Tratamiento antipalúdico preventivo intermitente para niños con anemia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Trial design: Individually randomized, controlled double‐blind trial Multicentre trial: Yes Trial duration: 2 years | |
| Participants | Recruitment: Children presenting to the out‐patient clinic or ward at the Royal Teaching Hospital, Banjul; Medical Research Council Hospital, Fajara; and major health centres at Birkama, Essau and Faji Kunda during 2003 and 2004 transmission period (July to December), Sibanor was added during the 2004 period. Inclusion criteria:
Other co‐morbidities: Not reported Sample size: 1200 enrolled | |
| Interventions | Total number of intervention groups: 2 Presumptive treatment for all participants:
Interventions:
Dose and timing of intervention:
Duration of intervention period: until the end of the transmission season (July to December) Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): Yes | |
| Outcomes | Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
Interviews with mothers at end of dry season | |
| Notes | Country: The Gambia Setting: Urban and peri‐urban Transmission area: Seasonal transmission Source of funding: Gates Malaria partnership Conflict of interest stated: Authors state that they have no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "children were individually randomised into either the SP or the placebo group in a 1:1 ratio at the time of admission, using permuted blocks of 12 generated by computer using the STATA program. Blockswere not split across centres". |
| Allocation concealment (selection bias) | Low risk | "Tablets (enough for 6 doses) were packed into envelopes bearing the randomisation number by MRC staff not involved in the trial in any other way. The next envelope in sequence was assigned to the child at the time of their admission to hospital". |
| Blinding of participants and personnel (performance bias) | Low risk | "None of the investigators, health care centre staff or laboratory staff participating in the trial had access to the code during the trial". Placebo and active tablet were identical in shape and colour. |
| Blinding of outcome assessment (detection bias) | Low risk | "None of the investigators, health care centre staff or laboratory staff participating in the trial had access to the code during the trial". |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up was similar in both groups (SP: 23%; placebo: 21.5%), but was over 20% in each group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported and checked with protocol. |
| Other bias | Low risk | Discrepancy between text and flow‐chart regarding number of children seen at follow‐up. |
| Methods | Trial design: Population‐based RCT (proof of concept study) Multicentre trial: No Trial duration: 2007 to 2008 | |
| Participants | Recruitment: Eligible children identified through active and passive malaria surveillance in participating communities (West Kiang district, lower river region, The Gambia) Inclusion criteria:
Exclusion criteria:
Other co‐morbidities: Not reported Sample size: Enrolled into trial: 132; randomized to receive IPT/placebo: 96 | |
| Interventions | Baseline treatment for all children:
Total number of intervention groups: 2
Dose and timing of intervention:
Duration of intervention period: 90 days (12 weeks) Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Children with positive tests were treated with either CQ/SP (2007) or ACT (2007 and 2008) Co‐interventions equal in each arm? (if not, describe): Yes | |
| Outcomes | Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
| |
| Notes | Country: The Gambia Setting: Rural Transmission area: Seasonal transmission Source of funding: UK Medical Research Council Conflict of interest stated: Authors stated no competing interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The block randomisation to the post‐malaria treatment of weekly CQ or placebo in both 2007 and 2008 was double blinded and was carried out in blocks of eight. The randomisation codes were generated by a staff member independent of the study team and held by the external trial monitor". |
| Allocation concealment (selection bias) | Low risk | "Treatment codes were labelled A to H and placed in sequentially numbered, opaque, sealed envelopes held by the study nurses. Allocation to the treatment was by matching the code in the envelope to a bottle of the intervention labelled with the same code and then labelled with the subject ID". |
| Blinding of participants and personnel (performance bias) | Low risk | Both participants and personnel were blinded, intervention and placebo syrups were in similar amber‐coloured bottles with matching caps and labels. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded trial, treatment codes held by external trial monitor. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, loss to follow‐up: 2% in IPT group; 7% in placebo group. All lost due to second malaria episode. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported on. |
| Other bias | Low risk | No other sources of bias identified. |
| Methods | Trial design: RCT with 2 X 2 factorial design Multicentre trial: no Trial duration: Children were screened between April to November 1999 | |
| Participants | Recruitment: All resident children in 15 villages in Asembo, Bondo district, Kenya were screened Inclusion criteria:
Other co‐morbidities: Not reported Sample size: 554 participants randomized; 546 enrolled; 491 followed up at 12 weeks; 468 followed up at 24 weeks. | |
| Interventions | Total number of intervention groups: 4 Presumptive treatment for all participants: single dose of SP (500 mg sulphadoxine and 25 mg pyrimethamine per tablet). Children ≤ 10kg received half a tablet, children > 10kg received one tablet
Dose, and timing of intervention: IPT with SP (or placebo) at 4 and 8 weeks; iron (or placebo) given daily for 12 weeks (3 to 6 mg/kg/day, orally). IPT given as crushed tablets mixed with water. Duration of intervention period: 12 weeks Place and person delivering intervention:
Co‐interventions:
Additional treatments: Children with symptomatic malaria (temp ≥ 37.5°C with any malaria parasitaemia or parasitaemia > 5000 parasites/mm3) received oral quinine (10 mg/kg, 3 times/day for 7 days). Children who developed severe malaria, severe anaemia (Hb < 5.0g/dL) or other severe disease requiring hospitalization were referred for further treatment. Co‐interventions equal in each arm? (if not, describe): Yes | |
| Outcomes | Outcomes not specified according to primary and secondary outcomes:
Measurement time points: Every 4 weeks How were outcomes assessed?
| |
| Notes | Country: Western Kenya Setting: Rural Transmission area: Perennial transmission Source of funding: US agency for International Development, Netherlands Foundation for the advancement of Tropical research Conflict of interest stated: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Balanced block randomisation (8 children/block) and a random number listing generated independently before the study". |
| Allocation concealment (selection bias) | Unclear risk | Children were assigned to 1 of the 4 groups sequentially according to the random number listing by one author. Drugs and placebos were identical. Code to true drug and placebo assignment was revealed only after completion of analysis. Still unclear whether allocation was concealed sufficiently – did they use numbered envelopes or bottles? |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo and trial drugs were identical; participants (or their mothers) and staff administering the drugs were thus not aware of the study group. The code was only broken after data analysis. |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo and trial drugs were identical; staff assessing outcomes were thus not aware of the trial group. The code was only broken after data analysis. |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up at 12 weeks: 4% (IPT + iron), 8.6% (iron), 6.6% (IPT) and 20.5% (double placebo). Reported reason: mostly migration (23/28 for double placebo group), loss to follow‐up at 24 weeks – data missing. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not listed in Methods section (protocol?). |
| Other bias | Low risk | No. |
| Methods | Trial design: Randomized double‐blind, placebo‐controlled trial Multicentre trial: Yes Trial duration: June 2006 to August 2009 | |
| Participants | Recruitment: Children were recruited from four hospital in southern Malawi: Queen Elizabeth Central hospital (Blantyre), Chikwawa District hospital, Thyolo District hospital, Zomba Central hospital. Inclusion criteria:
Exclusion criteria:
Other co‐morbidities: 8% of children were infected with HIV Sample size: 1431 randomized, 1414 allocated to groups; analysed 4310 | |
| Interventions | Presumptive treatment for all participants: Six doses of AL as part of the standard 3 day course in hospital. Children < 15 kg received one tablet; children > 15 kg received 2 tablets, once every 12 hours for 3 days. Total number of intervention groups: 2
Dose and timing of intervention:
Duration of intervention period: 3 months Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): Yes | |
| Outcomes | Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
| |
| Notes | Country: Malawi Setting: Urban/peri‐urban/rural Transmission area: Perennial Source of funding: Netherlands African Partnership for Capacity Development and Clinical Interventions against Poverty‐related Diseases; UBS Optimus foundation; Gates Malaria Partnership. Conflict of interest stated: Authors declared no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers, "stratified by hospital and weight group (< 15 kg and 15 kg or more) in randomly varying block sizes of two, four, or six". |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes containing AL or placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | Authors did not mention that placebo and AL were identical tablets, but described the trial as "double‐blind". |
| Blinding of outcome assessment (detection bias) | Low risk | Masking was maintained and the code only broken once all data sets were closed. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for. Loss to follow‐up rates similar across groups (7% at 6 months). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported on. |
| Other bias | Low risk | No. |
| Methods | Trial design: Randomized double‐blind trial Multicentre trial: No Trial duration: March 1998 to May 1998 | |
| Participants | Recruitment: Children living in refugee camp diagnosed with clinical anaemia Inclusion criteria:
Exclusion criteria
Other co‐morbidities: Hookworm Sample size: 238 randomized, 215 analysed | |
| Interventions | Presumptive treatment for all participants:
Total number of intervention groups: 3
Dose, and timing of intervention:
Duration of intervention period: 3 months Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): No | |
| Outcomes | Outcomes not specified as primary or secondary outcomes
Measurement time points:
How were outcomes assessed?
| |
| Notes | Country: Tanzania Setting: Rural Transmission area: Not reported Source of funding: Woodruff Foundation, Atlanta, Georgia. Conflict of interest stated: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Personnel distributing medications had access to the list of group assignment; group 1 did not receive SP placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | "The study coordinator and the study nurse, who were responsible for distributing medications, were the only team members with access to the register containing participants' names and group assignment. All other research team members were blinded to participants’ treatment group assignment". Laboratory workers were not aware of group assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | All participants accounted for. Loss to follow‐up rates: Group 1: 6%, Group 2: 10%; Group 3: 13% reasons for loss to follow‐up not stratified according to groups. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported on. |
| Other bias | Low risk | No. |
| Methods | Trial design: Double‐blind, placebo controlled trial with a 2x2 factorial design Multicentre trial: No Trial duration: 1998 to 2000 | |
| Participants | Recruitment: Children were recruited (randomly selected) from communities neighbouring the research clinic in the Mtito Andei Division, Eastern Province, Kenya at the start of the rainy season Inclusion criteria:
Other co‐morbidities: Not reported Sample size: 328 randomized, 307 analysed | |
| Interventions | Total number of intervention groups: 4
Dose, and timing of intervention:
Duration of intervention period: 12 weeks Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): Yes | |
| Outcomes | Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
Surveillance and monitoring of illness episodes and adverse effects at twice weekly meetings with community healthworker (questionnaires) | |
| Notes | Country: Kenya Setting: Unclear – rural? Transmission area: Seasonal transmission Source of funding: Netherlands foundation for the Advancement of Tropical Research Conflict of interest stated: Declared no conflicts. Author contacted for follow‐up Hb concentration values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation schedule was generated by one of us (HV) for each block, by means of tables with randomised permutations, and only after acceptance of all children making up a block". |
| Allocation concealment (selection bias) | Unclear risk | "The order of the children listed in each block was concealed from the person generating the allocation schedule" – unclear, was this not the same person? |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded, placebos and active compounds were indistinguishable in taste and appearance. |
| Blinding of outcome assessment (detection bias) | Low risk | None of the field investigators was aware of the code until after crude analysis and a plan for further analysis had been prepared. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for. Loss to follow‐up rates similar across groups. |
| Selective reporting (reporting bias) | High risk | Do not report on mean Hb concentrations (primary outcome), only on the difference in Hb concentration between groups. |
| Other bias | Low risk | No. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Intervention is chemoprophylaxis, not IPT. | |
| Anaemia not part of inclusion criteria ‐ study assessed effects on preventing anaemia. | |
| Anaemia not part of inclusion criteria. | |
| Anaemia not part of inclusion criteria. | |
| Anaemia not part of the inclusion criteria. | |
| No control group ‐ comparing two malaria treatment regimes. | |
| Anaemia not part of the inclusion criteria. | |
| Subanalysis of Desai 2003 KEN. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality plus hospital admissions at 6 months Show forest plot | 3 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| Analysis 1.1  Comparison 1 IPT versus placebo, Outcome 1 All‐cause mortality plus hospital admissions at 6 months. | ||||
| 1.1 Iron | 2 | 1474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.67] |
| 1.2 No iron | 2 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
| 2 Children with anaemia at 12 weeks Show forest plot | 4 | 2237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| Analysis 1.2 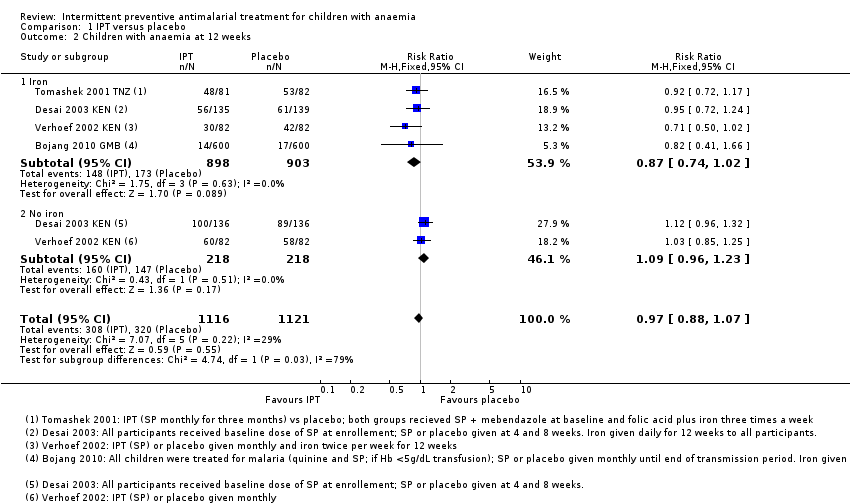 Comparison 1 IPT versus placebo, Outcome 2 Children with anaemia at 12 weeks. | ||||
| 2.1 Iron | 4 | 1801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 2.2 No iron | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.23] |
| 3 Mean change in Hb (baseline to 12 weeks) Show forest plot | 4 | 1672 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.19, 0.45] |
| Analysis 1.3  Comparison 1 IPT versus placebo, Outcome 3 Mean change in Hb (baseline to 12 weeks). | ||||
| 3.1 Iron | 3 | 1341 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.15, 0.45] |
| 3.2 No iron | 2 | 331 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.12, 0.65] |
| 4 Mean Hb at 12 weeks Show forest plot | 4 | 1672 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| Analysis 1.4 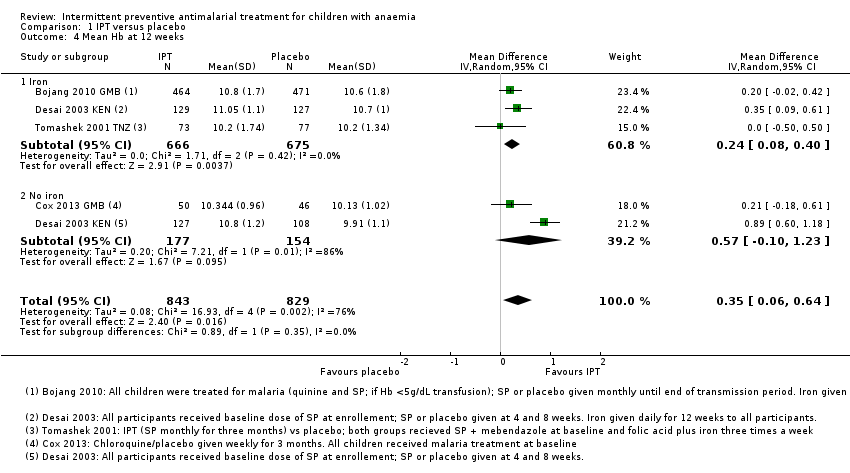 Comparison 1 IPT versus placebo, Outcome 4 Mean Hb at 12 weeks. | ||||
| 4.1 Iron | 3 | 1341 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 4.2 No iron | 2 | 331 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.10, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality plus hospital admissions at 6 months Show forest plot | 3 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| Analysis 2.1 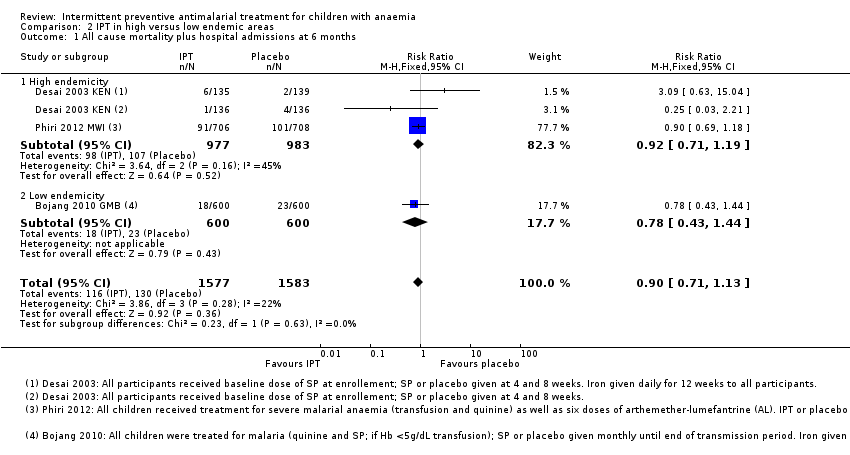 Comparison 2 IPT in high versus low endemic areas, Outcome 1 All cause mortality plus hospital admissions at 6 months. | ||||
| 1.1 High endemicity | 2 | 1960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 1.2 Low endemicity | 1 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.44] |
| 2 Children with anaemia at 12 weeks Show forest plot | 4 | 2237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| Analysis 2.2  Comparison 2 IPT in high versus low endemic areas, Outcome 2 Children with anaemia at 12 weeks. | ||||
| 2.1 High endemicity | 2 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 2.2 Low endemicity | 2 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
| 3 Mean change in Hb (baseline to 12 weeks) Show forest plot | 4 | 1672 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.19, 0.45] |
| Analysis 2.3  Comparison 2 IPT in high versus low endemic areas, Outcome 3 Mean change in Hb (baseline to 12 weeks). | ||||
| 3.1 High endemicity | 2 | 641 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.17, 0.53] |
| 3.2 Low endemicity | 2 | 1031 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.10, 0.48] |
| 4 Mean Hb at 12 weeks Show forest plot | 4 | 1672 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| Analysis 2.4  Comparison 2 IPT in high versus low endemic areas, Outcome 4 Mean Hb at 12 weeks. | ||||
| 4.1 High endemicity | 2 | 641 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.03, 0.91] |
| 4.2 Low endemicity | 2 | 1031 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children with anaemia at 12 weeks Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| Analysis 3.1  Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 1 Children with anaemia at 12 weeks. | ||||
| 2 Mean change in Hb (baseline to 12 weeks) Show forest plot | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.48, 0.48] |
| Analysis 3.2  Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 2 Mean change in Hb (baseline to 12 weeks). | ||||
| 3 Mean Hb at 12 weeks Show forest plot | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| Analysis 3.3  Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 3 Mean Hb at 12 weeks. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Comparison 1 IPT versus placebo, Outcome 1 All‐cause mortality plus hospital admissions at 6 months.
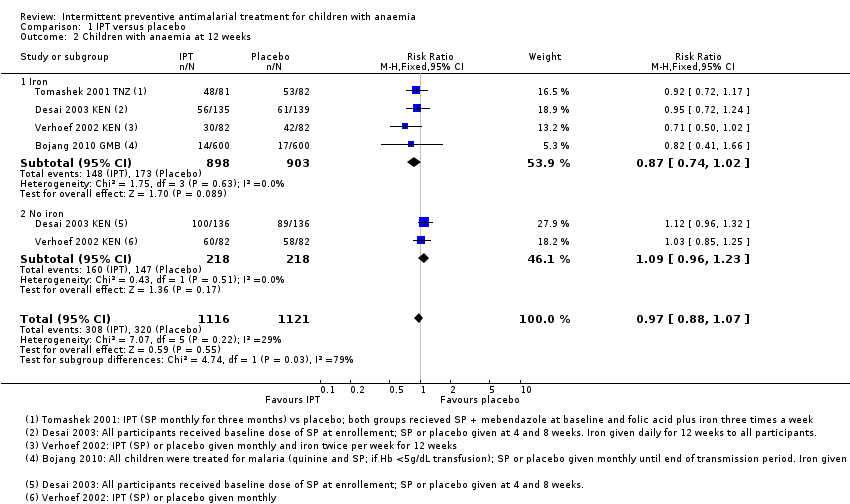
Comparison 1 IPT versus placebo, Outcome 2 Children with anaemia at 12 weeks.
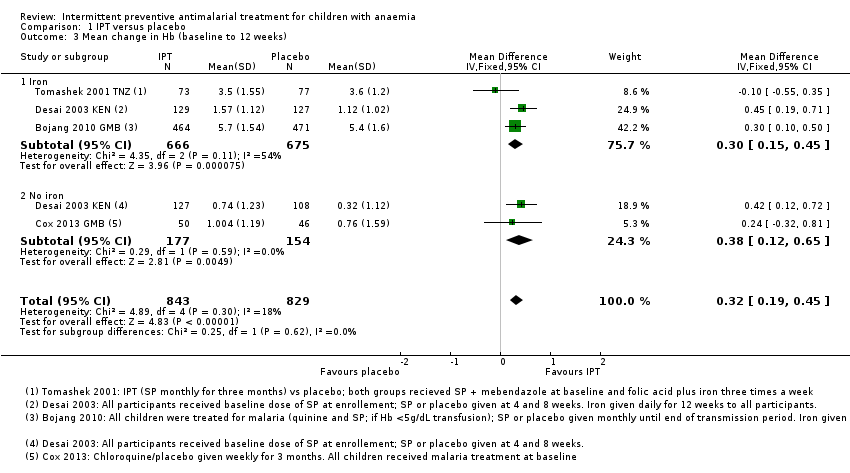
Comparison 1 IPT versus placebo, Outcome 3 Mean change in Hb (baseline to 12 weeks).

Comparison 1 IPT versus placebo, Outcome 4 Mean Hb at 12 weeks.
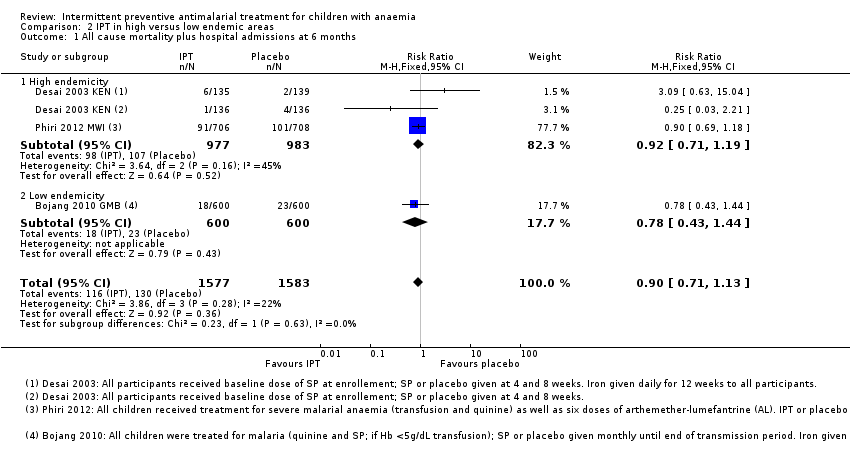
Comparison 2 IPT in high versus low endemic areas, Outcome 1 All cause mortality plus hospital admissions at 6 months.

Comparison 2 IPT in high versus low endemic areas, Outcome 2 Children with anaemia at 12 weeks.
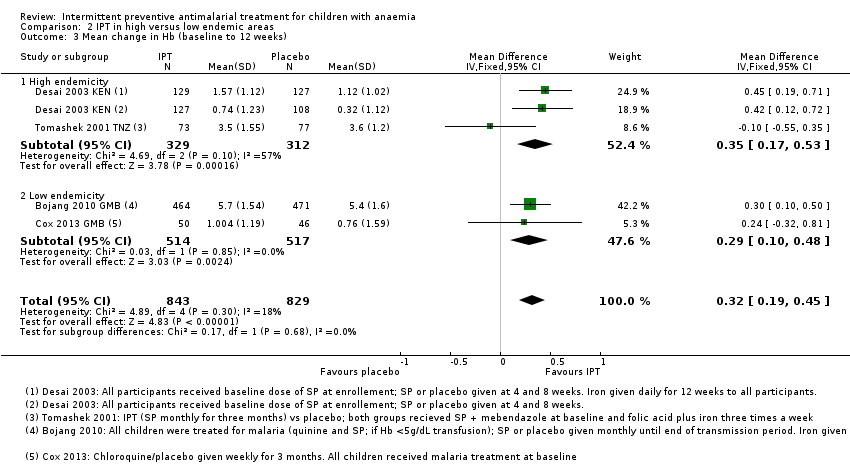
Comparison 2 IPT in high versus low endemic areas, Outcome 3 Mean change in Hb (baseline to 12 weeks).

Comparison 2 IPT in high versus low endemic areas, Outcome 4 Mean Hb at 12 weeks.

Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 1 Children with anaemia at 12 weeks.
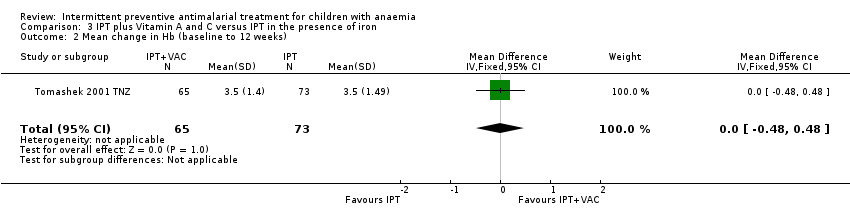
Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 2 Mean change in Hb (baseline to 12 weeks).

Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 3 Mean Hb at 12 weeks.
| Intermittent preventive treatment compared to placebo for children with anaemia | |||||
| Patient or population: Children with anaemia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | IPT | ||||
| Death or hospital admission Follow up at 6 months | 34 per 1000 | 31 per 1000 | RR 0.9 | 3160 | ⊕⊕⊕⊝ |
| Children with anaemia Follow up at 12 weeks | 579 per 1000 | 561 per 1000 | RR 0.97 | 2237 | ⊕⊕⊕⊝ |
| Mean change in Hb from baseline Follow up: 12 weeks | The mean change ranged across control groups from | The mean change in the intervention groups was | ‐ | 1672 | ⊕⊕⊕⊝ |
| Mean Hb Follow up at 12 weeks | The mean Hb concentration ranged across control groups from | The mean Hb concentration in the intervention groups was | ‐ | 1672 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk is the median control group risk across trials. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: The largest trial was at low risk of bias. The two smaller trials were at high risk of attrition bias, but exclusion of these trials does not change the result. 8 No serious imprecision. A small effect was seen although this disappears when we removed trials at high risk of bias from the analysis. | |||||
| Trial | ||||||
| Sample size (n randomized) | 1200 | 96 | 554 | 1431 | 328 | 238 |
| Country | The Gambia (Banjul) | The Gambia | Kenya (Western Kenya) | Malawi (southern Malawi) | Kenya (Eastern province) | Tanzania (Kigoma region) |
| Endemicity | low* | low* | high* | high* | low* | moderate/high (50% parasitaemia)** |
| Age | 3 months to 9 years | 12 to 72 months | 2 to 36 months | 4 to 59 months | 2 to 36 months | 6 to 59 months |
| Anaemia | Hb < 7 g/dL | Hb 69 to 110 g/L | Hb 7.0 to 10.9 g/dL | All children treated for severe malarial anaemia with transfusion and completed the course of intravenous quinine with subsequent Hb > 5g/dL | Hb 60 to 110 g/L | Hb 5.0 to 8.0 g/dL |
| Malaria | Not criteria for inclusion. Children with malaria were treated | Uncomplicated malaria | No malaria (aparasitaemic) or parasite counts < 20,000 parasites/mm3 | No clinical malaria | Not described as part of eligibility | |
| Recruitment | Hospital or OPD admission | Active and passive case finding of children in community | Community: resident children screened | Hospital admissions | Community: randomly selected | Health care worker diagnosed children with clinical anaemia and referred them to the trial |
| Trial intervention |
|
|
|
|
|
|
| Iron and other supplementation during trial period (all participants) | Yes: oral iron for 28 days | No | No ‐ part of trial intervention | Not reported | No – part of trial intervention | Yes: Iron and folic acid for 12 weeks |
| Baseline treatment for all participants | Some were treated with quinine and SP or CQ and SP. Not all children | Either CQ and SP or AL | Single dose of SP | 3 day course of AL | None | Single dose of SP; mebendazole for participants > 12 months |
| *endemicity derived from the Malaria Atlas Project (Gething 2011). **as reported in the trial (Tomashek 2001 TNZ). Abbreviations: Hb: Haemoglobin OPD: Out patient department IPT: Intermittent preventive treatment SP:Sulfadoxine‐pyrimethamine CQ: Chloroquine AL: Arthemeter‐lumefantrine VAC: Vitamin A and C | ||||||
| Outcomes | Trial | ||||||
| Haematological outcomes | Mean Hb at end of follow‐up | Mean Hb level at end of transmission period | ‐ | Hb concentration (measured in g/dL) | ‐ | Hb concentration at the end of follow‐up (12 weeks)* | Mean Hb |
| Mean change of Hb from baseline to follow‐up | ‐ | Mean change of Hb from baseline to follow‐up | ‐ | ‐ | ‐ | ‐ | |
| Children with anaemia at follow‐up | Proportion of children with moderate or severe anaemia at the end of the transmission period* | ‐ | Hematological recovery (Hb ≥ 11g/dL before or at week 12) Severe anaemia (Hb < 7 g/dL before or at week 12) | ‐ | Anaemia (Hb < 11.0 g/dL) | Prevalence of anaemia (Hb < 11.0 g/dL) | |
| Other | ‐ | Change in erythropoietic response Hb change from baseline to follow‐up in two placebo arms to investigate the effect of antimalarial therapy | MCV (measured in fL) sTfR concentration (measured in µg/mL) | ‐ | Iron deficiency (serum ferritin concentration < 12 µg/L) | Mean TfR level Prevalence of iron deficiency (TfR < 8.5 µg/mL) | |
| Malaria outcomes | Clinical malaria | Clinical episodes of malaria during the surveillance period | ‐ | Clinical malaria (axillary temperature 37.5 with co‐existing malaria parasitaemia) | ‐ | Proportion of children with at least one malaria attack (defined as presence of fever, that is temperature ≥ 37.5°C, and a positive dipstick result)* | ‐ |
| Malaria parasitaemia | Prevalence of parasitaemia and splenomegaly | Prevalence of submicroscopic malaria parasitaemia | Prevalence of malaria parasitaemia Parasite density (parasites/mm3) | ‐ | ‐ | ‐ | |
| Hospital admissions or clinic visits related to malaria | ‐ | ‐ | ‐ | Hospital re‐admission because of all‐cause severe anaemia or severe malaria Clinic visits because of microscopically confirmed non‐severe malaria | ‐ | ‐ | |
| Other | ‐ | ‐ | ‐ | ‐ | Time to first occurrence of malaria attack | ‐ | |
| All‐cause mortality and hospital admission | ‐ | ‐ | ‐ | Composite outcome of all‐cause mortality and hospital readmission because of all‐cause severe anaemia or severe malaria between 1 and 6 months* All‐cause hospital admission All‐cause mortality | ‐ | ‐ | |
| Visits to healthcare facilities | Outpatient attendance | ‐ | Clinic visits (incidence, number of episodes) | All‐cause sick child clinic visits | ‐ | ‐ | |
| Other | Nutritional status at the end oft he transmission period Compliance with treatment regimen | Change in urinary neopterin | ‐ | ‐ | Adverse drug reactions | ‐ | |
| *indicates primary outcomes Abbreviations: Hb: haemoglobin TfR: transferrin receptor | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality plus hospital admissions at 6 months Show forest plot | 3 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| 1.1 Iron | 2 | 1474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.67] |
| 1.2 No iron | 2 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
| 2 Children with anaemia at 12 weeks Show forest plot | 4 | 2237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 2.1 Iron | 4 | 1801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 2.2 No iron | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.23] |
| 3 Mean change in Hb (baseline to 12 weeks) Show forest plot | 4 | 1672 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.19, 0.45] |
| 3.1 Iron | 3 | 1341 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.15, 0.45] |
| 3.2 No iron | 2 | 331 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.12, 0.65] |
| 4 Mean Hb at 12 weeks Show forest plot | 4 | 1672 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| 4.1 Iron | 3 | 1341 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 4.2 No iron | 2 | 331 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.10, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality plus hospital admissions at 6 months Show forest plot | 3 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| 1.1 High endemicity | 2 | 1960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 1.2 Low endemicity | 1 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.44] |
| 2 Children with anaemia at 12 weeks Show forest plot | 4 | 2237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 2.1 High endemicity | 2 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 2.2 Low endemicity | 2 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
| 3 Mean change in Hb (baseline to 12 weeks) Show forest plot | 4 | 1672 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.19, 0.45] |
| 3.1 High endemicity | 2 | 641 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.17, 0.53] |
| 3.2 Low endemicity | 2 | 1031 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.10, 0.48] |
| 4 Mean Hb at 12 weeks Show forest plot | 4 | 1672 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| 4.1 High endemicity | 2 | 641 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.03, 0.91] |
| 4.2 Low endemicity | 2 | 1031 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children with anaemia at 12 weeks Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| 2 Mean change in Hb (baseline to 12 weeks) Show forest plot | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.48, 0.48] |
| 3 Mean Hb at 12 weeks Show forest plot | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |

