Intervenciones cognitivo‐conductuales para el trastorno de déficit de atención e hiperactividad (TDAH) en adultos
Resumen
Antecedentes
El trastorno de déficit de atención e hiperactividad (TDAH) es una afección del desarrollo caracterizada por síntomas de falta de atención, hiperactividad e impulsividad, junto con déficits en la función ejecutiva, la regulación emocional y la motivación. La persistencia del TDAH en la edad adulta es un problema clínico grave.
El TDAH afecta deforma significativa las interacciones sociales, el estudio y el rendimiento laboral.
Los estudios anteriores indican que la terapia cognitivo‐conductual (TCC) podría ser efectiva para tratar a los adultos con TDAH, especialmente cuando se combina con tratamiento farmacológico. La TCC procura cambiar los pensamientos y los comportamientos que refuerzan los efectos perjudiciales del trastorno al enseñarles a los pacientes técnicas para controlar los síntomas centrales. La TCC también procura ayudar a los pacientes a enfrentar las emociones, como la ansiedad y la depresión y a mejorar la autoestima.
Objetivos
Evaluar los efectos de la terapia cognitivo‐conductual para el TDAH en adultos.
Métodos de búsqueda
En junio 2017, se hicieron búsquedas en CENTRAL, MEDLINE, Embase, en otras siete bases de datos y en tres registros de ensayos. También se verificaron las listas de referencias, los resúmenes de congresos obtenidos mediante búsquedas manuales y se contactó con expertos e investigadores de este tema.
Criterios de selección
Ensayos controlados aleatorios (ECA) que evalúan cualquier forma de TCC para adultos con TDAH, ya sea como monoterapia o junto con otro tratamiento, versus uno de los siguientes: condiciones de control no específicas (que comprenden psicoterapias de apoyo, ningún tratamiento o lista de espera) u otras intervenciones específicas.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar recomendados por la Colaboración Cochrane.
Resultados principales
Se incluyeron 14 ECA (700 participantes), 13 de los cuales se realizaron en el hemisferio norte y uno en Australia.
Resultados primarios: Síntomas de TDAH
TCC versus condiciones de control no específicas (psicoterapias de apoyo, lista de espera o ningún tratamiento)
‐ TCC versus psicoterapias de apoyo: La TCC fue más efectiva que el tratamiento de apoyo para mejorar los síntomas de TDAH informados por el médico (1 estudio, 81 participantes; evidencia de baja calidad) pero no para los síntomas del TDAH autoinformados (DME ‐0,16; IC del 95% ‐0,52 a 0,19; dos estudios, 122 participantes; evidencia de baja calidad; tamaño pequeño del efecto).
‐ TCC versus lista de espera: La TCC dio lugar a un beneficio más grande en los síntomas del TDAH informados por el médico (DME ‐1,22; IC del 95% ‐2,03 a ‐0,41; dos estudios, 126 participantes; evidencia de muy baja calidad; tamaño grande del efecto). También se encontraron diferencias significativas a favor de la TCC para los síntomas del TDAH autoinformados (DME ‐0,84; IC del 95% ‐1,18 a ‐0,50; cinco estudios, 251 participantes; evidencia de calidad moderada; tamaño grande del efecto).
TCC más farmacoterapia versus farmacoterapia sola: La TCC con farmacoterapia fue más efectiva que la farmacoterapia sola para los síntomas centrales informados por el médico (DME ‐0,80; IC del 95% ‐1,31 a ‐0,30; dos estudios, 65 participantes; evidencia de muy baja calidad; tamaño grande del efecto), los síntomas centrales autoinformados (DM ‐7,42 puntos, IC del 95% ‐11,63 puntos a ‐3,22 puntos; 2 estudios, 66 participantes, evidencia de baja calidad) y la falta de atención autoinformada (un estudio, 35 participantes).
TCC versus otras intervenciones que incluyen componentes terapéuticos específicamente dirigidos al TDAH: Se encontró una diferencia significativa a favor de la TCC para los síntomas del TDAH informados por el médico (DME ‐0,58; IC del 95% ‐0,98 a ‐0,17; dos estudios, 97 participantes; evidencia de baja calidad; tamaño del efecto moderado) y para la gravedad de los síntomas del TDAH autoinformados (DME ‐0,44; IC del 95% ‐0,88 a ‐0,01; cuatro estudios, 156 participantes; evidencia de baja calidad; tamaño pequeño del efecto).
Resultados secundarios
TCC versus condiciones de control no específicas: se encontraron diferencias a favor de la TCC comparada con el control en lista de espera para la depresión autoinformada (DME ‐0,36; IC del 95% ‐0,60 a ‐0,11; cinco estudios, 258 participantes; tamaño pequeño del efecto) y para la ansiedad autoinformada (DME ‐0,45; IC del 95% ‐0,71 a ‐0,19; cuatro estudios, 239 participantes; tamaño pequeño del efecto). También se observaron diferencias a favor de la TCC para el estado de ira autoinformado (1 estudio, 43 participantes) y la autoestima autoinformada (1 estudio, 43 participantes) en comparación con la lista de espera. No se encontró ninguna diferencia entre la TCC y el tratamiento de apoyo (1 estudio, 81 participantes) para la depresión autocalificada, la ansiedad calificada por el médico o la autoestima autocalificada. Además, no hubo diferencias entre la TCC y la lista de espera para el rasgo de ira autoinformado (1 estudio, 43 participantes) o la calidad de vida autoinformada (DME 0,21; IC del 95% ‐0,29 a 0,71; dos estudios, 64 participantes; tamaño pequeño del efecto).
TCC más farmacoterapia versus farmacoterapia sola: Se encontraron diferencias a favor de la TCC más farmacoterapia para la puntuación de la Clinical Global Impression (DM ‐0,75 puntos, IC del 95% ‐1,21 puntos a ‐0,30 puntos; 2 estudios, 65 participantes), la depresión autoinformada (DM ‐6,09 puntos, IC del 95% ‐9,55 puntos a ‐2,63 puntos; 2 estudios, 66 participantes) y la ansiedad autoinformada (DME ‐0,58; IC del 95% ‐1,08 a ‐0,08; dos estudios, 66 participantes; tamaño moderado del efecto). También se observaron diferencias a favor de la TCC más farmacoterapia (1 estudio, 31 participantes) para la depresión informada por el médico y la ansiedad informada por el médico.
TCC versus otras intervenciones específicas: No se encontraron diferencias para ninguno de los resultados secundarios, como la depresión autoinformada y la ansiedad, y los resultados en la calidad de vida autoinformada variaron a través de diferentes estudios.
Conclusiones de los autores
Hay evidencia de baja calidad de que la terapia cognitivo‐conductual puede ser beneficiosa para el tratamiento de los adultos con TDAH a corto plazo. Las reducciones en los síntomas centrales de TDAH fueron bastante consistentes a través de las diferentes comparaciones: en la TCC más farmacoterapia versus farmacoterapia sola y en la TCC versus lista de espera. Hay evidencia de baja calidad de que la TCC también puede mejorar los trastornos secundarios comunes en los adultos con TDAH, como la depresión y la ansiedad. Sin embargo, la escasez de datos de seguimiento a largo plazo, la naturaleza heterogénea de los resultados medidos y la ubicación geográfica limitada (hemisferio norte y Australia) limitan la generalizabilidad de los resultados. Ninguno de los estudios incluidos informó los eventos adversos graves, aunque cinco participantes que recibieron diferentes modalidades de TCC describieron algún tipo de evento adverso, como dificultad y ansiedad.
PICO
Resumen en términos sencillos
Terapia cognitivo‐conductual para el trastorno de déficit de atención e hiperactividad (TDAH) en adultos
Antecedentes
Los pacientes con TDAH tienen dificultad para prestar atención, concentrarse, manejar la hiperactividad (p.ej. esperar en las filas) y actuar sin pensar (es decir impulsividad). En los adultos, el TDAH afecta de forma significativa las interacciones sociales, el estudio y el rendimiento laboral.
Los estudios anteriores indican que la terapia cognitivo‐conductual (TCC) podría ser efectiva para el tratamiento en los adultos con TDAH, especialmente cuando se combina con tratamiento farmacológico (es decir fármacos). La TCC procura cambiar los pensamientos y los comportamientos que refuerzan los efectos perjudiciales del trastorno al enseñarles a los pacientes técnicas para controlar los síntomas centrales. La TCC también procura ayudar a los pacientes a enfrentar las emociones, como la ansiedad y la depresión y a mejorar la autoestima.
Pregunta de la revisión
¿La TCC, sola o en combinación con tratamiento farmacológico, reduce los síntomas centrales del TDAH en adultos más que otros tratamientos o ningún tratamiento específico?
Fechas de la búsqueda
La evidencia está actualizada hasta junio de 2017.
Características de los estudios
Se encontraron 14 ensayos controlados aleatorios (estudios en los que los participantes son asignados al azar a diferentes grupos de tratamiento) que describieron los efectos de la TCC en 700 adultos con TDAH, de entre 18 y 65 años de edad. Trece ensayos se llevaron a cabo en el hemisferio norte y uno en Australia.
De los estudios incluidos, tres compararon TCC versus otras intervenciones específicas y siete versus condiciones de control no específicas (tratamiento de apoyo no específico, lista de espera o ningún tratamiento). Además, dos compararon TCC más farmacoterapia versus farmacoterapia sola. Un ensayo comparó TCC versus dos grupos de control, uno de los cuales recibió otro tratamiento específico no farmacológico y uno de los cuales fue un control de ningún tratamiento.
Calidad de la evidencia
Debido a la imprecisión (es decir resultados inexactos), la inconsistencia (es decir los resultados difieren a través de los ensayos) y las limitaciones metodológicas, se consideró que la calidad de la evidencia de los estudios incluidos varió de muy baja a moderada.
Resultados clave
Los hallazgos indican que la TCC podría mejorar los síntomas centrales del TDAH, y reducir la falta de atención, la hiperactividad y la impulsividad.
Cuando se combina con farmacoterapia, hubo evidencia de una mejoría en el funcionamiento global (es decir un nivel general de funcionamiento del paciente en la vida) y una reducción en la depresión y la ansiedad en comparación con lo observado con la farmacoterapia sola.
Ninguno de los estudios incluidos informó eventos adversos graves. Sin embargo, cinco participantes describieron algún tipo de evento adverso, como dificultad y ansiedad.
Authors' conclusions
Summary of findings
| Cognitive‐behavioural interventions versus unspecific control for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| CBT versus supportive therapy | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 12 weeks | — | The mean ADHD observer‐rated symptoms score in the intervention groups was 0.56 standardised deviations lower (1.01 lower to 0.12 lower) | — | 81 | ⊕⊕⊝⊝ | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 12 to 14 weeks | — | The mean ADHD self‐rated symptoms score in the intervention groups was 0.16 standardised deviations lower (0.52 lower to 0.19 higher) | — | 122 | ⊕⊕⊝⊝ | Small effect sizeb |
| CBT versus waiting list control | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 8 to 12 weeks | ‐ | The mean ADHD self‐rated symptoms score in the intervention groups was 1.22 standardised deviations lower (2.03 lower to 0.41 lower) | — | 126 | ⊕⊝⊝⊝ | Large effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks | — | The mean ADHD self‐rated symptoms score in the intervention groups was 0.84 standardised deviations lower (1.18 lower to 0.50 lower) | — | 251 | ⊕⊕⊕⊝ | Large effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded the quality of evidence due to imprecision (considering the width of the CI), methodological limitations (due to high risk of bias in blinding of participants and personnel), and because the evidence is based on a single study. | ||||||
| Cognitive‐behavioural therapy plus pharmacotherapy versus pharmacotherapy alone for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT plus pharmacotherapy | |||||
| ADHD symptoms: clinician rated Assessed by: various scales Follow‐up: 8 to 15 weeks | — | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.80 standardised deviations lower (1.31 lower to 0.30 lower) | — | 65 | ⊕⊝⊝⊝ | Large effect sizec |
| ADHD symptoms: self‐reported Assessed by: ADHD Current Symptoms Scale (range 0 (best) to 54 (worst)) Follow‐up: 8 to 15 weeks | The mean ADHD self‐rated symptoms score in the control groups ranged from 14.75 to 17.22. | The mean ADHD self‐rated symptoms score in the intervention groups was 7.42 lower (11.63 lower to 3.22 lower) | — | 66 | ⊕⊕⊝⊝ | Large effect size |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the quality of evidence due to methodological limitations (high risk of bias in blinding of participants and personnel, and the fact that Emilsson 2011 had a high risk of bias in three domains in one of the two included studies). | ||||||
| Cognitive‐behavioural therapy versus other interventions for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| ADHD symptoms: clinician‐rated Assessed by: various scales Follow‐up: 10 to 12 weeks | — | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.58 standardised deviations lower (0.98 lower to 0.17 lower) | — | 97 | ⊕⊕⊝⊝ | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks | — | The mean ADHD self‐reported symptoms score in the intervention groups was 0.44 standardised deviations lower (0.88 lower to 0.01 lower) | — | 156 | ⊕⊕⊝⊝ | Small effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded the quality of evidence because of imprecision (considering the width of the CI) and methodological limitations (due to the high risk of bias in blinding of participants and personnel and three other domains with unclear risk of bias). | ||||||
Background
Description of the condition
According to the Diagnostic and Statistical Manual of Mental Disorders, currently in its fifth edition (DSM‐5), attention deficit hyperactivity disorder (ADHD) is a developmental condition characterised by symptoms of inattention, hyperactivity and impulsivity (DSM‐5 2013). Using these criteria, ADHD can be divided into three types: combined type, predominantly inattentive type and predominantly hyperactive‐impulsive type. The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993), but the required number of symptoms and the age of onset are different. Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Davidson 2008; Torrente 2011; Wender 2001). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood and around 2.5% in adulthood (Polanczyk 2014; Simon 2009).
Evidence on gender differences in ADHD is controversial. Some authors suggest that there are no differences between females and males (Biederman 2002; Seidman 2006). Other authors, such as Gershon 2002, argue that there are quantitative and qualitative differences in executive functions.
Still 1902 is commonly accredited as the first description of a syndrome in children that included some of the characteristics of ADHD. However, the characterisation of the disorder in adults was more recent, with Adler 2002 attributing it to Wood 1976. Since then, many papers have provided evidence on the diagnostic validity of ADHD in adults (Spencer 1998). The validity of the diagnosis in adulthood is supported by clinical correlates, family history, treatment response and experimental studies (Faraone 2000). Additionally, longitudinal studies have demonstrated the persistence of the disorder in large proportions of adults who were diagnosed with ADHD during childhood (Barkley 1999).
As Brassett‐Harknett 2007 noted, there are diagnostic difficulties with ADHD in adults because the current diagnostic criteria were originally designed for children. ADHD in adulthood has particular characteristics that differ from the syndrome in childhood. For example, hyperactivity tends to decrease in adulthood (Achenbach 1998), with some studies showing that 90% of adults with ADHD present predominantly with inattentive symptoms (Millstein 1997).
Importantly, different authors have recognised the persistence of ADHD in adulthood as a clinical problem with serious health consequences (Davidson 2008; Wilens 2004). Barkley 2008 highlights the severe occupational consequences of the disorder, such as lower occupational status and annual salaries than in a control group, worse employer‐rated job performance, more job dismissals and frequent changes of job. Those who suffer from ADHD are less capable of fulfilling work demands, less likely to be working independently, completing tasks, and getting along well with supervisors, as rated by employers. They have poorer performance at job interviews and find certain tasks at work too difficult. Additionally, Woods 1986 suggests that people with ADHD experience anger dysregulation as a highly associated psychosocial problem. ADHD also carries psychological consequences since repeated life experiences of frustration undermine self‐concept and self‐esteem, leading to the formation of negative beliefs about the self, which, in turn, affect quality of life and emotional adjustment (Torrente 2012).
Description of the intervention
Diverse psychological treatments have been developed for for adults with ADHD in recent years (Knouse 2008; Weiss 2008). Most have been inspired by cognitive‐behavioural therapy (CBT) and designed as adjunct interventions to pharmacological treatment (Safren 2006). As is usual in CBT treatments, the interventions are organised into relatively brief and focused, structured protocols. Most CBT programmes for adults with ADHD take 8 to 12 sessions and can be delivered on an individual or group basis. The main objectives of the treatment are to change the behaviours that reinforce detrimental effects of the disorder by teaching people techniques to control ADHD's core symptoms, improving emotional adjustment, self‐esteem and common comorbid symptoms such as anxiety and depression. Proposed psychotherapeutic techniques include psychoeducation for increasing awareness and understanding of the disorder and cognitive techniques for restructuring the dysfunctional thoughts and maladaptive beliefs that reinforce emotional maladjustment. Finally, behavioural interventions and cognitive remediation methods intend to provide new, healthy, compensatory strategies and skills for deficient attention, executive functioning, impulse control and emotion regulation (Ramsay 2010).
Investigators have applied variants of the classical CBT approach to this population: Hesslinger 2002 and Philipsen 2007 have experimented with dialectical behavioural therapy and Solanto 2010 with meta‐cognitive therapy. These variants emphasise specific types of interventions such as emotion regulation skills in dialectical behavioural therapy and cognitive training methods in meta‐cognitive therapy, but because they share the general model and procedures of CBT, previous, non‐systematic reviews have usually included these methods within the broad spectrum of CBT interventions (Knouse 2008; Weiss 2008). However, no studies have ever directly compared these types of CBTs against each other, so it is unknown if they have different treatment effects. Moreover, comparing CBT versus placebo, waiting list and no treatment could produce different treatment effects for each comparison, and we plan to explore these potential differences in our study.
How the intervention might work
The cognitive‐behavioural approach provides a useful framework for understanding how negative life experiences may reinforce functional impairment and lead to increased emotional disturbance in adults with ADHD. Because of neurobiological deficits in attention, executive function and inhibitory control, failure and underachievement in different domains of function are common occurrences in people with ADHD as they enter adulthood (Barkley 2006a; Biederman 2006). According to the CBT model, such repeated life experiences of frustration undermine self‐concept and self‐esteem, leading to the formation of negative beliefs about the self, which, in turn, favour the expression of negative emotions such as depression and anxiety. Negative self‐beliefs can also lead to the adoption of maladaptive behavioural strategies, including negation, procrastination and extreme avoidance as a means of coping with difficult tasks (Ramsay 2008; Safren 2006; Young 2007a). In addition to emotional disturbances, negative expectations about the future, anticipation of failure and reduced self‐confidence can also affect motivation (Torrente 2011).
The proposed mechanisms of change entail the acquisition of compensatory behavioural and cognitive techniques for improving the core attention and executive deficits of ADHD and modifying distorted negative beliefs to promote emotional maladjustment (Ramsay 2010). CBT programmes are therefore usually organised into several modules with specific techniques for a series of target problems. Most treatments begin with a psychoeducational module in which patients are taught about the disorder and introduced to the rationale for the treatment. This is followed by an organisation module designed to aid the acquisition of different executive techniques such as goal setting, sequencing and prioritising, devising a time schedule, using a calendar or agenda, making 'to do' lists, monitoring progress, and planning breaks and rewards. Patients also learn problem‐solving techniques for articulating problems more clearly, generating a list of potential solutions, evaluating them and finally testing the chosen solution. The distraction management module helps patients to recognise their optimal attention span and organise the tasks according to it, and it introduces skills for dealing with distractions such as writing them down and going back to the task, using cues or alarms, or modifying environmental factors. The impulsivity management module includes strategies for self‐monitoring and self‐control. The self‐monitoring module involves the detection of cues and situations that act as triggers for impulsive behaviour, while self‐control strategies refer to the use of self‐instructions, relaxation techniques or other alternative behaviours. The cognitive restructuring module aims to help patients to become aware of the ideas that reinforce maladaptive behaviours and emotions and replace them with more adaptive thoughts.
Several pilot studies have demonstrated the feasibility and acceptability of the approach (Knouse 2008), and a series of randomised controlled trials have provided evidence for the efficacy of CBT in adults with ADHD (Safren 2005; Safren 2010; Solanto 2010; Stevenson 2002).
Why it is important to do this review
Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002). Also, pharmacological treatment is frequently associated with relevant side effects in both children and adults (AJCD 2001; Castells 2013; Cunill 2013; Graham 2011; King 2006; Lim 2006; Morton 2000; Perrin 2008; Prescrire 2007). Due to these concerns, it is important to have non‐pharmacological interventions for treating adults with ADHD.
The consequences of ADHD can also have an important and negative impact on different areas of a person's life, such as poor academic performance, deficits in social and occupational functioning, greater job insecurity and a greater number of legal problems (Barkley 2002; Davids 2004). An efficacious psychosocial intervention might be beneficial in one or more of these areas for adults with ADHD.
To our knowledge, three systematic reviews have compared the effects of CBT in adults with ADHD (Jensen 2016; Knouse 2017; Young 2016). However, there are important methodological differences between them, also with respect to our review. Both Jensen 2016 and Young 2016 employed more restrictive criteria for defining CBT treatments that excluded relevant CBT variants such as mindfulness‐based cognitive therapy and dialectical behavioural therapy. Knouse 2017 did not report grades of quality of evidence of the included studies.
Objectives
To assess the effects of cognitive‐behavioural‐based therapy for ADHD in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults aged 18 years and above diagnosed with ADHD or hyperkinetic disorder according to the established diagnostic criteria, whose medication was stable (less than 10% change in dose) in the two months prior to the initial evaluation.
Types of interventions
Individual and group treatments of CBT in any of its variants such as standard CBT, dialectical behavioural therapy, meta‐cognitive therapy, or mindfulness‐based cognitive therapy.
All included CBT interventions had to fulfil both of the following criteria.
-
Treatment was aimed at increasing knowledge on the disorder, identifying and restructuring dysfunctional thinking and maladaptive beliefs, and developing emotional and behavioural compensatory strategies for the core deficits.
-
The sequence of treatment modules was clearly defined.
We assessed 'CBT as a monotherapy' separately from 'CBT as part of a combined treatment' because the latter may present interactive effects that are not accounted for by any of the interventions alone. We evaluated these as follows.
-
CBT versus unspecific control conditions (supportive psychotherapies, waiting list or no treatment).
-
CBT plus pharmacotherapy versus pharmacotherapy alone.
-
CBT versus other specific interventions (control interventions that include therapeutic ingredients specifically targeted to ADHD).
We did not impose any restriction with regard to the format of the treatment (that is, the duration, quantity and frequency of sessions).
Types of outcome measures
We considered psychometrically validated self‐report measures or those completed by an independent rater or relative.
We present clinical and self‐reported outcomes separately, as do most studies about this topic, because assessing ADHD is more accurate when symptom information comes from more than one source (Barkley 1998a).
We considered the measures as short term (up to 6 months), medium term (6 months to 12 months) and long term (more than 12 months).
We included studies that assessed at least one primary outcome or at least one secondary outcome.
Primary outcomes
We assessed the core symptoms of ADHD (inattention, hyperactivity and impulsivity) as a whole. If the authors of a study reported these symptoms separately, we included the data in the analysis. We assessed the core symptoms using validated measures such as those listed below.
Continuous outcomes (efficacy)
-
Current Symptoms Scale (Barkley 1998a)
-
Conners' Adult ADHD Rating Scales ‐ Self‐Report: Long Version (Conners 1999a)
-
Conners' Adult ADHD Rating Scales ‐ Observer Report (Conners 1999a)
Dichotomous outcomes (safety)
-
All‐cause treatment discontinuation (proportion of patients randomised who dropped out from the study due to any cause, such as adverse effects of medication)
Secondary outcomes
We assessed the efficacy variables listed below as secondary outcomes. The listed measures are mentioned only as examples, and the list is not exclusive.
Continuous outcomes
-
Psychopathology (depression and anxiety)
-
Beck Depression Inventory II (Beck 1996)
-
Beck Anxiety Inventory (Beck 1988)
-
Hamilton Depression Scale (Hamilton 1960)
-
Hamilton Anxiety Scale (Hamilton 1959)
-
State‐Trait Anxiety Inventory (Speilberger 1989)
-
-
Anger: State‐Trait Anger Expression Inventory (Spielberger 1988)
-
Self‐esteem: Rosenberg Self‐Esteem Inventory (Rosenberg 1965a)
-
Quality of life: Adult Attention‐Deficit/Hyperactivity Disorder Quality‐of‐Life Scale (Brod 2005)
Dichotomous outcomes
-
Employment status (for example, working/not working, full‐time/part‐time, as defined by the authors of the study)
Considering that self‐ and clinician‐reported core symptoms are the main targets of CBT, we included them in the 'Summary of findings' tables. We prepared these tables using the GRADE methodology (Atkins 2004; Guyatt 2011). To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in section 12.6.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Re‐expressing SMDs using rules of thumb for effect sizes): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Higgins 2011).
Search methods for identification of studies
We used the following search terms and their synonyms: 'attention deficit disorder with hyperactivity', 'cognitive‐behavioural therapy' and 'adults' We used the Cochrane Highly Sensitive Search Strategy to identify RCTs in MEDLINE (Lefebvre 2011). We modified the search strategy as necessary for other databases.
Electronic searches
We searched the following databases in June 2017. We did not limit the searches by date or language (see search strategies in Appendix 1).
-
Cochrane Central Register of Controlled Studies (CENTRAL; 2017, Issue 6) in the Cochrane Library, which contains the Cochrane Developmental Psychosocial and Learning Problems Group Specialised Register (searched 13 June 2017).
-
MEDLINE PubMed, US National Library of Medicine (www.ncbi.nlm.nih.gov/pubmed; 1971 to 13 June 2017).
-
Embase Elsevier (1974 to 13 June 2017).
-
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 27 June 2017).
-
PsycINFO EBSCO (1967 to 15 June 2017).
-
BIOSIS Previews Web of Science (1926 to 16 June 2017).
-
Cochrane Database of Systematic Reviews (CDSR; 2017 Issue 6), part of the Cochrane Library (searched 13 June 2017).
-
Database of Abstracts of Reviews of Effects (DARE; 2015 Issue 2), part of the Cochrane Library (searched 13 June 2017).
-
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/es; searched 18 June 2017).
-
Networked Digital Library of Theses and Dissertations (NDLTD; www.ndltd.org/resources; searched 27 June 2017).
-
ClinicalTrials.gov (clinicaltrials.gov; searched 23 June 2017).
-
ISRCTN registry BioMed Central (www.isrctn.com; searched 23 June 2017).
-
World Health Organization International Clinical Trials Registry Portal (WHO ICTRP; apps.who.int/trialsearch; searched 23 June 2017).
Searching other resources
On 17 June 2017, we handsearched the World Congress of Behavioral and Cognitive Therapies from 1995 to 2016, together with the following websites.
-
Association for Behavioural and Cognitive Therapies (ABCT) Convention, 2008 to 2017 (www.abct.org/Conventions/?m=mConvention&fa=PastFutureConvention).
-
World Congress on ADHD, organised by the World Federation of ADHD, 2007 to 2017 (www.adhd‐federation.org/congresshistory).
-
Annual Meeting ‐ American Psychiatric Association (APA), 1973 to 2016 (www.psychiatry.org/psychiatrists/search‐directories‐databases/library‐and‐archive).
We also consulted experts and researchers in the field, including investigators from all review articles and primary studies identified through searches, about ongoing or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (PL and FT) independently screened the titles and abstracts using the Early Review Organizing Software (EROS) (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010). If it was clear from the title and abstract that the study did not meet the eligibility criteria, we rejected it. If it was not clear, then we obtained the full text of the study, and both review authors independently evaluated the paper using EROS to determine if the study should be included or excluded. If there was disagreement, the review authors tried to solve it by reaching a consensus. In the case that these two review authors could not reach a consensus, a third author (AC) independently assessed the study and resolved the disagreement. We recorded the results of this selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
Two review authors (PL and FT) independently extracted data from each included study and entered the information onto a pro‐forma document designed and piloted for this purpose. We extracted information about the 'Risk of bias' criteria and the methods of participant selection. We also extracted information about the populations, interventions, comparisons, outcomes, outcome data, study designs, gender, comorbidity, severity and baseline symptoms. The two review authors resolved any differences in opinion by consensus. If they were unable to do so, a third review author (AC) was included in the decision process, and all three review authors discussed the issue and made a final decision.
Assessment of risk of bias in included studies
We evaluated the risk of bias in each included trial using the seven criteria described in Table 8.5.d ('Criteria for judging risk of bias in the "Risk of bias" assessment tool') of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (PL and FT) independently assessed each included study as being at low, high or unclear (uncertain) risk of bias for each domain, using EROS software (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010); see Table 1. If there were discrepancies between their assessments, and the two review authors were unable to reach a consensus, a third review author (AC) joined the decision‐making process. All three review authors discussed the issue and made a final decision.
| Criteria | Description |
| Random sequence generation (selection bias ‐ biased allocation to interventions ‐ due to inadequate generation of a randomised sequence) |
|
| Allocation concealment (selection bias ‐ biased allocation to interventions ‐ due to inadequate concealment of allocations prior to assignment) |
|
| Blinding of participants and personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the study) |
|
| Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessors) |
|
| Incomplete outcome data (attrition bias due to the amount, nature or handling of incomplete outcome data) |
|
| Selective reporting (reporting bias due to selective outcome reporting) |
|
| Other bias (other sources of bias not captured by the other domains) |
|
Measures of treatment effect
Continuous data
We calculated mean differences (MD) when studies used the same measure and standardised mean differences (SMD) when studies used different measurement scales, and we present these with 95% confidence intervals (CIs). When necessary, we calculated the effect estimates from the P values, t statistics or other available statistics.
We interpreted the magnitude of effect for the SMD using a rule of thumb where we considered 0.2 as a small effect, 0.5 as a moderate effect, and 0.8 as a large effect (Cohen 1988).
For the studies that reported only change scores, we performed separate analyses from the studies that provided only final values. We combined both values using the generic inverse variance method (Higgins 2011).
In cases in which there were at least two studies pooled for the same comparison of results, we used the median change to facilitate readers' understanding.
We provide a table reporting relative effects in order to allow comparisons of effects of interventions across all outcomes (see Appendix 2). We reported the absolute and relative changes (95% CI) related to the control central estimates of each outcome (negative percentages indicate a reduction of symptoms).
Dichotomous data
We did not find dichotomous data to include in this review (see Lopez 2013; Table 2).
| Method | Approach |
| Measures of treatment effect | Dichotomous data We did not find dichotomous data. Should these data become available in future updates of this review, we will calculate the risk ratio (RR) with 95% confidence intervals (CI), as most readers find it easier to understand the RR and 95% CI than the odds ratio and risk difference. |
| Unit of analysis issues | Cluster‐RCTs We did not find cluster‐RCTs. This design is uncommon in this field. Should such studies become available in the future, and if the investigators report cluster‐randomised trial data as though the randomisation was performed on individuals rather than on clusters, we will request individual participant data to calculate an estimate of the intra‐cluster correlation coefficient (ICC). If the individual participant data are not available, we will obtain external estimates of the ICC from similar studies or available resources (Campbell 2000). Once established, we will use the ICC to re‐analyse the trial data to obtain approximate, correct analyses as described in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will combine the effect estimates and their corrected standard errors from cluster‐RCTs with those from parallel‐group designs using the generic inverse variance method (Higgins 2011). If the available information is insufficient to control for clustering in this manner, we will enter the data into Review Manager 5 (RevMan 2014), using individuals as the unit of analysis. We planned to perform sensitivity analyses to assess the potential bias that may occur as a result of the inadequately controlled clustered trials. Additionally, if the ICCs are obtained from external sources, we will perform sensitivity analyses to assess the potential biasing effects of inadequately controlled cluster‐randomised trials (Donner 2001). |
| Cross‐over trials We did not find cross‐over trials. Should we find these types of studies in future updates of this review, and as the duration of any effect of CBT is unknown, we will use only first‐period data from any cross‐over trials that fit the inclusion criteria to avoid a possible carry‐over effect. | |
| Dealing with missing data | If the studies do not report the standard deviation (SD), we will calculate it from the P values, t values, CIs or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). If this information is not reported or is unattainable, we will impute the SD from the study with the highest SD for that outcome, and assess the effects of this assumption on the analysis by conducting a sensitivity analysis. If the outcome data are reported as a median, a range or as a mean without a variance, we will report the data in additional tables. |
| Sensitivity analysis | We will conduct sensitivity analyses to:
|
CBT: cognitive‐behavioural intervention; CI: confidence interval; ICC: intracluster correlation coefficient; RCT: randomised controlled trial; SD: standard deviation.
See also Lopez 2013.
Unit of analysis issues
For each included study, we determined the appropriateness of the unit of analysis for the unit of randomisation and the design of each study (the number of observations had to match the number of units that were randomised). We expected to find trials with a simple parallel‐group design, with participants randomly allocated as individuals, and a single measurement collected and analysed for each outcome from each participant.
Cluster‐RCTs and cross‐over trials
We did not find cluster‐RCTs or cross‐over trials (see Lopez 2013; Table 2).
Multiple treatment groups
For trials with multiple treatment groups, we combined the results across all eligible treatment arms and compared them with the combined results across all eligible control arms, making single, pair‐wise comparisons. Where such a strategy prevented an investigation of the potential sources of heterogeneity, we analysed each treatment arm separately (against a common control group) but divided the sample size of the common comparator groups proportionately across each comparison (Higgins 2011, section 16.5.4). This approach prevented inappropriate double‐counting of individuals.
Dealing with missing data
When necessary, we attempted to contact the corresponding authors of the included studies up to three times to collect any unreported data.
We described missing data and dropouts for each included study in the 'Risk of bias' table (beneath the Characteristics of included studies tables), reporting the reasons for missing data and the number and characteristics of dropouts, and we discussed in the 'Quality of the evidence' section the extent to which the missing data could threaten our results due to attrition bias.
We made no assumptions about loss to follow‐up for continuous data, and we based the analyses on those participants who completed the trial.
See Lopez 2013 and Table 2.
Assessment of heterogeneity
We appraised the extent of clinical heterogeneity among the studies by comparing the distribution of participant characteristics (comorbidity, severity, baseline symptoms, ADHD subtype) and study factors (randomisation, allocation concealment, blinding of outcome assessment, loss to follow‐up, treatment type, type of control group, co‐interventions, different types of outcome measurements). We assessed these variables by subgroup analysis if I2 was more than 30%. Additionally, we deemed a low P value for the Chi2 test (< 0.10) as sufficient reason to explore causes of heterogeneity (Subgroup analysis and investigation of heterogeneity).
We described the statistical heterogeneity of the intervention effects by calculating the I2 statistic and using the Chi2 test. The thresholds used for the interpretation of I2 can be misleading because the importance of inconsistency depends on several factors. We interpreted it as follows.
-
0% to 40%: might not be important.
-
30% to 60%: may represent moderate heterogeneity.
-
50% to 90%: may represent substantial heterogeneity.
-
75% to 100%: represents considerable heterogeneity.
Assessment of reporting biases
Had there been at least 10 studies in a meta‐analysis, we would have used funnel plots to detect bias. Funnel plot asymmetry can be due to publication bias, but it can also be due to a real relationship between trial size and effect size, such as when larger trials have a lower adherence, and adherence is positively related to effect size. In general, asymmetry may be due to selection biases (publication bias, delayed publication bias, location bias, selective outcome reporting), poor methodological quality leading to spuriously inflated effects in smaller studies (poor methodological design, inadequate analysis, fraud), true heterogeneity or chance (Egger 1997). We used the test proposed by Egger 1997 for continuous outcomes to test for funnel plot asymmetry (Higgins 2011).
Data synthesis
We synthesised the results in a meta‐analysis using Review Manager 5 (RevMan 5) when we considered studies to be sufficiently homogenous in terms of population (regarding sex, age and diagnosis), interventions (comparable modalities of CBT) and comparisons (as a monotherapy or a part of a combined treatment) to avoid clinical heterogeneity, and in terms of outcome measurement methods to avoid methodological heterogeneity (RevMan 2014). Two authors assessed homogeneity independently and solved discrepancies by consensus. In the cases where comparisons had a considerable heterogeneity but the same direction, we present both the global results and the results of each study separately in order to show the range of effects comprised in the comparisons.
We used both a fixed‐effect model and a random‐effects model and compared them to assess the degree of statistical heterogeneity. Because we assumed that clinical heterogeneity was very likely to impact our review results, given the nature of the interventions included, we primarily reported the results of the random‐effects model, regardless of statistical evidence of heterogeneity. We calculated all effects using inverse variance methods. For continuous data, the change in score from baseline to postintervention was the main outcome of interest. We analysed separately continuous data reported as change scores in some studies and as final values in other studies. Additionally, we combined these values using the generic inverse variance method (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Where it was possible to secure the necessary data, we conducted subgroup analyses, classifying the trials as follows.
-
Type of ADHD subtype: inattentive, hyperactive‐impulsive or combined type.
-
Type of control group: other specific treatment or unspecific control conditions (supportive psychotherapies, waiting list or no treatment).
We calculated a pooled effect size for each subgroup.
Sensitivity analysis
We used sensitivity analyses to assess the impact of risk of bias on the results of the primary analyses. For this review, we undertook sensitivity analyses to determine the effect of removing from the analysis: studies with high risk of selection bias (associated with sequence generation or allocation concealment); studies with high risk of performance bias (associated with issues of blinding); and studies with high risk of attrition bias (associated with completeness of data). In addition, we assessed the sensitivity of findings to any imputed data within a study.
We investigated the impact of applying a fixed‐effect model on the results compared to that of a random‐effects model. We also compared the impact of using the odds ratio as an effect measure compared to the risk difference.
Summary of findings table
We prepared a 'Summary of findings' table for our three main comparisons (see Types of interventions) according to GRADE methodology (Atkins 2004; Guyatt 2011), using GRADEpro GDT software (GRADEpro 2015). We included our primary outcome, the core symptoms of ADHD (self‐, clinician‐ or observer‐reported), in the tables.
Two review authors (AC and PL) independently assessed the quality of the evidence as high, moderate, low or very low, downgrading the rating according to the presence of study limitations, including the studies' 'Risk of bias' level; imprecision; inconsistency of results; indirectness of evidence; and likely publication bias.
To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect.
Results
Description of studies
Results of the search
Our database searches returned 12,088 records (10,306 unique records). We did not find any relevant records by handsearching sources of congress or conference abstracts. After screening titles and abstracts, we deemed 10,237 records to be irrelevant and retrieved 69 full texts for further scrutiny. Of these 69 reports, 14 studies (from 15 reports) met our predefined inclusion criteria (Criteria for considering studies for this review); we considered one additional reference to be a secondary reference of Stevenson 2002 because the authors used the same data set and reported the same results. We excluded 49 reports as irrelevant (Excluded studies) and identified five ongoing studies. See Figure 1.

Study flow diagram.
Included studies
Study design
Authors of the 14 included studies described them as RCTs, with a parallel group design, blinded for participants; each lasted 8 to 15 weeks. We did not find any cluster‐RCTs or cross‐over trials.
Setting
Four trials were in outpatients in the USA (Fleming 2015; Safren 2005; Safren 2010; Solanto 2010), and three took place in Sweden (Hirvikoski 2011; Moëll 2015; Pettersson 2017). The seven remaining trials were carried out in: Australia (Stevenson 2002), China (Gu 2017), Finland (Virta 2010), Iceland (Emilsson 2011), the Netherlands (Hepark 2015; Schoenberg 2014), and Spain (Vidal Estrada 2013).
Participants
In total, the 14 trials recruited 700 participants aged 18 to 65 years.
The included trials used three different versions of DSM criteria.
-
Third Edition‐Revised (DSM‐III‐R 1989), used in one trial (Stevenson 2002);
-
Fourth Edition (DSM‐IV 1994), used in eight trials (Emilsson 2011; Hirvikoski 2011; Moëll 2015; Safren 2005; Safren 2010; Solanto 2010; Vidal Estrada 2013; Virta 2010); and IV‐Text Revision (DSM‐IV‐TR 2000), used in three trials (Hepark 2015; Pettersson 2017; Schoenberg 2014).
-
Fifth Edition (DSM‐5 2013) used in two trials (Fleming 2015; Gu 2017).
Intervention and comparisons
All of the trials reported the effects of CBT‐based treatments on adults with ADHD symptoms. We included studies that assessed mindfulness‐based interventions when authors explicitly stated that the treatment included CBT principles or techniques together with mindfulness procedures. We considered meta‐cognitive therapy and dialectical behaviour therapy as variants of CBT. The trials included the following interventions and comparisons.
-
CBT versus unspecific control conditions.
-
Gu 2017: mindfulness‐based cognitive therapy versus waiting list.
-
Hepark 2015: mindfulness‐based cognitive therapy versus waiting list.
-
Hirvikoski 2011: dialectical behavioural therapy‐based skills training versus structured discussion group.
-
Moëll 2015: CBT‐inspired Internet‐based course with support (Living Smart) versus waiting list.
-
Pettersson 2017: Internet CBT in a self‐help format (iCBT‐S) versus waiting list, and Internet CBT plus weekly group therapy sessions (iCBT‐G) versus waiting list.
-
Schoenberg 2014: mindfulness‐based cognitive therapy versus waiting list.
-
Solanto 2010: meta‐cognitive therapy versus supportive therapy.
-
Stevenson 2002: standard CBT versus waiting list.
-
Virta 2010: standard CBT versus no treatment.
-
-
CBT combined with pharmacotherapy versus pharmacotherapy alone.
-
CBT versus other specific interventions.
-
Fleming 2015: dialectical behavioural therapy versus a skills handouts control condition.
-
Safren 2010: standard CBT versus relaxation with educational support.
-
Vidal Estrada 2013: standard CBT plus limited psychoeducation versus psychoeducation.
-
Virta 2010: standard CBT versus cognitive training.
-
Outcome measures
The included trials used a diversity of outcome measures, which made it difficult to make statistical comparisons between treatment regimens. The outcomes were based on clinical assessments by a physician or by self‐report through the use of validated scales.
Primary outcomes
We defined treatment efficacy as an improvement in the core symptoms of ADHD, which was evaluated in terms of specific ADHD symptoms, namely hyperactivity, inattentiveness and impulsivity, using clinical, symptom‐specific scales and scores. The authors used a heterogeneous group of scales for each outcome (see details in Table 3).
| Outcomes | Studies and instruments |
| ADHD symptom severity |
|
| Inattention |
|
| Hyperactivity |
|
| Impulsivity |
|
| Hyperactivity‐impulsivity |
|
| Clinical Global Impression |
|
ADHD: attention deficit hyperactivity disorder; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders ‐ Third Edition ‐ Revised; NIMH: National Institutes of Mental Health.
Secondary outcomes
The authors used a heterogeneous group of scales to assess the secondary outcomes (see details in Table 4).
| Outcomes | Studies and instruments |
| Depression |
|
| Anxiety |
|
| State anger |
|
| Trait anger |
|
| Self‐esteem |
|
| Quality of life |
|
| Employment status | No study included this outcome. |
ADHD: attention deficit hyperactivity disorder.
Excluded studies
We excluded 49 full‐text reports as ineligible for this review for the following reasons.
-
Not an RCT (n = 8).
-
Not CBT (n = 14).
-
Comparison not considered in this review (n = 7).
-
Others (protocols, or not ADHD in adults) (n = 20).
We described seven of these studies, which initially seemed to merit inclusion but on closer inspection did not, in the Characteristics of excluded studies tables. We excluded one study because it compared group psychotherapy versus individual psychotherapy, thereby affecting the comparability of the intervention of interest of our review (Philipsen 2015). We excluded two studies because the authors explained that their goal was to assess the efficacy of mindfulness, in Mitchell 2013, and mindfulness plus virtual reality, in Serra‐Pla 2017, without introducing other treatment modalities such as CBT. We excluded four studies because the comparisons used in these studies did not correspond to the comparisons included in our protocol (Cherkasova 2016; Weiss 2012; Young 2015; Young 2017).
Ongoing studies
We also found five ongoing studies (ISRCTN03732556; NCT02463396; NCT02062411; NCT02210728; NCT02829970). See Characteristics of ongoing studies tables.
Risk of bias in included studies
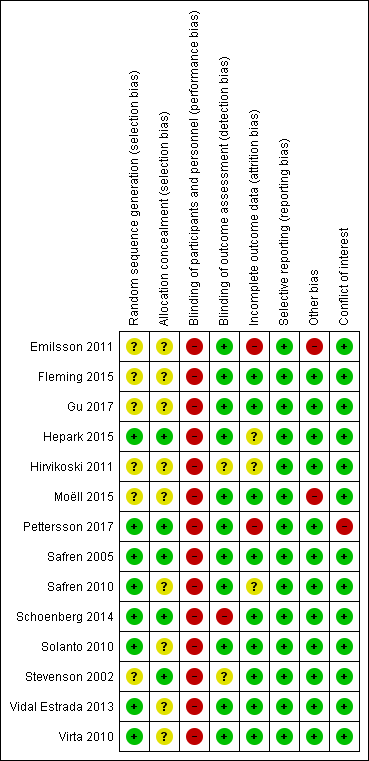
No trial was free from bias across all 'Risk of bias' domains. Authors often described randomisation and allocation concealment processes poorly (See Figure 2 and Figure 3 for 'Risk of bias' graphs). When the authors did not explicitly state the sequence generation method, we asked them for this information through email correspondence (Emilsson 2011; Gu 2017; Hepark 2015; Hirvikoski 2011; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
All included studies were at high risk of performance bias because it is not possible to blind personnel in psychotherapy. For 10 studies, this was the only domain at high risk of bias.
We considered four studies to be at high risk of bias for another domain. Emilsson 2011 was at high risk of attrition and other bias. Pettersson 2017 was at high risk of attrition bias and had conflicts of interest. These two studies had a higher number of domains with high risk of bias. Additionally, we considered Schoenberg 2014 to be at high risk of detection bias because the outcome assessor was not blinded. Finally, we judged Moëll 2015 to be at high risk of other bias.
Allocation
Sequence generation
Eight trials were at low risk of bias (Hepark 2015; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Vidal Estrada 2013; Virta 2010). Six trials were at unclear risk of bias because there was no description of the sequence generation process (Emilsson 2011; Fleming 2015; Gu 2017; Hirvikoski 2011; Moëll 2015; Stevenson 2002).
Allocation concealment
Five trials had an adequate description of the allocation concealment process, and we considered them to be at low risk of bias in this domain (Hepark 2015; Pettersson 2017; Safren 2005; Schoenberg 2014; Stevenson 2002).
We considered the remaining nine trials to be at unclear risk of bias. Solanto 2010 affirmed that individuals were stratified by whether or not they were currently receiving medication for ADHD and otherwise randomly assigned to either the CBT or the support group; however, the authors did not describe how they designed this process. Authors of the eight remaining trials provided no information about the randomisation process (Emilsson 2011; Fleming 2015; Gu 2017; Hirvikoski 2011; Moëll 2015; Safren 2010; Vidal Estrada 2013; Virta 2010).
Blinding
Blinding of participants and personnel
We judged all studies to be at high risk of performance bias because, as usual in psychotherapy, it is not possible to blind the personnel (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Moëll 2015; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
Blinding of outcome assessment
We considered 11 trials to be at low risk of detection bias because the assessors were blinded to group assignment (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Moëll 2015; Pettersson 2017; Safren 2005; Safren 2010; Solanto 2010; Vidal Estrada 2013; Virta 2010).
We considered the risk of detection bias to be unclear in two studies because authors did not adequately describe the blinding of the results (Hirvikoski 2011; Stevenson 2002).
Finally, we rated one study, Schoenberg 2014, at high risk of detection bias because the outcome assessor was not blinded.
Incomplete outcome data
We considered nine trials to be at a low risk of attrition bias since the effect size among the missing outcomes was not enough to have a clinically relevant impact on the observed effect size (Fleming 2015; Gu 2017; Moëll 2015; Safren 2005; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
We judged two studies to be at high risk of attrition bias because the dropout rate was around 40% (Emilsson 2011; Pettersson 2017), and the remaining three were at unclear risk of bias: Hepark 2015 and Safren 2010 performed an intention‐to‐treat (ITT) analysis, but there was unbalanced rate of dropouts, and Hirvikoski 2011 performed ITT analysis, but there was an important rate of dropouts (around 20% per group).
Selective reporting
We considered all trials to be free of reporting bias because the published results corresponded to those expected in these types of studies (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Moëll 2015; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010). Five studies prospectively registered the trial, but it was clear that the published reports included all expected outcomes, including those that were pre‐specified (Emilsson 2011; Moëll 2015; Safren 2005; Safren 2010; Solanto 2010); we assessed whether the outcome measures described in the Methods of the paper were reported in the Results section.
Other potential sources of bias
We considered 12 trials to be either free of other potential sources of bias or as being at low risk of other bias (Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
We considered two trials to be at high risk of other bias (Emilsson 2011; Moëll 2015). Emilsson 2011 did not ask the participants in either condition to refrain from engaging in other interventions during the study period. In Moëll 2015, the authors reported a lack of confirmed ADHD‐diagnoses for some of the participants, and 12% did not receive an ADHD diagnosis after their previous neuropsychiatric assessment and were thus classified as having sub‐clinical ADHD.
Conflicts of interest
One study had conflicts of interest, and we considered it to be at high risk of bias (Pettersson 2017). We rated all remaining studies at low risk of bias for this domain (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Moëll 2015; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
Effects of interventions
See: Summary of findings for the main comparison Cognitive‐behavioural interventions versus unspecific control for attention deficit hyperactivity disorder (ADHD) in adults; Summary of findings 2 Cognitive‐behavioural therapy plus pharmacotherapy versus pharmacotherapy alone for attention deficit hyperactivity disorder (ADHD) in adults; Summary of findings 3 Cognitive‐behavioural therapy versus other non‐pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in adults
CBT versus unspecific control conditions
Primary outcomes
ADHD symptoms
Aggregated ADHD symptoms
Three studies evaluated the effect of CBT on observer‐reported ADHD symptoms against unspecific control conditions (Analysis 1.1). Solanto 2010 compared CBT to supportive therapy for this outcome and found a significant effect of treatment (SMD −0.56, 95% CI −1.01 to −0.12; 81 participants; low‐quality evidence; moderate effect size, see Summary of findings table 1). Two studies (126 participants) comparing CBT to waiting list showed significant effects favouring CBT (SMD −1.22, 95% CI −2.03 to −0.41; I2 = 74%; very low‐quality evidence; large effect size; (see Summary of findings table 1): Hepark 2015 (SMD −0.85, 95% CI −1.30 to −0.40; 83 participants) and Stevenson 2002 (SMD −1.68, 95% CI −2.39 to −0.98; 43 participants). Considering that the results of Hepark 2015 and Stevenson 2002 are expressed as SMDs, in line with the methods described in Measures of treatment effect, we present the results of Solanto 2010 as an SMD (SMD −0.56, 95% CI −1.01 to −0.12) in the forest plot because it is not possible to present different statistical measures in the same graphic.
Seven studies assessed the effect of CBT versus unspecific control conditions on self‐reported ADHD symptoms (Analysis 1.2). Two studies (122 participants) compared CBT with supportive therapy (Hirvikoski 2011; Solanto 2010), finding no significant effect of treatment (SMD −0.16, 95% CI −0.52 to 0.19; I2 = 0%; low‐quality evidence; small effect size; see summary of findings Table for the main comparison). Analysis of the five studies (251 participants) that compared CBT to waiting list revealed a significant effect on this outcome favouring CBT (SMD −0.84, 95% CI −1.18 to −0.50; I2 = 38%; Gu 2017; Hepark 2015; Pettersson 2017; Schoenberg 2014; Virta 2010; moderate‐quality evidence; large effect size; see summary of findings Table for the main comparison).
Disaggregated ADHD symptoms
Two studies evaluated the effect of CBT on clinician‐reported inattention separately (Analysis 1.3). One study, Solanto 2010, compared CBT to supportive therapy using the Adult ADHD Investigator Symptom Rating Scale (AISRS) Inattention subscale (range 0 (best) to 27 (worst)) (MD −2.47 points, 95% CI −4.43 points to −0.51 points; 81 participants), and the other study, Hepark 2015, compared CBT to waiting list using Conners' Adult ADHD Rating Scale ‐ Investigator Rated (CAARS‐INV; range 0 (best) to 27 (worst)) (MD −4.10 points, 95% CI −6.00 points to −2.20 points; 83 participants). Both studies showed a significant effect of treatment.
Four studies (244 participants) compared the effect of CBT on self‐reported inattention to waiting list (Gu 2017; Hepark 2015; Moëll 2015; Schoenberg 2014). The analysis revealed a significant effect in favour of CBT (SMD −1.10, 95% CI −1.37 to −0.82; I2 = 0%; large effect size; Analysis 1.4).
Hepark 2015 assessed clinician‐reported hyperactivity‐impulsivity using the CAARS‐INV (range 0 (best) to 27 (worst)) in the comparison of CBT versus waiting list and found a significant effect of treatment on this outcome (MD −2.50 points, 95% CI −4.63 points to −0.37 points; 83 participants; see the illustrative forest plot in Analysis 1.5).
Four studies (244 participants) compared CBT to waiting list for self‐reported hyperactivity‐impulsivity (Gu 2017; Hepark 2015; Moëll 2015; Schoenberg 2014), finding a significant effect in favour of CBT (SMD −0.60, 95% CI −0.98 to −0.22; I2 = 53%; moderate effect size; Analysis 1.6).
All‐cause treatment discontinuation
Schoenberg 2014 reported excluding two participants because one did not attend the full 12‐week mindfulness‐based cognitive therapy intervention, and the second started extra mindfulness training outside the intervention; nine other participants dropped out of the study due to competing time commitments. Gu 2017 reported that two participants dropped out of mindfulness‐based cognitive therapy after six sessions and did not complete the post‐treatment or follow‐up assessments. There were no dropouts due to adverse events reported for this comparison.
Two of the 21 participants who completed the treatment from a study that evaluated dialectical behavioural therapy reported adverse events to the group leaders at the post‐treatment assessment. Both of them reported anxiety related to separation from the group (Hirvikoski 2011). They had both started pharmacological treatment during the ongoing group treatment. In the control group, two individuals (2/20 who completed the group) reported adverse events to the project leader (TH). These individuals also experienced temporary anxiety due to separation from the group, and they especially missed other participants in the group, rather than group leaders or the sessions themselves. No serious adverse events were reported.
Secondary outcomes
Psychopathology
Depression
Six studies assessed self‐reported depression. One study (81 participants) compared CBT to supportive therapy (Solanto 2010), finding no significant differences (SMD 0.07, 95% CI −0.36 to 0.51; small effect size). The remaining five studies (258 participants) compared CBT to waiting list (Gu 2017; Hepark 2015; Moëll 2015; Pettersson 2017; Virta 2010), finding a significant effect of treatment on self‐reported depression (SMD −0.36, 95% CI −0.60 to −0.11; I2 = 0%; small effect size; see Analysis 1.7).
Anxiety
One study (81 participants) assessed clinician‐rated anxiety using the Hamilton Anxiety Scale (HAM‐A; range 0 (best) to 56 (worst)) (Solanto 2010), finding no significant effect of CBT compared to supportive therapy (MD −0.81 points, 95% CI −3.21 points to 1.59 points; see the illustrative forest plot in Analysis 1.8).
Four studies (239 participants) compared CBT to waiting list for self‐reported anxiety (Gu 2017; Hepark 2015; Moëll 2015; Schoenberg 2014). The analysis revealed a significant effect favouring CBT on this outcome (SMD −0.45, 95% CI −0.71 to −0.19; I2 = 23%; small effect size; Analysis 1.9).
Anger
One study (43 participants) compared CBT to waiting list using the State‐Trait Anger Expression Inventory (STAXI; range 0 (best) to 66 (worst)) (Stevenson 2002), finding a significant effect of treatment on self‐reported state anger (MD −3.30 points, 95% CI −5.62 points to −0.98 points; see the illustrative forest plot in Analysis 1.10) and a non‐significant effect on self‐reported trait anger (MD −3.80 points, 95% CI −7.63 points to 0.03 points; see the illustrative forest plot in Analysis 1.11).
Self‐esteem
Two studies assessed this outcome (Analysis 1.12). One study, Solanto 2010, compared CBT to supportive therapy and found no significant effect of treatment (MD 0.00, 95% CI ‐1.85 to 1.85; 81 participants). The other study, Stevenson 2002, compared CBT to waiting list and found a significant effect favouring CBT (MD 12.40, 95% CI 4.55 to 20.25; 43 participants; large effect size).
Quality of life
Two studies (64 participants) evaluated CBT versus waiting list (Pettersson 2017; Virta 2010), finding no significant effect of treatment on self‐reported quality of life (SMD 0.21, 95% CI −0.29 to 0.71; I2 = 0%; small effect size; Analysis 1.13).
CBT plus pharmacotherapy versus pharmacotherapy alone
Primary outcomes
ADHD symptoms
Aggregated ADHD symptoms
Two studies compared CBT plus pharmacotherapy versus pharmacotherapy alone (Emilsson 2011; Safren 2005). The analysis revealed a significant effect of treatment on clinician‐reported ADHD symptoms (SMD −0.80, 95% CI −1.31 to −0.30; I2 = 0%; 65 participants; Analysis 2.1; very low‐quality evidence; large effect size) and self‐reported ADHD symptoms (MD −7.42 points, 95% CI −11.63 points to −3.22 points; 66 participants; I2 = 0%; Analysis 2.2; low‐quality evidence), as assessed using the Current Symptoms Scale (CSS; range 0 (best) to 54 (worst)). See summary of findings Table 2.
Disaggregated ADHD symptoms
Only one study (35 participants) compared CBT plus pharmacotherapy versus pharmacotherapy alone (Emilsson 2011). The study authors found a significant effect of the combined treatment for self‐reported inattention (MD −4.54 points, 95% CI −7.75 points to −1.33 points; see the illustrative forest plot in Analysis 2.3) but no significant effect for self‐reported hyperactivity‐impulsivity (MD −1.70 points, 95% CI −5.29 points to 1.89 points; see the illustrative forest plot in Analysis 2.4), as assessed using the CSS (range 0 (best) to 54 (worst)).
All‐cause treatment discontinuation
In one study, Emilsson 2011, one participant in the CBT plus pharmacotherapy condition reported severe distress at the end of treatment due to changes in personal circumstances. This participant then received individual treatment and was not assessed at follow‐up. Emilsson 2011 also described that four participants dropped out during the treatment phase without explanation, one dropped out upon moving out of the area, one due to illness in the family and one due to pregnancy. No dropouts due to adverse events were reported for this comparison, and no other study reported dropouts due to serious adverse events.
Secondary outcomes
Psychopathology
Clinical Global Impression
A meta‐analysis of two studies (65 participants) comparing CBT plus pharmacotherapy versus pharmacotherapy alone revealed a significant effect on the Clinical Global Impression scale (CGI; range 1 (best) to 7 (worst)) in favour of the combined treatment (MD −0.75 points, 95% CI −1.21 points to −0.30 points; Emilsson 2011; Safren 2005; I2 = 0%; Analysis 2.5).
Depression
Only one study (31 participants) evaluated clinician‐reported depression using the Hamilton Depression Scale (HAM‐D; range 0 (best) to 52 (worst)) (Safren 2005). The comparison of CBT plus pharmacotherapy versus pharmacotherapy alone showed a significant effect of treatment on this outcome (MD −5.56 points, 95% CI −9.71 points to −1.41 points; Analysis 2.6).
Two studies (66 participants) assessed self‐reported depression using the Beck Depression Inventory (BDI; range 0 (best) to 63 (worst)) (Emilsson 2011; Safren 2005), reporting a significant effect of CBT plus pharmacotherapy compared to pharmacotherapy alone (MD −6.09 points, 95% CI −9.55 points to −2.63 points; I2 = 0%; Analysis 2.7).
Anxiety
One study (31 participants) evaluated clinician‐reported anxiety using the HAM‐A (range 0 (best) to 56 (worst)) (Safren 2005), finding a significant effect favouring combined CBT plus pharmacotherapy compared to pharmacotherapy alone (MD −5.68 points, 95% CI −10.32 points to −1.04 points; Analysis 2.8).
The analysis of self‐reported anxiety in two studies (66 participants) comparing CBT plus pharmacotherapy versus pharmacotherapy alone revealed a significant effect favouring the combined treatment (SMD −0.58, 95% CI −1.08 to −0.08; I2 = 0%; moderate effect size; Emilsson 2011; Safren 2005; Analysis 2.9).
No studies reported data on our other secondary outcomes for this comparison: anger, self‐esteem or quality of life.
CBT versus other specific interventions
Primary outcomes
ADHD symptoms
Aggregated ADHD symptoms
Two studies, Safren 2010 and Virta 2010, assessed CBT compared with other specific non‐pharmacological interventions on clinician‐reported ADHD symptoms. The analysis of this comparison revealed a significant effect favouring CBT (SMD −0.58, 95% CI −0.98 to −0.17; 97 participants; I2 = 0%; Analysis 3.1; low‐quality evidence; moderate effect size; summary of findings Table 3).
Four studies (156 participants) evaluated the outcome of CBT on ADHD symptoms through self‐reported measures (Fleming 2015; Safren 2010; Vidal Estrada 2013; Virta 2010). The analysis showed a significant effect of CBT on self‐reported ADHD symptom severity when compared with other specific non‐pharmacological interventions (SMD −0.44, 95% CI −0.88 to −0.01; I2 = 41%; Analysis 3.2; low‐quality evidence; small effect size; summary of findings Table 3).
Disaggregated ADHD symptoms
Two studies (65 participants) compared self‐reported inattention symptoms separately (Fleming 2015; Vidal Estrada 2013). This comparison showed no significant differences between CBT and other specific interventions (SMD −0.12, 95% CI −0.61 to 0.37; I2 = 15%; small effect size; Analysis 3.3).
Only one study (32 participants) compared CBT to psychoeducation using the Conners' Adult ADHD Rating Scale ‐ Self‐Reported (CAARS‐SR; range 0 (best) to 52 (worst)) (Vidal Estrada 2013), finding no significant effect of treatment for self‐reported hyperactivity (MD 1.72 points, 95% CI −4.41 points to 7.85 points; see the illustrative forest plot in Analysis 3.4) or self‐reported impulsivity (MD 2.84 points, 95% CI −3.26 points to 8.94 points; Analysis 3.5).
All‐cause treatment discontinuation
One study, Vidal Estrada 2013, reported that in the psychoeducation group, one participant dropped out after five sessions because of timetable incompatibilities, and one participant was lost to follow‐up because he did not turn up for the post‐treatment assessment. In the CBT group, one participant dropped out because of illness at session six, and three were lost at follow‐up because they missed the post‐treatment evaluation. There were no dropouts due to adverse events reported for this comparison, and no other study reported adverse events for this comparison.
Secondary outcomes
Psychopathology
Clinical Global Impression
Two studies assessed psychopathology using the clinician‐reported CGI scale (range 1 (best) to 7 (worst)). Safren 2010, reported no significant effect when comparing CBT to relaxation plus educational support (MD −0.53 points, 95% CI −1.09 points to 0.03 points; 78 participants), nor did Vidal Estrada 2013, when comparing CBT to psychoeducation (MD 0.18 points, 95% CI −0.19 points to 0.55 points; 32 participants). See Analysis 3.6.
One study (32 participants) assessed psychopathology using the self‐reported CGI scale (range 1 (best) to 7 (worst)) but found no significant effect when comparing CBT to psychoeducation (Vidal Estrada 2013): MD 0.29 points, 95% CI −0.32 points to 0.90 points; see the illustrative forest plot in Analysis 3.7).
Depression
Three studies (84 participants) comparing CBT to other specific non‐pharmacological interventions reported non‐significant effects on self‐reported depression (SMD −0.27, 95% CI −0.70 to 0.16; I2 = 0%; small effect size; Fleming 2015; Vidal Estrada 2013; Virta 2010; Analysis 3.8).
Anxiety
Two studies (65 participants) comparing CBT to other specific non‐pharmacological interventions reported non‐significant effects of treatment on self‐reported anxiety (SMD −0.46, 95% CI −0.95 to 0.04; I2 = 0%; moderate effect size; Fleming 2015; Vidal Estrada 2013; Analysis 3.9).
Quality of life
Three studies evaluated this outcome through self‐reported measures. One study, Virta 2010, compared CBT to cognitive training and found a non‐significant effect on self‐reported quality of life (SMD −0.28, 95% CI −1.19 to 0.62; 19 participants; small effect size), as did another study, Vidal Estrada 2013, which compared CBT to psychoeducation (SMD 0.33, 95% CI −0.37 to 1.03; 32 participants; small effect size). In contrast, Fleming 2015, which compared CBT to skills handouts, found a significant effect in favour of CBT (SMD 1.17, 95% CI 0.42 to 1.92; 33 participants; large effect size). See Analysis 3.10.
Sensitivity analyses
Two trials presented with a high risk of attrition bias because they registered an important loss of participants in at least one group and/or no imputation (Emilsson 2011; Pettersson 2017).
CBT versus unspecific control conditions
ADHD symptoms (self‐reported)
Excluding Pettersson 2017 from Analysis 1.2.2 (subgroup: CBT versus waiting list) did not affect the conclusion (SMD −0.98, 95% CI −1.27 to −0.69; 4 studies, 206 participants; I2 =0%; large effect size) but did increase the I2 for the subgroup differences (analysis not shown).
Depression (self‐reported)
Excluding Pettersson 2017 from Analysis 1.7.2 (subgroup: CBT versus waiting list) did not affect the conclusion (SMD −0.40, 95% CI −0.67 to −0.12; 4 studies, 213 participants; I2 = 0%; small effect size) but did increase the I2 for the subgroup differences (analyses not shown).
Anxiety (self‐reported)
Excluding Pettersson 2017 from Analysis 1.9 (CBT versus waiting list) did not affect the conclusion (SMD −0.52, 95% CI −0.81 to −0.23; 3 studies, 194 participants; I2 = 24%; moderate effect size; analysis not shown).
Quality of life (self‐reported)
Excluding Pettersson 2017 from Analysis 1.13 (CBT versus waiting list) did not affect the conclusion (MD 1.70 points, 95% CI −14.70 points to 18.10 points; rated on Quality of Life Enjoyment and Satisfaction Questionnaire (higher scores indicate greater enjoyment or satisfaction. As Virta 2010 combined the work and school subscale into a work/study subscale, it is difficult to estimate the range) 1 study, 19 participants; analysis not shown).
CBT plus pharmacotherapy versus pharmacotherapy alone
For Analysis 2.1, when we removed Emilsson 2011 due to its dropout percentage, the difference in favour of CBT and pharmacotherapy treatment became non‐significant (SMD −0.60, 95% CI −1.32 to 0.12; 2 studies, 31 participants; I2 = 0%; large effect size). However, in Analysis 2.2 (MD −9.12 points, 95% CI −15.69 points to −2.55 points; rated on CSS (range 0 (best) to 54 (worst)); 2 studies, 31 participants; I2 = 0%), Analysis 2.5 (MD −0.82 points, 95% CI −1.51 points to −0.13 points; rated on CGI (range 1 (best) to 7 (worst)); 2 studies, 31 participants; I2 = 0%), Analysis 2.7 (MD −4.77 points, 95% CI −9.19 points to −0.35 points; rated on BDI (range 0 (best) to 63 (worst); 2 studies, 31 participants; I2 = 0%) and Analysis 2.9 (SMD −0.81, 95% CI −1.54 to −0.07; 2 studies, 31 participants; I2 = 100%; large effect size), removing this study did not have a significant impact on the results of the comparison (analyses not shown).
Following the criteria specified in the Methods section of our protocol (Lopez 2013), we did not perform an analysis of publication bias because the number of included studies per comparison was less than 10 (see Table 2).
CBT versus other specific interventions
There were not enough trials at high risk of attrition bias to run a sensitivity analysis for this comparison.
Discussion
Summary of main results
We identified 14 RCTs that fulfilled the inclusion criteria of this Cochrane Review (Criteria for considering studies for this review). We considered that Stevenson 2002 was had two relevant study reports; although the authors mentioned the use of two different samples, the reported data coincided exactly. Of the records we excluded, one study reported a comparison between group psychotherapy versus individual psychotherapy, affecting the comparability of the intervention of interest in our review (Philipsen 2015). We excluded four other studies because the employed comparison did not correspond to the comparisons included in our protocol (Cherkasova 2016; Weiss 2012; Young 2015; Young 2017), plus two more because their goals were to assess the efficacy of mindfulness and mindfulness plus virtual reality respectively, without introducing other treatment modalities such as CBT (Mitchell 2013; Serra‐Pla 2017).
The primary outcome of the included trials was the effect of different modalities of CBT‐based treatments on the core symptoms of ADHD in adults. The overall findings of this review suggest that CBT‐based treatments may improve the core symptoms of ADHD as assessed both by clinician‐ and self‐report.
The first main comparison was CBT versus unspecific control conditions. In this analysis, CBT was more effective than a waiting list or supportive therapy for improving observer‐rated core symptoms of ADHD. Additionally, CBT was better than waiting list for reducing self‐reported ADHD symptoms but not more effective than supportive therapy. When analysing symptoms of ADHD separately, we found that CBT was superior to supportive therapy regarding clinician‐reported inattention and superior to the waiting list regarding inattention (clinician‐ and self‐reported) and hyperactivity/impulsivity (clinician‐ and self‐reported). With respect to secondary outcomes, we found significant differences from waiting list in depression (self‐rated), anxiety (self‐rated), state anger (self‐rated) and self‐esteem (self‐rated). There were no differences between CBT and supportive therapy or the waiting list in depression (self‐rated). Additionally, we found no differences between CBT and supportive therapy in anxiety (clinician‐rated) or self‐esteem (self‐rated), or between CBT and waiting list in trait anger (self‐rated), self‐esteem (self‐rated) or quality of life (self‐rated).
The second main comparison was CBT plus pharmacotherapy versus pharmacotherapy alone. The results showed that combined treatment could be more effective than pharmacotherapy alone, not only for improving the core symptoms of ADHD but also for reducing the commonly associated symptoms of depression and anxiety (Emilsson 2011; Safren 2005). However, we found no significant differences in self‐reported hyperactivity‐impulsivity when evaluated separately.
The third main comparison of our review was CBT versus other specific interventions. In this comparison, we found that CBT was more effective than the comparison group in terms of improvement in core ADHD symptoms, in both clinician‐ and self‐reported questionnaires. With further analysis, however, we found no significant differences when separately evaluating self‐reported inattention, hyperactivity and impulsivity. Regarding secondary outcomes, we found no differences in self‐reported depression or anxiety and inconsistent results for self‐reported quality of life.
In the first and the third comparisons described above, and with the exception of Moëll 2015, study authors controlled the proportion of participants who received pharmacological treatment in the different groups.
None of the included studies reported severe adverse events in participants receiving the different modalities of CBT, but five participants described some type of adverse event, such as distress and anxiety.
The sensitivity analysis was limited due to the small number of studies for each outcome. However, the direction of the results was consistent with the primary analysis, except for the comparison between CBT plus pharmacotherapy versus pharmacotherapy alone, for the outcome of clinician‐rated ADHD symptoms.
Overall completeness and applicability of evidence
Our search found studies assessing CBT for adults with ADHD, and their reported results provided findings that could be applicable. However, these studies included heterogeneous clinician‐ and self‐reported measures of ADHD symptoms and associated dimensions. This heterogeneity might have an impact on the interpretation of the results.
Furthermore, our review found few trials, concentrated in the northern hemisphere; there is only one trial from the southern hemisphere and none from low‐ or lower‐middle‐income countries. Because of this, we suggest caution regarding the cross‐cultural applicability of the evidence.
We described the effects of interventions as meta‐analyses when possible and at a study level otherwise.
Quality of the evidence
Important methodological limitations reduced the certainty of the evidence offered by most included trials. Many of the trials were small and included different outcome measures, and selective outcome reporting was occasionally an issue.
We assessed the quality of the evidence using the GRADE approach and presented our ratings in 'Summary of findings' tables (see summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). Considering that self‐ and clinician‐reported core symptoms are the main target of CBT, we included them in the 'Summary of findings' tables.
None of the studies were at high risk of bias for random sequence generation, but six of them were at unclear risk because the authors did not describe the sequence generation process. We were able to record this factor as a low risk only when the study authors responded with more information about this via email.
Similarly, reports did not contain an adequate description of the allocation concealment process. We classified nine studies as being at unclear risk of bias. Five studies were at low risk, and we completed most of the information after contacting the study authors via email.
Due to the inherent characteristics of psychotherapy, we considered all studies to be at high risk of performance bias because it is not possible to blind personnel to this type of intervention.
We considered one trial to be at high risk of detection bias because the study authors described that the outcomes were potentially prone to risk of bias due to lack of blinding of the assessor (Schoenberg 2014).
Additionally, we rated two studies at high risk of attrition bias because the dropout rate was around 40% (Emilsson 2011; Pettersson 2017). Three studies were at unclear risk of bias: Hepark 2015 and Safren 2010 performed an ITT analysis, but the number of dropouts was unbalanced between groups; Hirvikoski 2011 performed an ITT analysis, but there was an important rate of dropouts (around 20% per group).
Five studies prospectively registered the trial and reported all planned outcomes (Emilsson 2011; Moëll 2015; Safren 2005; Safren 2010; Solanto 2010). We considered all studies as free of the risk of reporting bias because the published reports clearly included all expected outcomes.
Two studies presented a high risk of 'other bias' (Emilsson 2011; Moëll 2015). Emilsson 2011 reported not asking participants in either group to refrain from engaging in other interventions during the study period; therefore, they could not establish the impact of a possible second intervention. In Moëll 2015, the study authors did not confirm the diagnosis of ADHD for some of the participants after their previous neuropsychiatric assessment.
We were unable to assess publication bias as planned (Lopez 2013), as there were fewer than 10 included studies per comparison. However, the search strategy was very sensitive, and the field is not so large. Therefore, we consider that there is no evidence of publication bias.
Finally, we considered one study, Pettersson 2017, to be at high risk of bias due to conflicts of interest, because the main author was a partner and shareholder in the company that constructed and owned the rights to the Internet‐based treatment programme, In Focus. This author was also involved in the design and construction of the programme.
Potential biases in the review process
We designed the search process in conjunction with a specialised librarian, and the Cochrane Developmental, Psychosocial and Learning Problems Information Specialist supervised it with the objective of minimising bias in the compilation of potentially relevant references.
In all cases, we repeatedly tried to contact the authors of the included studies by email to obtain more information, when needed. In five cases, we did not receive a reply. The replies we did receive helped us to describe the random allocation process and the random sequence generation.
The high number of outcomes analysed could generate type 1 errors, but the consistency in the direction of most results reduces this possibility.
Finally, we did not identify other significant potential biases in the review process.
Agreements and disagreements with other studies or reviews
According to Chandler 2013, there is a need for a meta‐analysis of CBT versus other psychotherapies. To our knowledge, there are three meta‐analyses of RCTs on psychotherapy for adults with ADHD. In the first one (Jensen 2016), the authors only included studies that evaluated the effects of standard CBT, using a more restrictive criteria for CBT treatments. With respect to search results, these authors found a smaller number of studies, and, after full‐text evaluation, they only included two (Emilsson 2011; Safren 2005). The results of their review are similar to the results of our comparison between CBT with pharmacotherapy versus pharmacotherapy alone (which includes these two studies), except for clinician‐reported ADHD symptoms. This difference might be because Jensen 2016 only reported the data from Safren 2005, leaving out the data from Emilsson 2011. When only considering Safren 2005, the results were not significant, but as a whole (including Emilsson 2011), the results were significant.
The second meta‐analysis reported similar findings even though the eight included studies and comparison groups differed from the present review (Young 2016). The first difference between our reviews was that Young 2016 did not include mindfulness‐based cognitive therapy or dialectical behavioural therapy. Second, those authors defined the control conditions as 'inactive' (waiting list or treatment as usual) or 'active' (alternative treatment to CBT). These categories resemble the ones employed in our review (unspecific control conditions and other specific interventions), but the inclusion of studies was not homologous (i.e. Young 2016 classified supportive therapy as an active control, while we considered it an unspecific control condition). Finally, Young 2016 did not discriminate between studies based on whether or not they employed CBT as an adjunctive treatment to pharmacotherapy. Beyond these differences, the overall findings point to the same conclusions.
The third meta‐analysis included controlled and uncontrolled studies, but the authors meta‐analysed controlled and uncontrolled (pre‐ to‐post) effect sizes separately (Knouse 2017). They also included controlled studies that did not use random assignment. Even though the global results were similar, these authors failed to assess the quality of the evidence, limiting the findings of the review.
Additionally, previous non‐systematic reviews on this topic such as Knouse 2010 and Mongia 2012 showed similar results to our findings.
Our results agree with Antshel 2011 and Ramsay 2007, who stated that CBT plus pharmacotherapy improved treatment outcomes more than medication alone; however, these authors found only limited evidence that CBT was efficacious on its own.
In contrast to Philipsen 2012, our results did not clearly show whether specific treatment for ADHD in adults significantly reduced the commonly associated symptoms (depression, anxiety and anger).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
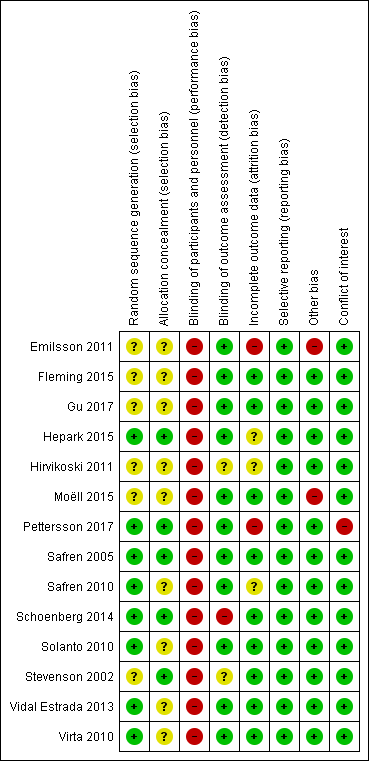
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
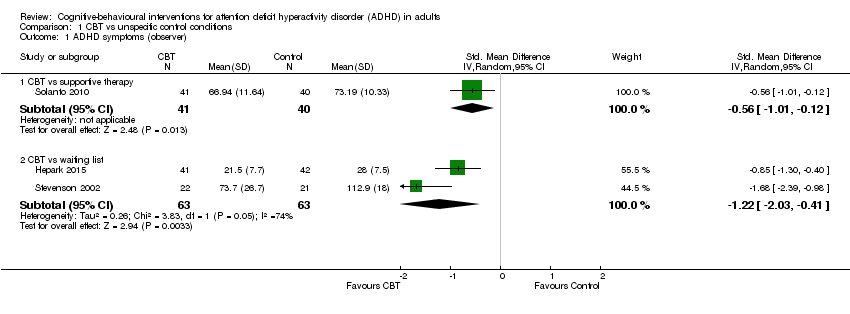
Comparison 1 CBT vs unspecific control conditions, Outcome 1 ADHD symptoms (observer).

Comparison 1 CBT vs unspecific control conditions, Outcome 2 ADHD symptoms (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 3 Inattention (clinician).

Comparison 1 CBT vs unspecific control conditions, Outcome 4 Inattention: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician).

Comparison 1 CBT vs unspecific control conditions, Outcome 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 7 Depression (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 8 Anxiety: CBT vs supportive therapy (clinician).

Comparison 1 CBT vs unspecific control conditions, Outcome 9 Anxiety: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 10 State anger: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 11 Trait anger: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 12 Self‐esteem (self‐reported).
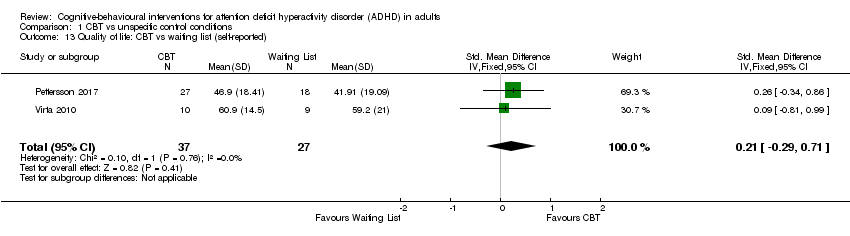
Comparison 1 CBT vs unspecific control conditions, Outcome 13 Quality of life: CBT vs waiting list (self‐reported).
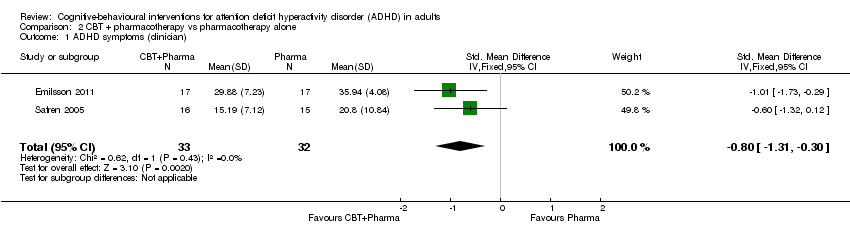
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 1 ADHD symptoms (clinician).
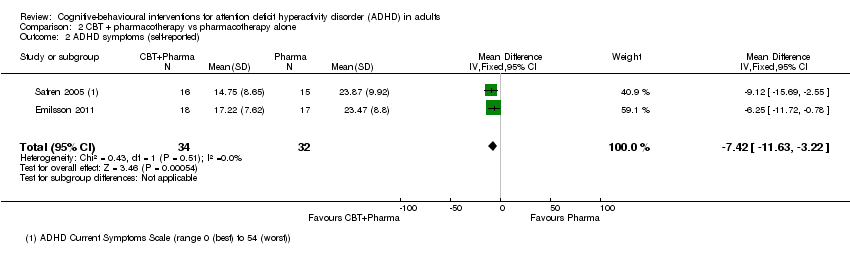
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 2 ADHD symptoms (self‐reported).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 3 Inattention (self‐reported).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 4 Hyperactivity‐impulsivity (self‐reported).
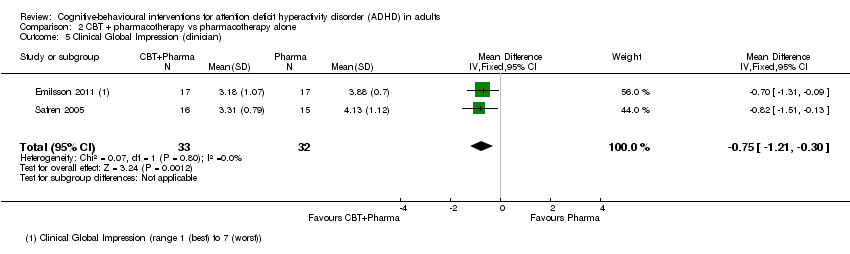
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 5 Clinical Global Impression (clinician).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 6 Depression (clinician).
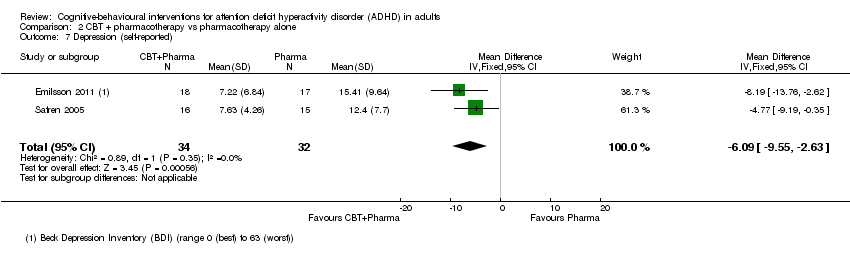
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 7 Depression (self‐reported).
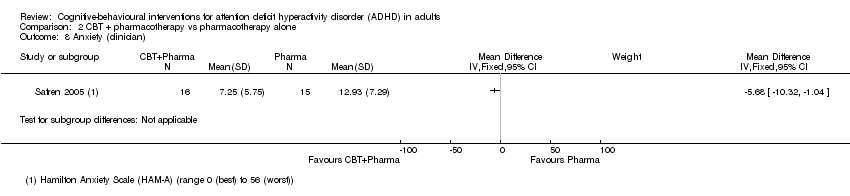
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 8 Anxiety (clinician).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 9 Anxiety (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 1 ADHD symptoms (clinician).

Comparison 3 CBT vs other specific interventions, Outcome 2 ADHD symptoms (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 3 Inattention (self‐reported).
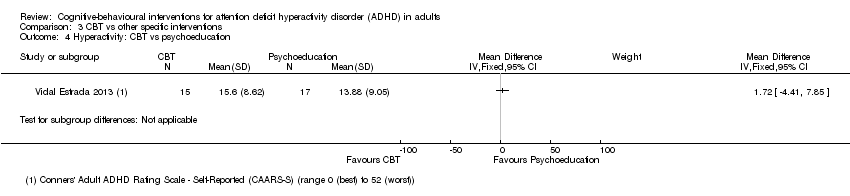
Comparison 3 CBT vs other specific interventions, Outcome 4 Hyperactivity: CBT vs psychoeducation.

Comparison 3 CBT vs other specific interventions, Outcome 5 Impulsivity: CBT vs psychoeducation.

Comparison 3 CBT vs other specific interventions, Outcome 6 Clinical Global Impression (clinician).
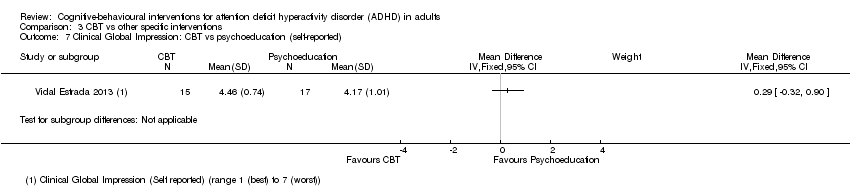
Comparison 3 CBT vs other specific interventions, Outcome 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 8 Depression (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 9 Anxiety (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 10 Quality of life (self‐reported).
| Cognitive‐behavioural interventions versus unspecific control for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| CBT versus supportive therapy | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 12 weeks | — | The mean ADHD observer‐rated symptoms score in the intervention groups was 0.56 standardised deviations lower (1.01 lower to 0.12 lower) | — | 81 | ⊕⊕⊝⊝ | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 12 to 14 weeks | — | The mean ADHD self‐rated symptoms score in the intervention groups was 0.16 standardised deviations lower (0.52 lower to 0.19 higher) | — | 122 | ⊕⊕⊝⊝ | Small effect sizeb |
| CBT versus waiting list control | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 8 to 12 weeks | ‐ | The mean ADHD self‐rated symptoms score in the intervention groups was 1.22 standardised deviations lower (2.03 lower to 0.41 lower) | — | 126 | ⊕⊝⊝⊝ | Large effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks | — | The mean ADHD self‐rated symptoms score in the intervention groups was 0.84 standardised deviations lower (1.18 lower to 0.50 lower) | — | 251 | ⊕⊕⊕⊝ | Large effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded the quality of evidence due to imprecision (considering the width of the CI), methodological limitations (due to high risk of bias in blinding of participants and personnel), and because the evidence is based on a single study. | ||||||
| Cognitive‐behavioural therapy plus pharmacotherapy versus pharmacotherapy alone for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT plus pharmacotherapy | |||||
| ADHD symptoms: clinician rated Assessed by: various scales Follow‐up: 8 to 15 weeks | — | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.80 standardised deviations lower (1.31 lower to 0.30 lower) | — | 65 | ⊕⊝⊝⊝ | Large effect sizec |
| ADHD symptoms: self‐reported Assessed by: ADHD Current Symptoms Scale (range 0 (best) to 54 (worst)) Follow‐up: 8 to 15 weeks | The mean ADHD self‐rated symptoms score in the control groups ranged from 14.75 to 17.22. | The mean ADHD self‐rated symptoms score in the intervention groups was 7.42 lower (11.63 lower to 3.22 lower) | — | 66 | ⊕⊕⊝⊝ | Large effect size |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the quality of evidence due to methodological limitations (high risk of bias in blinding of participants and personnel, and the fact that Emilsson 2011 had a high risk of bias in three domains in one of the two included studies). | ||||||
| Cognitive‐behavioural therapy versus other interventions for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| ADHD symptoms: clinician‐rated Assessed by: various scales Follow‐up: 10 to 12 weeks | — | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.58 standardised deviations lower (0.98 lower to 0.17 lower) | — | 97 | ⊕⊕⊝⊝ | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks | — | The mean ADHD self‐reported symptoms score in the intervention groups was 0.44 standardised deviations lower (0.88 lower to 0.01 lower) | — | 156 | ⊕⊕⊝⊝ | Small effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded the quality of evidence because of imprecision (considering the width of the CI) and methodological limitations (due to the high risk of bias in blinding of participants and personnel and three other domains with unclear risk of bias). | ||||||
| Criteria | Description |
| Random sequence generation (selection bias ‐ biased allocation to interventions ‐ due to inadequate generation of a randomised sequence) |
|
| Allocation concealment (selection bias ‐ biased allocation to interventions ‐ due to inadequate concealment of allocations prior to assignment) |
|
| Blinding of participants and personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the study) |
|
| Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessors) |
|
| Incomplete outcome data (attrition bias due to the amount, nature or handling of incomplete outcome data) |
|
| Selective reporting (reporting bias due to selective outcome reporting) |
|
| Other bias (other sources of bias not captured by the other domains) |
|
| Method | Approach |
| Measures of treatment effect | Dichotomous data We did not find dichotomous data. Should these data become available in future updates of this review, we will calculate the risk ratio (RR) with 95% confidence intervals (CI), as most readers find it easier to understand the RR and 95% CI than the odds ratio and risk difference. |
| Unit of analysis issues | Cluster‐RCTs We did not find cluster‐RCTs. This design is uncommon in this field. Should such studies become available in the future, and if the investigators report cluster‐randomised trial data as though the randomisation was performed on individuals rather than on clusters, we will request individual participant data to calculate an estimate of the intra‐cluster correlation coefficient (ICC). If the individual participant data are not available, we will obtain external estimates of the ICC from similar studies or available resources (Campbell 2000). Once established, we will use the ICC to re‐analyse the trial data to obtain approximate, correct analyses as described in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will combine the effect estimates and their corrected standard errors from cluster‐RCTs with those from parallel‐group designs using the generic inverse variance method (Higgins 2011). If the available information is insufficient to control for clustering in this manner, we will enter the data into Review Manager 5 (RevMan 2014), using individuals as the unit of analysis. We planned to perform sensitivity analyses to assess the potential bias that may occur as a result of the inadequately controlled clustered trials. Additionally, if the ICCs are obtained from external sources, we will perform sensitivity analyses to assess the potential biasing effects of inadequately controlled cluster‐randomised trials (Donner 2001). |
| Cross‐over trials We did not find cross‐over trials. Should we find these types of studies in future updates of this review, and as the duration of any effect of CBT is unknown, we will use only first‐period data from any cross‐over trials that fit the inclusion criteria to avoid a possible carry‐over effect. | |
| Dealing with missing data | If the studies do not report the standard deviation (SD), we will calculate it from the P values, t values, CIs or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). If this information is not reported or is unattainable, we will impute the SD from the study with the highest SD for that outcome, and assess the effects of this assumption on the analysis by conducting a sensitivity analysis. If the outcome data are reported as a median, a range or as a mean without a variance, we will report the data in additional tables. |
| Sensitivity analysis | We will conduct sensitivity analyses to:
|
| CBT: cognitive‐behavioural intervention; CI: confidence interval; ICC: intracluster correlation coefficient; RCT: randomised controlled trial; SD: standard deviation. See also Lopez 2013. | |
| Outcomes | Studies and instruments |
| ADHD symptom severity |
|
| Inattention |
|
| Hyperactivity |
|
| Impulsivity |
|
| Hyperactivity‐impulsivity |
|
| Clinical Global Impression |
|
| ADHD: attention deficit hyperactivity disorder; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders ‐ Third Edition ‐ Revised; NIMH: National Institutes of Mental Health. | |
| Outcomes | Studies and instruments |
| Depression |
|
| Anxiety |
|
| State anger |
|
| Trait anger |
|
| Self‐esteem |
|
| Quality of life |
|
| Employment status | No study included this outcome. |
| ADHD: attention deficit hyperactivity disorder. | |
| Section of Review | Protocol | Full review |
| "The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993). Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Wender 2001; Davidson 2008; Torrente 2011). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood (Polanczyk 2007) and approximately 2.5% in adulthood (Simon 2009)." | "The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993), but the required number of symptoms and the age of onset are different. Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Davidson 2008; Torrente 2011; Wender 2001). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood and around 2.5% in adulthood (Polanczyk 2014; Simon 2009)." | |
| "Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002)." | "Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002). Also, pharmacological treatment is frequently associated with relevant side effects in both children and adults (AJCD 2001; Castells 2013; Cunill 2013; Graham 2011; King 2006; Lim 2006; Morton 2000; Perrin 2008; Prescrire 2007). Due to these concerns, it is important to have non‐pharmacological interventions for treating adults with ADHD." | |
| "To date, no systematic review has examined the effects of CBT in adults with ADHD. The growing number of randomised controlled trials assessing the efficacy of CBT for this population (Knouse 2008) suggest that this review is timely." | "To our knowledge, three systematic reviews have compared the effects of CBT in adults with ADHD (Jensen 2016; Knouse 2017; Young 2016). However, there are important methodological differences between them, also with respect to our review. Both Jensen 2016 and Young 2016 employed more restrictive criteria for defining CBT treatments that excluded relevant CBT variants such as mindfulness‐based cognitive therapy and dialectical behavioural therapy. Knouse 2017 did not report grades of quality of evidence of the included studies." | |
| We considered the following comparisons: Monotherapy
Combined therapy
| We considered the following comparisons:
| |
| We considered psychometrically validated self‐report measures or those completed by an independent rater or relative. The measures were considered short‐ (up to six months), medium‐ (six months to 12 months) and long‐term (more than 12 months)." | "We considered psychometrically validated self‐report measures or those completed by an independent rater or relative. "We presented clinical and self‐reported outcomes separately, as do most studies about this topic, because assessing ADHD is more accurate when symptom information comes from more than one source (Barkley 1998a). "We considered the measures as short term (up to 6 months), medium term (6 months to 12 months) and long term (more than 12 months). "We included studies that assessed at least one primary outcome or at least one secondary outcome." | |
| "We will include studies that have assessed at least one primary or secondary outcome." | "We included studies that assessed at least one primary outcome or at least one secondary outcome." | |
| The safety outcome 'All‐cause treatment discontinuation' was considered as secondary outcome | We considered the safety outcome 'All‐cause treatment discontinuation' to be a primary outcome. | |
| We planned to search Ovid MEDLINE | We used MEDLINE PubMed because of the availability of this interface. | |
| "Additionally, we searched dissertations and abstracts from the following.
| "Additionally, we searched dissertations and abstracts from the following.
| |
| "We independently evaluated the risk of bias using EROS. This process followed the six criteria described in Table 8.5.d, "Criteria for judging risk of bias in the 'Risk of bias' assessment tool" of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (See Table 1)." | "We evaluated the risk of bias in each included trial using the seven criteria described in Table 8.5.d ('Criteria for judging risk of bias in the "Risk of bias' assessment tool") of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (PL and FT) independently assessed each included study as being at low, high or unclear (uncertain) risk of bias for each domain, using EROS software (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010); see Table 1. If there were discrepancies between their assessments, and the two review authors were unable to reach a consensus, a third review author (AC) joined the decision‐making process. All three review authors discussed the issue and made a final decision." | |
| The bands that we reported for I2 were:
| The bands that we reported for I2 were:
| |
| "When we considered studies to be sufficiently homogenous in terms of participants, interventions and outcomes, we synthesised the results in a meta‐analysis using RevMan (RevMan 2014)." | "We synthesised the results in a meta‐analysis using Review Manager 5 (RevMan 5) when we considered studies to be sufficiently homogenous in terms of population (regarding sex, age and diagnosis), interventions (comparable modalities of CBT) and comparisons (as a monotherapy or a part of a combined treatment) to avoid clinical heterogeneity, and in terms of outcome measurement methods to avoid methodological heterogeneity (RevMan 2014). Two authors assessed homogeneity independently and solved discrepancies by consensus." | |
| We did not include a subsection about summary of findings | We included a 'Summary of findings table' subsection: "We prepared a 'Summary of findings' table for our three main comparisons (see Types of interventions) according to GRADE methodology (Atkins 2004; Guyatt 2011), using GRADEpro GDT software (GRADEpro 2015). We included our primary outcome, the core symptoms of ADHD (self‐, clinician‐ or observer‐reported), in the tables. "Two review authors (AC and PL) independently assessed the quality of the evidence as high, moderate, low or very low, downgrading the rating according to the presence of the following criteria: study limitations, in which we considered the studies' 'Risk of bias' level; imprecision; inconsistency of results; indirectness of evidence; and likely publication bias. "To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect." | |
| We considered the type of CBT as a possible subgroup analysis | We did not include the type of CBT as a possible subgroup analysis after we redefined the comparisons. | |
| For this review, we had planned to undertake sensitivity analyses to determine the effect of restricting the analysis to: "(a) only studies with low risk of selection bias (associated with sequence generation or allocation concealment), (b) only studies with low risk of performance bias (associated with issues of blinding), and (c) only studies with low risk of attrition bias (associated with completeness of data). In addition, we will assess the sensitivity of findings to any imputed data within a study." | "[W]e undertook sensitivity analyses to determine the effect of removing from the analysis: studies with high risk of selection bias (associated with sequence generation or allocation concealment); studies with high risk of performance bias (associated with issues of blinding); and studies with high risk of attrition bias (associated with completeness of data). In addition, we assessed the sensitivity of findings to any imputed data within a study." | |
| The transformation of the continuous results to relative percentage changes had not been foreseen. | We included the transformation of continuous results to relative percentage changes in Appendix 2. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (observer) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.01, ‐0.12] |
| 1.2 CBT vs waiting list | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.03, ‐0.41] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 CBT vs supportive therapy | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.52, 0.19] |
| 2.2 CBT vs waiting list | 5 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.18, ‐0.50] |
| 3 Inattention (clinician) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 CBT vs supportive therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.47 [‐4.43, ‐0.51] |
| 3.2 CBT vs waiting list | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.00, ‐2.20] |
| 4 Inattention: CBT vs waiting list (self‐reported) Show forest plot | 4 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.37, ‐0.82] |
| 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported) Show forest plot | 4 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.98, ‐0.22] |
| 7 Depression (self‐reported) Show forest plot | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.36, 0.51] |
| 7.2 CBT vs waiting list | 5 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.60, ‐0.11] |
| 8 Anxiety: CBT vs supportive therapy (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Anxiety: CBT vs waiting list (self‐reported) Show forest plot | 4 | 239 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.71, ‐0.19] |
| 10 State anger: CBT vs waiting list (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Trait anger: CBT vs waiting list (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Self‐esteem (self‐reported) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 CBT vs Supportive Therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.85, 1.85] |
| 12.2 CBT vs Waiting list | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [4.55, 20.25] |
| 13 Quality of life: CBT vs waiting list (self‐reported) Show forest plot | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.29, 0.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (clinician) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.31, ‐0.30] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐7.42 [‐11.63, ‐3.22] |
| 3 Inattention (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Hyperactivity‐impulsivity (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Clinical Global Impression (clinician) Show forest plot | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.21, ‐0.30] |
| 6 Depression (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Depression (self‐reported) Show forest plot | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐6.09 [‐9.55, ‐2.63] |
| 8 Anxiety (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Anxiety (self‐reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.08, ‐0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (clinician) Show forest plot | 2 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.98, ‐0.17] |
| 1.1 CBT vs relaxation + educational support | 1 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.97, ‐0.06] |
| 1.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.78, 0.11] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 4 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.88, ‐0.01] |
| 2.1 CBT vs relaxation + educational support | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.26, ‐0.29] |
| 2.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.10, 0.71] |
| 2.3 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.57, 0.82] |
| 2.4 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.42, ‐0.00] |
| 3 Inattention (self‐reported) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.61, 0.37] |
| 3.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.54, 0.85] |
| 3.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 4 Hyperactivity: CBT vs psychoeducation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Impulsivity: CBT vs psychoeducation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Clinical Global Impression (clinician) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 CBT vs relaxation + educational support | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.09, 0.03] |
| 6.2 CBT vs psychoeducation | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.19, 0.55] |
| 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Depression (self‐reported) Show forest plot | 3 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.70, 0.16] |
| 8.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.32, 0.51] |
| 8.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.59] |
| 8.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐1.04, 0.34] |
| 9 Anxiety (self‐reported) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.95, 0.04] |
| 9.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.04, 0.36] |
| 9.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.27, 0.13] |
| 10 Quality of life (self‐reported) Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐1.19, 0.62] |
| 10.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.37, 1.03] |
| 10.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.42, 1.92] |

