Productos biológicos, colchicina, corticosteroides, inmunosupresores e interferón alfa para el síndrome neuro‐Behçet
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010729.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esclerosis múltiple y enfermedades raras del sistema nervioso central
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Concept ‐ GF, IT
Title registration ‐ GF, IT, FN, FG
Protocol draft ‐ GF, IT, FN, FG
Protocol editing ‐ GF, IT
Data abstraction ‐ FN, FG
Data entry ‐ FN, FG
Drafting the review ‐ IT, LM
Editing and revising the review ‐ GF, IT, LM, GH, ADB, CM
Sources of support
Internal sources
-
Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta Milano, Italy.
Support the editorial base of "Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group"
External sources
-
Protocol submitted to the independent research programme of the Italian Medicines Agency (AIFA), Italy.
Financially support the reviewers
Declarations of interest
FN ‐ none
FG ‐ none
LM ‐ none
GH ‐ none
ADB ‐ none
CM ‐ none
GF ‐ none
IT ‐ none
Acknowledgements
We thank Peter Tugwell and Richard Wormald for useful comments during the preparation of the manuscript.
We thank Andrea Fittipaldo, Trials Search Co‐ordinator of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group, for her help and support in developing this review.
We thank Yoshifumi Tada (Department of Rheumatology, Faculty of Medicine, Saga University, Saga, Japan), and Corynne Marchal (Managing Editor ‐ Cochrane Lung Cancer Group, Besancon University Hospital ‐ University of Franche‐Comte, CHRU Besançon, France ‐ Service Pneumologie) for having read and extracted data from original articles.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 18 | Biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha for Neuro‐Behçet's Syndrome | Review | Francesca Nava, Francesca Ghilotti, Lorenzo Maggi, Gulen Hatemi, Alessandra Del Bianco, Chiara Merlo, Graziella Filippini, Irene Tramacere | |
| 2013 Sep 05 | Biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha for Neuro‐Behçet's Syndrome | Protocol | Francesca Nava, Francesca Ghilotti, Gulen Hatemi, Alessandra Del Bianco, Chiara Merlo, Graziella Filippini, Irene Tramacere | |
Differences between protocol and review
None.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
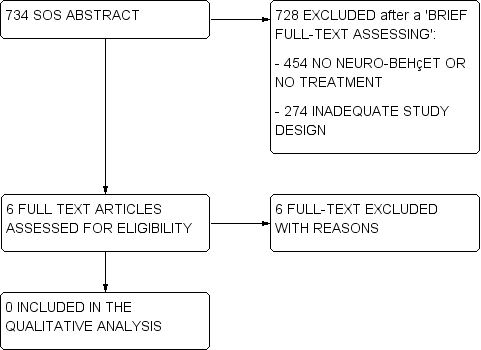
Flow diagram (2).


