% de los niños nacen con trastornos congénitos o genéticos. Las afecciones autosómicas recesivas más frecuentes son la talasemia, la anemia de células falciformes, la fibrosis quística y la enfermedad de Tay‐Sachs, con mayores tasas de portadores en poblaciones específicas de pacientes. La identificación y el asesoramiento de las parejas con riesgo genético de afecciones antes del embarazo les permite tomar decisiones reproductivas completamente fundamentadas, y algunas de estas opciones no están disponibles si el consejo genético solamente se ofrece en un contexto prenatal. Esta es una actualización de una revisión publicada anteriormente.
Objetivos
Evaluar la efectividad de la evaluación preconceptiva sistemática del riesgo genético para mejorar los resultados reproductivos en las mujeres y sus parejas identificados como portadores para talasemia, anemia de células falciformes, fibrosis quística y enfermedad de Tay‐Sachs en contextos de asistencia sanitaria en comparación con atención habitual.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Fibrosis Quística y Enfermedades Genéticas (Cochrane Cystic Fibrosis and Genetic Disorders Group). Además, se buscaron todos los ensayos relevantes desde 1970 (o desde la primera fecha en la que la base de datos estuvo disponible después de 1970) hasta la actualidad, mediante bases de datos electrónicas (MEDLINE, Embase, CINAHL, PsycINFO), bases de datos de ensayos clínicos (National Institutes of Health, Clinical Trials Search portal of the World Health Organization, metaRegister of controlled clinical trials), y búsquedas manuales en revistas clave y libros de resúmenes de congresos desde 1998 hasta la actualidad (European Journal of Human Genetics, Genetics in Medicine, Journal of Community Genetics). También se buscó en las listas de referencias de artículos relevantes, revisiones y guías y también se contactó con expertos en el tema para solicitar cualquier ensayo no publicado u otros ensayos publicados.
Fecha de la última búsqueda de los registros: 20 de junio de 2017.
Fecha de la última búsqueda en todas las otras fuentes: 16 de noviembre de 2017.
Criterios de selección
Cualquier ensayo controlado aleatorizado o cuasialeatorizado (publicado o no publicado) que comparara resultados reproductivos de la evaluación preconceptiva sistemática del riesgo genético para talasemia, anemia de células falciformes, fibrosis quística y enfermedad de Tay‐Sachs en comparación con atención habitual.
Obtención y análisis de los datos
Se identificaron 25 trabajos, que describen 16 ensayos únicos que eran potencialmente elegibles para su inclusión en la revisión. Sin embargo, después de la evaluación, no se encontraron ensayos controlados aleatorizados de evaluación preconceptiva del riesgo genético para talasemia, anemia de células falciformes, fibrosis quística y enfermedad de Tay‐Sachs.
Resultados principales
No se encontraron ensayos controlados aleatorizados de evaluación preconceptiva del riesgo genético para talasemia, anemia de células falciformes, fibrosis quística y enfermedad de Tay‐Sachs. Se ha identificado un ensayo en curso que podría ser elegible para su inclusión una vez terminado.
Conclusiones de los autores
Como no se encontraron ensayos controlados aleatorizados de evaluación preconceptiva del riesgo genético para talasemia, anemia de células falciformes, fibrosis quística, o enfermedad de Tay‐Sachs para inclusión en esta revisión, la evidencia de los estudios de investigación para las recomendaciones de las políticas actuales está limitada a los estudios no aleatorizados.
Es conveniente obtener información de ensayos aleatorizados bien diseñados, con poder estadístico suficiente, para establecer recomendaciones más consistentes para la práctica. Sin embargo, dichos ensayos también deben considerar las barreras legales, éticas y culturales para la realización de la evaluación preconceptiva del riesgo genético.
PICOs
Resumen en términos sencillos
Identificación del estado de portador para talasemia, anemia de células falciformes, fibrosis quística, o enfermedad de Tay‐Sachs en mujeres no embarazadas y sus parejas
Pregunta de la revisión
Se buscó evidencia que mostrara si la identificación de las personas que son portadores para talasemia, anemia de células falciformes, fibrosis quística, o enfermedad de Tay‐Sachs, antes del embarazo mejora las opciones reproductivas y los resultados del embarazo.
Antecedentes
En todo el mundo, cerca del 5% de los niños nacen con trastornos genéticos. Estos trastornos pueden pasar de los padres al niño. Hay pruebas para identificar el riesgo de los trastornos genéticos más frecuentes (talasemia, anemia de células falciformes, fibrosis quística, o enfermedad de Tay‐Sachs) antes del embarazo. En estos trastornos, llamadas afecciones autosómicas recesivas, los padres de los niños afectados son "portadores" de la afección, lo que significa que generalmente ellos no presentan síntomas. Todas las parejas "portadoras" tendrán una probabilidad del 25% de tener un niño afectado. La evaluación del riesgo de estos trastornos genéticos antes del embarazo beneficiaría a los padres potenciales que pueden ser portadores. Esta información le daría a la pareja en riesgo la oportunidad de tomar decisiones completamente fundamentadas acerca de la planificación familiar. Sin embargo, la evaluación del riesgo genético antes del embarazo potencialmente puede tener una repercusión psicológica negativa. Esta es una versión actualizada de la revisión original.
Fecha de la búsqueda
La última búsqueda de evidencia fue el 16 de noviembre de 2017.
Características de los estudios
No se encontró ningún ensayo que se pudiera incluir en esta revisión, pero hay un ensayo en curso que tal vez se pueda incluir una vez que se haya completado.
Resultados clave
Aunque no se identificaron ensayos en los que los voluntarios tendrían iguales probabilidades de estar en cualquier grupo, hay varios estudios que no se diseñaron de una manera tan estricta que apoyan las recomendaciones de políticas actuales de la evaluación del riesgo genético antes del embarazo en la práctica clínica habitual. Cualquier ensayo futuro debe considerar las barreras legales, éticas y culturales para la implementación.
Authors' conclusions
Background
A glossary of terms is available as an appendix (Appendix 1).
Description of the condition
Genetic medicine is expanding into almost every aspect of health care; reproductive risk assessment during the preconception period is a prime example. Identifying genetic risks before pregnancy or conception might produce significant benefits, such as providing information about the risk of having children with genetic conditions and thus giving couples or prospective parents the opportunity to make more informed reproductive decisions. It has been estimated that a couple has a baseline risk of 2% to 3% of having a child with a congenital or genetic disorder (Teeuw 2010). The probability of having an affected child increases when there is a family history of genetic disorders (Shapira 2006; Teeuw 2010). Globally, about 5% of children are born with congenital or genetic disorders (WHO 1999).
Preconception risk assessment for autosomal recessive genetic disorders would benefit couples who may be carriers. The most common examples of these autosomal recessive disorders are thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease. In these disorders, such carriers are usually asymptomatic; however, their child will be affected if he or she inherits the affected genes from both parents. All carrier couples have a 25% chance of having an affected child. These conditions have a high morbidity risk, are potentially life‐threatening and have a significant psychological impact not only on the affected child, but also on their families or care givers. These diseases are also more prevalent in individuals of particular ethnic backgrounds (WHO 2000).
The need for medical care, as well as psychological interventions to offer behavioural and emotional support, imposes a potentially high economic and public health burden. In view of the magnitude of these conditions and their implications, there have been considerable efforts to identify the genetic reproductive risk for the four specified conditions and offer support to potential parents before the birth of an affected child. Women and couples at increased genetic risk, as well as healthcare professionals, have recognised the importance of preconception assessment (Boulton 1996; Henneman 2001; Locock 2008; Mennie 1998; Poppelaars 2004; Watson 1999; Wille 2004). To date, the practical experience of reproductive genetic risk assessment for autosomal recessive disorders focuses mainly on the antenatal and newborn period (Qureshi 2004). Identifying genetic reproductive risk during the antenatal period leaves the couple a short period of time to make difficult or limited choices, such as terminating the pregnancy or continuing with the pregnancy and caring for the affected child (Dormandy 2008). Identifying couples who have confirmed genetic carrier status before conception provides an opportunity for individuals or couples to make fully informed reproductive choices including avoiding pregnancy, pre‐implantation diagnosis and in vitro fertilization, arranging early prenatal diagnosis and antenatal care and also considering adoption of a child (Jones 2002; Wille 2004).
Thalassaemia
According to the WHO, every year 300,000 infants are born with major haemoglobin disorders, the most common being thalassaemia and sickle cell disease (WHO 1999). Thalassaemia is characterised by the defects or absence of synthesis in one of the two globin chains (α or β) which form the normal adult human haemoglobin molecule; this leads to haemolytic anaemia (Peters 2012). Thalassaemia can be diagnosed by measuring fractions of haemoglobin A and haemoglobin F with high performance liquid chromatography (HPLC) or electrophoresis. In addition, DNA analysis is required to detect an α or β globin chain mutation (Peters 2012). It is estimated that between two and five per cent of the world's population are carriers and this is more prevalent in the Mediterranean and Southern Asian populations (Modell 2001). Morbidity is related to severe anaemia and an affected child will require lifelong blood transfusions. Multiple blood transfusions may eventually result in iron overload and potentially cause heart failure, infection, hypogonadism, infertility, diabetes mellitus, and hypothyroidism. Affected individuals may die prematurely, unless given optimal medical management. In individuals with thalassaemia and their families or care givers, psychosocial problems have also been reported, for example stigmatisation, isolation, family adjustment, coping with school and education, and social interaction (Gharaibeh 2009; Ratip 1996; Telfer 2005).
Sickle cell disease
Sickle cell disease is caused by a mutation in the haemoglobin gene (βS) which individuals inherit from both parents (Weatherall 1997). The WHO estimates that sickle cell disease affects 275,000 conceptions each year globally (Modell 2008; Yusuf 2011). Diagnosis is confirmed using HPLC or electrophoresis with the detection of haemoglobin S and C fraction. It affects mainly individuals of African origin, but is also found in Indian and some Mediterranean populations. The reported prevalence of carrier frequency ranges from one to 40 per cent, depending on the population group. The condition causes the red blood cells to have a sickle shape which results in premature haemolysis, and can lead to life‐threatening acute and chronic vaso‐occlusion, including renal and cardiovascular complications. Individuals with this condition are also susceptible to serious septicaemia. Like thalassaemia, individuals and their families are also confronted with psychosocial challenges which include the disruption of school and work, social isolation and loneliness, stigmatisation, bullying, and rejection by peers (Barbarin 1999).
Cystic fibrosis
Cystic fibrosis is caused by a mutation in the gene cystic fibrosis transmembrane conductance regulator (CFTR); more than 1500 CFTR mutations have now been identified. Diagnosis of cystic fibrosis is indicated by phenotypic features (chronic sino‐pulmonary disease, gastrointestinal and nutritional abnormalities, obstructive azoospermia and salt‐loss syndromes), a family history of cystic fibrosis or a positive newborn screening test, together with laboratory evidence of a CFTR abnormality. Abnormalities in the CFTR can be identified by elevated sweat chloride concentrations (sweat test), identification of two CFTR mutations, or in vivo demonstration of characteristic abnormalities in ion transport across the nasal epithelium. Carriers are confirmed by identification of a CFTR mutation from the blood or saliva (CDC 2004).
Cystic fibrosis is most common among people of European descent with a carrier frequency of 1 in 25 (Murray 1999). This condition is commonly associated with recurrent pulmonary infections, which potentially lead to bronchiectasis and atelectasis, and also pancreatic exocrine insufficiency. There is currently no cure for the disease, with treatment mainly aimed at improving a person's quality of life. The need for emotional and social adjustment is a significant psychosocial consequence for people with cystic fibrosis (Bregnballe 2007; Glasscoe 2008). In addition, poor adherence to treatment has also been reported due to the burden of treatment and the long‐term management of the condition (Abbot 1996).
Tay‐Sachs disease
Tay‐Sachs disease is caused by a genetic mutation in the α chains of the hexosaminidase A (Hex A) isozyme in the gangliosides in nerve cells of the brain (Bach 2001). The disease is diagnosed by measuring the activity of hexosaminidase A and further identification of a genetic mutation in Hex A (ACOG Committee Opinion 2005). It is most prevalent in the Ashkenazi Jewish and French Canadian populations, with a carrier frequency of around 1 in 30 (Petersen 1983; Palomaki 1995). The condition leads to a progressive deterioration of mental and physical abilities. Death usually occurs before five years of age. At present, there is no cure or available treatment.
Description of the intervention
Women and their partners can be assessed during the preconception period to identify if they are carriers of one of these four autosomal recessive conditions. These four conditions represent the most common autosomal recessive conditions globally. Cystic fibrosis is most common in Northern European populations; sickle cell disease and thalassaemia are most common non‐Northern European populations, and Tay‐Sachs disease is most common in individuals of Ashkenazi Jewish and French Canadian ancestry. Approaches to improve health outcomes and reproductive choice in couples who carry these genetic conditions should be generalizable to other, but rarer, autosomal recessive conditions. In populations with high carrier rates or significant burden of affected individuals, or both, carrier screening may be offered during preconception to all women in some healthcare settings (PFASP England 2013). More commonly, women and their partners may be assessed on the need for carrier testing. This assessment would be based firstly on a review of the family history for any of the autosomal recessive conditions or their carrier status; and, secondly, on the ethnic origin of the woman and her partner (Dyson 2006). This assessment of ancestry will identify if the individual originates from an ethnic group with a greater probability of being a healthy carrier of any of the four autosomal recessive disorders; for example those with Ashkenazi Jewish ancestry are more likely to carry Tay‐Sachs disease, whilst those of African descent may carry sickle cell trait. The benefits of recording family history as one of the components of preconception health checks have been reported in previous observational community‐based studies for a broad range of genetic conditions in both the United Kingdom (Rose 1999) and Hungary (Czeizel 2012).
Overall, previous interventions have involved genetic carrier testing or screening (with or without educational support and genetic counselling) or both. There is often confusion between the terms genetic carrier testing and screening (Nuffield Council on Bioethics 1993). Genetic carrier testing refers to the testing of individuals to determine the presence or absence of the carrier status (Human Genetics Commission 2011). This testing could, for example, be in the context of a family history of the autosomal recessive condition or relevant ethnicity. On the other hand, genetic carrier screening involves offering or testing the whole population group irrespective of individual risk (Castellani 2010; Human Genetics Commission 2011). Both genetic carrier testing and screening involves the analysis of blood, tissue or bodily fluid samples.
With regards to the actual genetic carrier tests, currently either HPLC or electrophoresis is used to detect haemoglobin variants and to confirm carrier status for thalassaemia and sickle cell disease (NHS Screening Programme 2013). Carrier status for cystic fibrosis is confirmed by analysing the mutations in the gene CFTR, using DNA commonly obtained from white blood cells, mouthwashes and buccal swabs (Murray 1999). Confirmation of Tay‐Sachs disease carrier status comprises of molecular analysis to detect genetic mutations in the α chains of the hexosaminidase A (Hex A) isozyme (ACOG Committee Opinion 2005). To improve detection rate, this should be combined with biochemical tests (ACOG Committee Opinion 2005).
For each condition, as well as confirmed carrier status identified by genetic carrier tests, there are other laboratory investigations that could indicate a probable carrier state. A microcytic or hypochromic blood picture, or both, without anaemia suggests a probable thalassaemia carrier, whilst a probable sickle cell carrier is indicated by a positive sickle solubility test. Elevated sodium chloride levels in sweat can indicate a probable cystic fibrosis carrier state.
At present, there is no formal or standard recommendation that fully addresses preconception genetic risk assessment (NHS Screening Programme 2013). There is variability in how preconception genetic risk assessment is offered across countries. For example, in Iran, screening for haemoglobinopathies is offered in pre‐marital clinics (Samavat 2004), whereas in the United Kingdom, screening for any reproductive genetic disorder may be offered opportunistically in a range of settings such as family planning clinics (Watson 1999). Similarly, in current clinical practice, preconception risk assessment is not offered systematically, but most commonly offered opportunistically, for example when the issue is brought up by the couple (Heyes 2004).
How the intervention might work
In the specified autosomal recessive disorders (thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease), preconception genetic risk assessment ensures at‐risk couples, in which both the women and her partner are carriers of the specified conditions, are aware that they have a one in four chance of an affected child prior to pregnancy, enabling them to make fully informed reproductive choices (Christie 2009; Czeizel 2012; Lena‐Russo 2002; Massie 2009; Mitchell 1996). This offers the at‐risk couples the opportunity to consider the full range of reproductive options (Borry 2011); for instance, couples may choose to have in vitro fertilisation (IVF) with pre‐implantation genetic diagnosis, use donor gametes, adopt a child or remain childless (Human Genetics Commission 2011; Jones 2002; Wille 2004). These options are not available to couples who are only made aware of their reproductive genetic risk during the pregnancy. Of equal importance, if couples who have already been informed of their risk, decide to carry on with pregnancy they may consider and be offered prenatal diagnosis earlier in pregnancy. This enables the option of termination in early gestation, or can enhance preparation for foetal and maternal well‐being throughout pregnancy, preparation following the birth of an infant, and postnatal support. (Wille 2004). If the carrier testing is implemented in the antenatal period, all of these decisions are delayed (Qureshi 2004). With regards to family history assessment, participants have acknowledged that this intervention enables pregnancy planning (Rose 1999) and early identification of couples at reproductive genetic risk (Czeizel 2012).
At a societal level, preconception carrier state identification has reduced the rate of affected births (Angastiniotis 1998; Samavat 2004). Although, it is estimated that preconception screening programmes worldwide have caused a small decrease in affected births for haemoglobin disorders from 2.7 per 1000 conceptions to 2.55 per 1000 conceptions over a five‐year period from 1998 to 2003, more data and across all common autosomal recessive conditions need to be explored (Modell 2008). Similarly, early observational studies involving genetic carrier screening programmes for Tay‐Sachs disease and thalassaemia in Canada and France carried out in high school students were associated with increased rate of early prenatal diagnosis and termination of affected pregnancies (Lena‐Russo 2002; Mitchell 1996; Zeesman 1984). In Cyprus and Iran, the incidence of thalassaemia has fallen with the introduction of mandatory pre‐marital genetic carrier screening programmes (Alswaidi 2009; Angastiniotis 1998; Samavat 2004). In Hungary, preconception screening has resulted in improved identification of carrier couples and access to genetic counselling services (Czeizel 2012). However, such observational studies are subject to bias.
Why it is important to do this review
While a number of observational studies have reported the potential benefits of preconception risk assessment for genetic conditions in general (Czeizel 2012), and specifically for cystic fibrosis (Christie 2009; Massie 2009), haemoglobinopathies (Cao 1996) and Tay‐Sachs disease (Mitchell 1996), as with other programmes for genetic testing or screening, this has potential adverse effects. Genetic assessment for reproductive risk has been linked to psychological distress such as anxiety; however, the raised anxiety was a transient phenomenon (Archibald 2011; Bekker 1994). Further, it has been reported that carrier status may be associated with a poor perception of health (Henneman 2001) and may have an impact on the carrier's relationships with their partner (Fanos 1995). Social impacts such as stigmatisation and discrimination have been reported with mandatory carrier screening (Bonham 2010; Kenen 1978; Whitten 1973). Despite these reported adverse effects, there are numerous psychological benefits including the opportunity for informed decision‐making and reproductive autonomy in prospective parents (Anido 2005; Archibald 2011; Lewis 2011).
With regards to the economic implications, as for other programmes for genetic testing and screening, there is an opportunity cost for redistributing resources from medical care to preconception risk assessment (WHO 1968). Several economic appraisals of haemoglobinopathies screening in the antenatal and neonatal settings have indicated that these strategies are cost‐effective (Davies 2000; Zeuner 1999). A recent review of existing screening programmes in Australia has shown that targeted preconception screening in certain ethnic groups demonstrates both clinical and cost‐effectiveness (Lew 2014).
At a policy level, preconception genetic risk assessment has been recommended in clinical practice in the Netherlands, the United States of America and the United Kingdom (ACOG Committee Opinion 2009; Health Council of Netherlands 2007; Human Genetics Commission 2011). However, a comprehensive review of the current evidence still needs to be undertaken to directly inform healthcare practice.
This review will explore if robust trial evidence exists on the effect of preconception genetic risk assessment for genetic disorders, particularly before its widespread routine implementation in current healthcare settings. This is an update of a previously published review (Hussein 2015).
Objectives
The purpose of this review is to assess the effectiveness of systematic preconception genetic risk assessment to improve reproductive outcomes in women and their partners who are identified as carriers of thalassaemia, sickle cell disease, cystic fibrosis or Tay‐Sachs disease in healthcare settings, when compared to usual care.
Methods
Criteria for considering studies for this review
Types of studies
We planned for this review to include all relevant randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Women and their partners of reproductive age (aged 16 to 50 years old) who are carriers for thalassaemia, sickle cell disease, cystic fibrosis or Tay‐Sachs disease, accessing any healthcare services which include hospitals and community‐based healthcare settings. Community‐based healthcare settings include family or general practices, community health centres, community health services, community or outpatient clinics and ambulatory care services. Settings outside of healthcare do not directly inform healthcare practice, and thus will be excluded as being outside the scope of this review. If trials contain both eligible and ineligible participants, they will be included if data on eligible participants can be extracted.
Types of interventions
We planned to assess the effects of systematic preconception genetic risk assessment for thalassaemia, sickle cell disease, cystic fibrosis or Tay‐Sachs disease, in any healthcare setting. We define systematic preconception genetic risk assessment as a package of risk assessment including one or more of these components:
-
family history assessment;
-
assessment of ethnicity background;
-
genetic carrier testing;
-
genetic carrier screening.
Risk assessment can be offered at anytime prior to conception.
We planned to compare systematic preconception genetic risk assessment with standard care. We define standard care as where people receive usual or alternative care in any healthcare setting, that does not involve a specific systematic approach to preconception genetic risk assessment.
Types of outcome measures
The listed outcomes do not form part of the eligibility criteria for the included trials.
Primary outcomes
-
Reproductive outcomes in women and their partners who are carriers of thalassaemia, sickle cell disease, cystic fibrosis or Tay‐Sachs disease identified during or after pregnancy
-
number of infants born with genetic conditions
-
number of infants born with congenital anomalies
-
number of infants born with low birth weight
-
number of infants born prematurely
-
-
Decisions about future conception and pregnancy in women and their partners who are carriers for thalassaemia, sickle cell disease, cystic fibrosis or Tay‐Sachs disease
-
number of women or couples who would make use of prenatal diagnosis
-
number of women or couples who would make use of prenatal diagnosis and consider termination of pregnancy if the child is affected
-
number of women or couples who would consider pre‐implantation genetic diagnosis and in vitro fertilization
-
number of women or couples who would conceive using donated gametes
-
number of women or couples who would consider adoption
-
number of women or couples who would refrain from having any children
-
Secondary outcomes
-
During pregnancy following intervention
-
gestational date of prenatal diagnosis in at‐risk women
-
gestational date of prenatal counselling in at‐risk women or couples
-
-
Self‐reported measures (short‐term change from baseline)
-
any objective measures of health‐related quality of life resulting from preconception genetic risk assessment, using validated tools such as Short Form Health Survey 36 (SF36) and Health Questionnaire EQ‐5D
-
any objective measures of quantifying psychological or social outcomes or both resulting from preconception genetic risk assessment using validated tools such as Spielberger State‐Trait Anxiety Inventory (STAI), Perceived Stress Questionnaire (PSQ)
-
knowledge (any measures of the women's or couples' or both, knowledge of reproductive genetic risk associated with carrier status for thalassaemia, sickle cell disease, cystic fibrosis or Tay‐Sachs disease using validated self‐reported questionnaire)
-
satisfaction (any measures of the women's or couples' or both, satisfaction with the intervention using validated self‐reported questionnaire)
-
-
Cost of the intervention (including follow‐up visits and tests)
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status. If we identify potentially eligible non‐English language trials in future searches, we will source a person who can read the language in order to assess these trials for possible inclusion and data extraction.
Electronic searches
We sought trials from the relevant Cystic Fibrosis and Genetic Disorders Group's Trials Registers using the terms: ((carrier* OR trait OR risk assessment OR Tay‐Sachs):kw) AND ((cystic fibrosis OR haemoglobinopathies OR Tay‐Sachs):kw). For full details of all searching activities for the registers, please see the relevant section of the Cystic Fibrosis and Genetic Disorders Group's website.
Date of latest search: 20 June 2017.
In addition, we searched all relevant trials from the following databases:
-
Ovid MEDLINE (1970 until 16 November 2017);
-
Ovid Embase ((1974 until 16 November 2017);
-
CINAHL (1970 until 16 November 2017);
-
Ovid PsycINFO (1970 until 16 November 2017).
The search strategies are available in the appendices (Appendix 2). Start dates for database searches are set to when carrier screening or testing was first available. Based on WHO reports, earliest carrier status assessment was introduced for Tay‐Sachs disease and haemoglobinopathies from the early 1970s (Angastiniotis 1995; Kaback 2000). We searched for relevant trials in the databases from 1970 or from when the date of the database was first available if after 1970.
We searched the following clinical trial databases for ongoing and unpublished trials:
Searching other resources
We planned to examine the reference lists of eligible published trials to identify further relevant trials. We hand searched the key journals European Journal of Human Genetics, Genetics in Medicine and the Journal of Community Genetics from 1998 to November 2017. We complemented the search by contacting subject experts or centres in the field to request any unpublished or other published trials that we may not have identified.
Data collection and analysis
Selection of studies
We saved the results of the searches in the Endnote reference managing software (EndNote X3). Two review authors (one content expert and one methodologist) independently screened the citations and article abstracts of every retrieved record. We would have resolved any disagreements on eligibility by discussion and if doubt remained, we would have acquired the relevant full article(s) for further inspection. Two review authors independently screened all full text articles of the eligible trials. We aimed to resolve any disagreement by discussion. If required, we would have consulted a third review author. If necessary, we planned to contact the authors of the articles for further information and clarification of trials. We have reported reasons for excluding trials and provided a PRISMA flowchart (Figure 1).
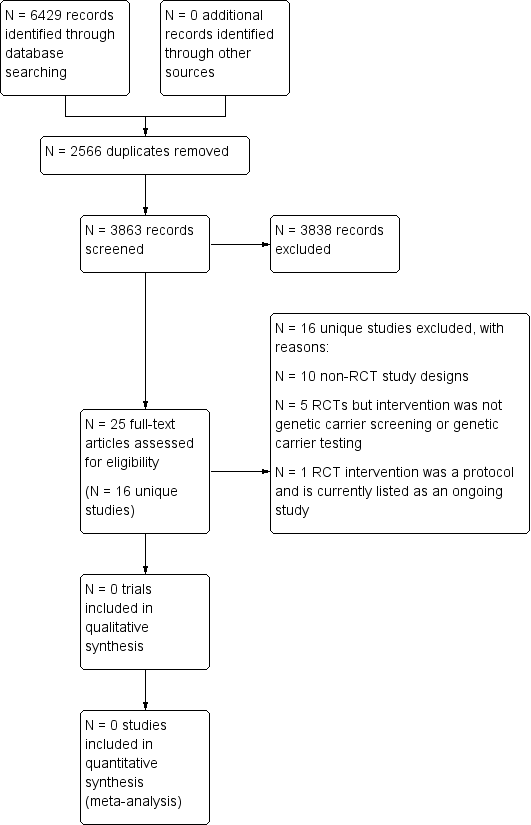
Study flow diagram
We did not identify any trials for inclusion in this version of the review. However, if we identify any trials for future updates of the review, we plan to undertake the following.
Data extraction and management
Two review authors will independently extract data from each included trial using an agreed data extraction form. We will collect data on trial population characteristics (including sample size, participants' ethnic or cultural characteristics, geographic locations), intervention characteristics (including process and duration of intervention) and primary and secondary outcome measures of interest. We plan to report short‐term outcomes post intervention up to six months. We plan to report long‐term outcomes post intervention from six months up to 12 months, and then annually thereafter.
We will settle any disagreements about the data extracted through discussion by the two review authors, and if necessary by arbitration with a third author. We will enter all the data into the Review Manager software (RevMan 2014).
Assessment of risk of bias in included studies
We will construct a risk of bias table for each trial as outlined in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Two review authors will independently assess and record the following six domains in the risk of bias table:
-
random sequence generation;
-
allocation sequence concealment;
-
blinding of participants, trial personnel, outcome assessors;
-
incomplete outcome data;
-
selective outcome reporting;
-
other sources of bias.
We will judge the methods used in the trials for each domain as having either a low, high or unclear risk of bias. Two review authors will aim to resolve any disagreements in the judgement of the domains through discussion. If no agreement can be reached, then they will consult a third author and aim to resolve the disagreement by consensus.
We will record the information in the 'Risk of bias' tables in Review Manager (RevMan 2014). We aim to resolve any disagreement by consensus or arbitration by a third author. We will use the results of the risk of bias assessment to provide an evaluation of the overall risk of bias of the included trials based on the approach outlined in the chapter 8 (Table 8.7a) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We will extract all the main results of the included trials as mentioned below. We will contact relevant authors of the original reports for data or any missing relevant information or when clarification is needed. We will settle any disagreements about the data extracted through discussion and if necessary by arbitration by a third author. We will enter all the data into the Review Manager software (RevMan 2014).
Continuous data
For scale‐derived data, we will include continuous data from rating scales only if the measuring instrument has been validated. We will include endpoint data and only use change data if the former are not available. For continuous outcomes we will record mean, standard deviation (SD) and number of participants for each group and report effect size using the mean difference (MD) for the same units of measurement or the standardised mean difference (SMD) when different scales are used to evaluate the same outcome, with 95% confidence intervals (CI). The MD measures the absolute difference between the means in two groups, whereas the SMD is the MD relative to variability observed in that trial.
Dichotomous data
We will report dichotomous data using the risk ratio (RR) and the corresponding 95% CIs.
Unit of analysis issues
We anticipate cluster‐randomised designs to be used in the included trials; for example, groups of patients of a single doctor or practice. If available, we will extract the direct estimate of the effect (RR with CI) that accounts for a cluster design. We will contact the primary authors of the included trials to obtain the intra‐cluster correlation coefficient (ICC) which will describe the relative variability within and between clusters, to adjust for clustering effect (Donner 1980). We will meta‐analyse the appropriate analyses of cluster randomised trials using the generic inverse variance method. Alternatively, we will estimate an ICC to describe the relative variability within and between clusters (Donner 1980). An ICC usually derives from the trial or from other sources (ICC from a similar trial in an existing database) (Ukoumunne 1999). If the ICC is derived from other sources, we will report this and conduct a sensitivity analysis. If the trials were analysed as if the randomisation was performed on the individuals rather than the clusters, we will re‐calculate the correct analysis if we are able to extract the following information: the number of clusters randomised to each intervention group; the mean size of each cluster; and the outcome data ignoring the cluster design for the total number of individuals.
If we identify more than one intervention group of interest in a trial, we will analyse the effect of the additional intervention group using pair‐wise comparisons. If the additional intervention group is irrelevant, we will not reproduce the data.
Dealing with missing data
Whenever possible, we will contact the original investigators and the authors of the included trials to request any missing data. If this is unsuccessful we will deal with missing data as mentioned below.
Overall loss of credibility
We will choose that, if for any particular outcome there is a high risk bias for missing data according to the risk of bias assessment, we will not use these data in the analyses and will present the results in the form of a narrative synthesis.
Continuous data
If SDs are not reported or available, we will first look for statistics that allow the calculation of the SD (for example, the CI and the standard error (SE) of group means, as well as P values and T values for the differences in means). If this is not possible, we will consider imputing SDs of other included trials. We will examine the consequences of imputations in a sensitivity analysis.
Assessment of heterogeneity
Clinical heterogeneity
We will consider clinical heterogeneity which can result from differences between trials in characteristics of the populations, interventions and outcomes. We will fully discuss the influence of clinical heterogeneity on the observed effects.
Methodological heterogeneity
We will assess for methodological heterogeneity, which can result from differences in characteristics of the trial designs. We will fully discuss the influence of methodological heterogeneity on the observed effects.
Statistical heterogeneity
We plan to examine graphs or summary tables of the trials to investigate the possibility of statistical heterogeneity. We plan to consider the I2 statistic which estimates the proportion of variability in effect estimates that is due to heterogeneity (Higgins 2002). We will determine the level of heterogeneity by the following reference ranges: low 0% to 40%; moderate 41% to 75%; and high 76% to 100%. We also plan to use the Chi2 statistic and if the P value is less than 0.10 it will be considered an indication of heterogeneity. If there is a high level of heterogeneity between trials, it may not be appropriate to conduct a meta‐analysis, thus we will present results in a qualitative analysis. These trials will be entered into RevMan and presented on a forest plot with their individual effect sizes, but with no combined effect to give an overall picture of evidence (RevMan 2014).
Assessment of reporting biases
If the review includes more than 10 trials, we will create a funnel plot to investigate the possibility of small trial effects (a tendency for the intervention effects estimated in smaller trials to differ from those estimated in larger trials) (Sterne 2011).
Data synthesis
We will summarise all trials using narrative synthesis methods. This will involve the use of narrative text and tables to summarise data, consider outcomes in the light of differences in trial designs and address potential sources of bias for each of the trials being reviewed. We will group trials according to types of genetic conditions, and then organise them in terms of intervention and outcomes. We will summarise the results of the trials, including the range and size of any reported associations and important trial characteristics. We will also include a detailed commentary on the major methodological problems or biases affecting the trials, together with a description of how these may have affected the individual trial results.
We will use a random‐effects model to conduct the meta‐analysis due to anticipated differences between trial location and population. If there is substantial variation in results, particularly if there is inconsistency in the direction of effect, we will not perform a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
The authors will perform subgroup analyses where sufficient data are available. In the subgroup analyses, the authors will analyse the data in pre‐specified subgroups of trials that share characteristics of interest, to see whether the intervention effect remains consistent or whether it varies for particular characteristics of trials. For this review, the authors aim to compare the effects of interventions on outcome measures in the following groups by:
-
healthcare setting (primary, secondary, tertiary care or other);
-
intensity of the intervention (number or duration of intervention sessions);
-
nature of carrier status testing (confirmed genetic carrier status compared to probable carrier status);
-
type of condition.
Sensitivity analysis
If there is a spread of bias across the trials, we will provide two estimates of the intervention effect; firstly for all included trials, and secondly only including trials with an overall assessment of a low risk of bias.
Results
Description of studies
Results of the search
Database searching identified 6429 records. After screening 3863 unique records, 24 full‐text articles describing 16 unique trials were retrieved for further analysis; one of which is listed as ongoing (Kauffman 2017). No RCTs were found that were eligible for inclusion in the review. A flow diagram illustrates the search flow process (Figure 1).
Included studies
No RCTs were found to be eligible for inclusion in the review.
Excluded studies
We excluded 10 studies due to their non‐RCT study designs (Alhamdan 2007; Bekker 1993; Childs 1976; Clayton 1996; Hegwer 2006; Henneman 2001; Honnor 2000; Payne 1997; Tambor 1994; Watson 1991), while five RCTs were excluded because the intervention was not preconception genetic carrier testing or genetic carrier screening (Castellani 2011; Cheuvront 1998; Fisher 1981;Temme 2015Wilkie 2013). One RCT was a protocol (Kauffman 2017). The tables summarise the study details and reasons for exclusions (Characteristics of excluded studies).
Ongoing studies
One protocol for a trial has been identified and will be fully assessed for eligibility once completed (Kauffman 2017). The protocol describes a randomised controlled trial for genomic carrier screening in healthy individuals seeking preconception genetic testing for cystic fibrosis. It is due to report on a number of patient‐reported and economic outcomes. It is being conducted by Centre for Health Research, Kaiser Permanente Northwest in the USA.
Risk of bias in included studies
No trials were included in this review.
Effects of interventions
No trials were eligible for inclusion in this review.
Discussion
Summary of main results
Identifying those couples before pregnancy, who have a confirmed genetic carrier status for thalassaemia, sickle cell disease, cystic fibrosis, or Tay‐Sachs disease may provide the opportunity for individuals or couples to make fully informed reproductive choices such as avoiding pregnancy, pre‐implantation diagnosis, in vitro fertilisation, arranging early prenatal diagnosis, or consideration of adoption. However, there is currently no evidence from randomised controlled trials (RCTs) for the impact of genetic risk assessment for these conditions in non‐pregnant women on pregnancy outcomes, informed reproductive choices or psychological adverse effects.
Overall completeness and applicability of evidence
To date, in many countries, reproductive genetic risk assessment for autosomal recessive disorders has focused on the antenatal period and carrier status that has emerged as an incidental finding in neonatal screening. In the antenatal period, carrier status is identified either through formal screening programmes, or opportunistically during antenatal follow up in women at increased risk based on ancestry. During the antenatal period, if both parents are found to be carriers of the genes (at‐risk couples), prenatal diagnostic tests, such amniocentesis, may only be available either late in the first trimester or in the second trimester of pregnancy, which leaves the couple only a short period of time to make limited and difficult choices about termination or continuation of the pregnancy. This limits reproductive choices, with the potential of increased psychological distress in at‐risk couples (Modell 1980a; Modell 1980b). The incidental finding of the carrier state during neonatal screening for the specific disorders has highlighted concerns from at‐risk couples about the failure to offer this information prior to pregnancy (Locock 2008).
The evidence supporting genetic risk assessment before pregnancy has largely been from a series of observational studies (Alhamdan 2007; Bekker 1993; Childs 1976; Clayton 1996; Hegwer 2006; Henneman 2001; Honnor 2000; Payne 1997; Tambor 1994; Watson 1991). The majority of the observational studies have used before and after intervention designs (Bekker 1993; Clayton 1996; Hegwer 2006; Henneman 2001; Honnor 2000; Payne 1997; Tambor 1994; Watson 1991), while two studies utilised cross‐sectional designs (Alhamdan 2007; Childs 1976). Six RCTs were also identified (Castellani 2011; Cheuvront 1998; Fisher 1981; Kauffman 2017; Temme 2015; Wilkie 2013). However, five of these studies were not preconception genetic risk assessment (Castellani 2011; Cheuvront 1998; Fisher 1981; Temme 2015; Wilkie 2013) and one study was a protocol (Kauffman 2017). Only a few studies have assessed reproductive intentions (Cheuvront 1998; Henneman 2001; Watson 1991), whilst no studies have assessed actual reproductive outcomes. The limited duration of follow up in these studies would make assessment of latter outcomes unrealistic. All of the above studies have assessed psychological, attitudes, or knowledge outcomes, but there was some heterogeneity in these outcomes between and within studies. Further, none of the outcome measures for knowledge had used validated instruments. Although study participants recognised the importance of identifying genetic carrier states before pregnancy, different attitudes towards genetic testing were elicited and reproductive intentions varied following positive test results. In the Netherlands, study participants would consider prenatal diagnosis and abortion if an affected foetus is identified (Henneman 2001). In contrast, in the US state of Tennessee in a study of cystic fibrosis screening, reproductive intentions were limited by cultural and socio‐political factors, such as, insurability, being labelled as 'at risk', a lack of understanding, and religious beliefs about abortion (Clayton 1996). In addition, barriers to implementation may be due to fears of stigma (Kenen 1978), legal discrimination (Lapham 1996), or religious restrictions on abortion (Fowzan 2001).
Despite the absence of robust and relevant RCT evidence, a number of international organisations have recommended offering preconception genetic risk assessment routinely at the population level (ACOG Committee Opinion 2005; ACOG Committee Opinion 2009; Health Council of Netherlands 2007; Human Genetics Commission 2011; Johnson 2006). In the United States of America, the recommendations to improve preconception health care were developed through collaborative efforts of the Centres for Disease Control and Prevention (CDC), March of Dimes and the American College of Obstetrics and Gynaecology (ACOG) (ACOG Committee Opinion 2005; Johnson 2006). For instance, the ACOG has recommended in couples planning pregnancy identify if either member of the couple are of Eastern European (Ashkenazi) Jewish ancestry or have a family history of relevant recessive genetic diseases (such as Tay‐Sachs disease and Cystic Fibrosis), and such couples should be offered carrier screening before conception or early in pregnancy (ACOG Committee Opinion 2009).
Similarly the Health Council of Netherlands has recognised the seriousness of these conditions and high prevalence in local population groups, advocating preconception genetic risk assessment for cystic fibrosis, sickle cell and thalassaemia (Health Council of Netherlands 2007). However, the paucity of high‐quality studies limits the justification for national implementation.
The WHO's Regional Office of Eastern Mediterranean recommend preconception genetic risk assessment for sickle cell and thalassaemia ideally before marriage, taking account of the socio‐cultural issues in the region, in particular religious reservations towards termination of pregnancy (Alwan 1997). Since the 1970s, the Cyprus Thalassaemia Control Programme has been at the forefront of premarital genetic screening and this has contributed to a fall in the prevalence of thalassaemia in the country (Angastiniotis 1981). This universal premarital approach to thalassaemia carrier screening has also been adopted by Sardinia, Italy (Cao 1996) and Greece (Loukopoulos 1996).
In line with international policy recommendations, the UK Human Genetics Commission has recognised that since antenatal screening is currently already offered for genetic conditions such as sickle cell disease and thalassaemia, there are no ethical, legal or social issues with regards to the implementation of a preconception screening programme which would provide the advantage of improving reproductive choices (Human Genetics Commission 2011).
In South East Asia, the Family Planning Association of Hong Kong has recognised the benefits of preconception screening of genetic risk due to the high prevalence of thalassaemia carriers, accounting for up to eight per cent of the local population (Lau 1997).
In the absence of high quality randomised controlled trials of preconception genetic risk assessment, as demonstrated in this systematic review, international policy makers must base recommendations on observational studies and consensus agreements.
Quality of the evidence
It has been suggested that the optimum evidence to evaluate the reproductive and psychological outcomes as a result of preconception screening, compared to standard practice, is a systematic review of RCTs, or a high quality RCT with a large enough sample size to ensure the control of potential confounding factors (National Screening Committee 2003). Such trials address methodological issues that are particularly associated with screening interventions such as ascertainment bias due to non‐randomisation, with individuals joining screening programmes tending to have healthier lifestyles and better adherence to interventions (Smith 2003). In addition, none of the excluded studies identified in the searches for this review have evaluated reproductive outcomes. This is possibly related to the limited duration of follow up in these studies. Although preconception genetic carrier tests and screening have been shown to be to highly accurate and efficient in determining carrier status (Bach 2001; CDC 2004; Peters 2012; Weatherall 1997), the effectiveness of such interventions is ultimately measured by their ability to reduce morbidity and mortality of the diseases. Therefore, reproductive outcomes are essential to addressing this question.
Since this systematic review shows that there is a complete lack of RCTs in the field of preconception genetic risk assessment for autosomal recessive conditions, healthcare providers need to assess whether the information provided in published policy recommendations and non‐randomised studies is relevant to inform their preconception screening practice. Furthermore, healthcare providers have to balance the benefits of increasing reproductive choice against the potential psychological adverse effects from preconception genetic risk assessment, whilst taking into account the legal and socio‐cultural context of their healthcare setting and patient population.
Potential biases in the review process
The review authors have attempted to limit the bias in the review process through multiple authors and non‐author contributors who independently searched for trials, screened titles and abstracts, selected full‐text articles and extracted data. Any disagreements were resolved by group discussion and consensus, and therefore it was unlikely that trials have been incorrectly excluded. Although all clinical trials should be registered, there is always the potential of publication bias. However, attempts have been made to minimise publication bias through searching the grey literature and contacting key experts in the field.
Agreements and disagreements with other studies or reviews
This is the only systematic review looking at preconception genetic risk assessment for thalassaemia, sickle cell disease, cystic fibrosis and Tay‐Sachs disease and there were no randomised controlled trials eligible for inclusion, and therefore no comparisons could be made to other reviews or studies.

