Productos biológicos, colchicina, corticosteroides, inmunosupresores e interferón alfa para el síndrome neuro‐Behçet
Resumen
Antecedentes
El síndrome neuro‐Behçet (SNB) es una vasculopatía inflamatoria crónica grave que compromete el sistema nervioso central (SNC), y es un trastorno invalidante que da lugar a discapacidad y a un impacto enorme sobre la calidad de vida. Las recomendaciones sobre los tratamientos para el SNB incluyen el uso de tratamientos que modifican la enfermedad en general, aunque no son apoyadas por una revisión sistemática de las pruebas.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de los tratamientos disponibles para el SNB, incluidos los productos biológicos, la colchicina, los corticosteroides, los inmunosupresores y el interferón alfa.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos hasta el 30 septiembre 2014: Se hicieron búsquedas en el registro especializado de ensayos del Grupo Cochrane de Esclerosis Múltiple y Enfermedades Raras del Sistema Nervioso Central (Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group), CENTRAL, MEDLINE, EMBASE, CINAHL, LILACS, ORPHANET, Clinicaltrials.gov y el World Health Organization (WHO) International Clinical Trials Registry Portal.
Criterios de selección
Los ensayos controlados aleatorios (ECA), los ensayos clínicos controlados (ECC) y los estudios de cohortes controlados prospectivos y retrospectivos eran aptos para evaluar los beneficios. Pacientes a partir de los 13 años de edad con diagnóstico de SNB. Para la evaluación de los efectos perjudiciales, se programó evaluar de forma adicional la extensión de diseño abierto (EDA), los estudios de casos y controles, los registros basados en la población, las series de casos y los informes de casos.
Obtención y análisis de los datos
La selección de estudios, la extracción de datos y la evaluación del riesgo de sesgo se planificó que fueran realizadas de forma independiente por dos autores de la revisión. Se siguieron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane. Se programó realizar metanálisis estándar por pares para los ECA y metanálisis basados en los cálculos ajustados mediante el método del promedio ponderado de la varianza inversa para los estudios no aleatorios (ENA). Se planificó presentar los principales resultados de la revisión en una tabla de "Resumen de los hallazgos" utilizando el enfoque GRADE.
Resultados principales
Ningún ECA, ECC ni estudio controlado de cohortes sobre el beneficio de los tratamientos para el SNB cumplió con los criterios de inclusión de la revisión. Se identificó sólo un estudio potencialmente elegible, aunque no informó detalles suficientes sobre las características de los pacientes. El autor de este estudio no proporcionó los datos adicionales solicitados y, por lo tanto, fue excluido. Por lo tanto, no se incluyeron estudios en la presente revisión. Como no se incluyeron estudios sobre la evaluación de los beneficios, no se realizaron búsquedas adicionales para recopilar datos sobre los efectos perjudiciales.
Conclusiones de los autores
No existen pruebas para apoyar o refutar el beneficio de los productos biológicos, la colchicina, los corticosteroides, los inmunosupresores y el interferón alfa para el tratamiento de los pacientes con SNB. Por lo tanto, se necesitan ECA multicéntricos bien diseñados para informar y guiar la práctica clínica.
PICOs
Resumen en términos sencillos
Modificación de tratamientos como los productos biológicos, la colchicina, los corticosteroides, los inmunosupresores y el interferón alfa para el síndrome neuro‐Behçet
El síndrome neuro‐Behçet (SNB) es un trastorno invalidante con un impacto enorme sobre la calidad de vida. Las recomendaciones sobre los tratamientos para el SNB incluyen el uso de tratamientos que modifican la enfermedad, como los productos biológicos, la colchicina, los corticosteroides, los inmunosupresores y el interferón alfa. Para evaluar sus efectos beneficiosos y perjudiciales, los revisores decidieron realizar una revisión sistemática de los tratamientos disponibles para el SNB. No se encontraron estudios que reunieran los criterios de inclusión de esta revisión, lo cual indica que no existen pruebas para apoyar o refutar el beneficio de estos tratamientos para los pacientes con SNB. Por lo tanto, se necesita más investigación bien realizada antes de poder realizar recomendaciones basadas en pruebas.
Authors' conclusions
Background
Description of the condition
Behçet's Syndrome (BS) is a chronic, relapsing, inflammatory vascular disease characterised by ulcers in the mouth and on the genitals, and inflammation in specific parts of the eye (uveitis) as well as arthritis (swollen, painful, stiff joints), skin problems, and involvement of the digestive tract, brain, and spinal cord. The typical histopathologic feature of BS is a vasculitis affecting veins and arteries of different sizes and presenting mainly with vein thrombosis and, to a lesser degree, arterial aneurysm or thrombosis. Onset most commonly occurs in adults, but paediatric cases have been reported. Both genders are affected equally, but the disease runs a more severe course in males (Yazici 2012). The disease course is characterised by exacerbations and remission ending in a total remission in at least 60% of patients at 20 years of follow‐up (Kural‐Seyahi 2003).
The disease is of unknown origin. There is no clear evidence showing the role of infections in the pathogenesis. A correlation between genetic predisposition and triggering extrinsic factors has been suggested, because more than 60% of BS patients are associated with HLA‐B 51 (Gül 2012; Kose 2012; Yazici 1980). Some clinical features show distinct geographical differences. The prevalence is high in Turkey (> 1/1000 people). Fewer cases of intestinal disease are reported in the Mediterranean area; eye disease causes considerable morbidity in Turkish patients (Kural‐Seyahi 2003; Tugal‐Tutkun 2004), but is rarely a severe problem among Italian (Salvarani 2007) or American patients (Calamia 2009). A positive skin pathergy test is less frequent among patients from northern Europe, America or Japan (Hatemi 2012; Yazici 2012).
The diagnostic criteria for BS were defined by the International Study Group (ISG) for Behçet's Disease in 1990, and include the presence of recurrent oral ulceration, with at least three episodes over 12 months, in addition to two of the following features: recurrent genital ulcers, eye lesions, skin lesions and a positive pathergy test (ISG 1990). An international team (from 27 countries) has recently proposed a revision of the ISG criteria (ICBD 2013), in which eye lesions, oral ulcers and genital ulcers are each assigned two points, while skin lesions, central nervous system (CNS) involvement and vascular manifestations are assigned one point each. The pathergy test, when used, was assigned one point. A patient scoring four or more points is classified as having BS. These new criteria have higher sensitivity over the ISG criteria, but considerably lower specificity (Yazici 2014).
When the disease involves the CNS it is defined as Neuro‐Behçet Syndrome (NBS). Sporadic neurological manifestations are frequent (> 20%), often occurring one to 10 years after initial symptoms, but in some cases neurological symptoms are the first manifestation of BS (Akman‐Demir 1999; Al‐Araji 2009). NBS is more frequent in men than women and it usually occurs at between 20 and 40 years of age ( Al‐Araji 2009; Dalvi 2012).
There are two main categories of NBS that should be considered separately: parenchymal and non‐parenchymal (Serdaroglu 1998). Parenchymal NBS includes the following four syndromes.
-
Brainstem involvement that includes ophthalmoparesis, cranial neuropathy, and cerebellar or pyramidal dysfunction.
-
Cerebral hemispheric involvement that presents with encephalopathy, hemiparesis, hemisensory loss, seizures, dysphasia, cognitive dysfunction and psychosis.
-
Spinal cord involvement that occurs with movement disorders, sensory dysfunctions, and, commonly, sphincter dysfunction.
-
Evidence for cerebral or spinal cord involvement in addition to the brainstem signs and symptoms.
Non‐parenchymal NBS occurs as cerebral venous thrombosis or intracranial and extracranial aneurysm (Al‐Araji 2009). Patients with non‐parenchymal NBS have a significantly better prognosis than those with parenchymal NBS (Siva 2001).
Around a third of NBS patients have single episodes, a third have repeated relapses with remission, and a third undergo a progressive disease course with accrual of multiple functional disability (Akman‐Demir 1999; Al‐Fahad 1999; Kidd 1999; Kural‐Seyahi 2003; Siva 2001). High cellular and/or protein content in the cerebrospinal fluid and parenchymal involvement of the brain, especially of the brainstem, are associated with a worse prognosis (Akman‐Demir 1999).
Description of the intervention
There is no cure for BS. Treatments focus on relieving the symptoms and preventing worsening or complications.
Treatments for BS include: biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha. Corticosteroids are prescribed for rapid suppression of inflammatory process during acute exacerbations, to reduce severe joint pain, skin sores, eye disease, or CNS symptoms. Long‐term use of corticosteroids in addition to immunosuppressants is also reported. However, long‐term corticosteroids may cause several side effects such as diabetes, osteoporosis, weight gain, infections, delayed wound healing, persistent heartburn, elevated blood pressure and mental disorders (Mat 2006). Immunosuppressants such as azathioprine, cyclophosphamide, chlorambucil, cyclosporine‐A, methotrexate, micophenolate, mitoxantrone, levamisole and tacrolimus are used for patients with BS to reduce inflammation, and prevent exacerbations and complications, but they also cause serious adverse events (Hatemi 2008).
In recent years, there has been considerable interest in evaluating the efficacy of biologics for BS. These compounds have been licensed for use in other conditions, e.g. rheumatoid arthritis, psoriasis, psoriatic arthritis and inflammatory bowel disease (Singh 2011). Biologics are a group of medications that suppress the immune system and reduce inflammation. Even though suppressing the immune system can increase the risk of infections, it also helps to stabilise an overactive immune system. The following biologics are used off‐label for patients with BS: anti‐CD20 (rituximab), anti‐interleukin (IL)‐1 (anakinra, canakinumab, gevokizumab, rilonacept), anti‐IL‐2 (daclizumab), anti‐IL‐6 (tocilizumab), and anti‐IL‐17 (secukinumab), and Tumor Necrosis Factor (TNF) inhibitors (adalimumab, etanercept, infliximab). Biologics are administered subcutaneously except for infliximab and rituximab, which are administered as intravenous infusions. Several adverse events such as tuberculosis reactivation with biologics are drug‐specific. However, some adverse events such as increased risk of infection are related to a general immunomodulator or immunosuppressive effect and are common to all biologics (Singh 2011).
How the intervention might work
Immunosuppressants are agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others act through activation of T‐cells or by inhibiting the activation of helper T‐cells, targeting immune mechanisms important in BS pathogenesis (Abbas 2001; Kose 2012).
Biologics are highly specific molecules targeting various immune cells that play a key role in local and systemic inflammation (Singh 2011). Anti‐TNF blockers include both soluble receptors that serve as decoy receptors competing with TNF‐receptors (etanercept) and monoclonal antibodies targeting the TNF‐receptors (adalimumab and infliximab). Rituximab is a monoclonal antibody against CD20, which is found primarily on B‐cells. Clinical and laboratory observations have suggested an important role of TNF‐mediated process in the pathogenesis of BS (Kose 2012). Increased levels of TNF, soluble TNF receptors, and TNF‐producing cells were found in the peripheral blood of patients with active disease. Among inflammatory cytokine‐related genes, TNF blockade reduced expression of IL‐1 receptor type 2, interferon γ receptors, IL‐6 receptors, and IL‐17 receptors (Keino 2011). It was found that infliximab is capable of interfering with gamma delta T cell function in BS characterised by dysregulated cell‐mediated immunity (Accardo‐Palumbo 2010). Arida and colleagues analysed published data on 369 patients treated with either adalimumab, etanercept or infliximab, and reported that the majority of patients showed improvement of their mucocutaneous manifestations (Arida 2011). Rituximab was found effective in retinal vasculitis and ocular manifestations in BS (Davatchi 2010). Rilonacept and canakinumab are human anti‐IL‐1β monoclonal antibodies, targeting a cytokine implicated in the pathogenesis of many inflammatory diseases. Reports from clinical trials suggest that rilonacept and canakinumab are well tolerated in patients with BS and no serious adverse effects were reported (Dubois 2011).
Why it is important to do this review
A Cochrane review, Saenz 1998, on general management of BS was published in 1998 but it did not consider NBS. NBS occurs in about one third of patients affected by BS and, together with gastrointestinal system and blood vessels involvement, seems to represent the main important prognostic factor of BS (Berlit 2010). A systematic review of all available studies is warranted to evaluate the benefit and harms of the treatments for patients with NBS.
Objectives
To assess the benefit and harms of biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha to improve health condition in patients with Neuro‐Behçet Syndrome (NBS).
Methods
Criteria for considering studies for this review
Types of studies
Benefit. Randomised controlled trials (RCTs), controlled clinical trials (CCTs), prospective and retrospective controlled cohort studies were eligible.
We classified a trial as a CCT if the author(s) did not state explicitly that the trial was randomised, but randomisation could not be ruled out. The classification of CCT was also applied to quasi‐randomised studies, where the method of allocation was reported in the primary study, but we did not judge it as strictly random (Higgins 2011).
We classified as a controlled cohort study a study in which a defined group of people (the cohort) was followed over time, to examine associations between different interventions received by patients and subsequent outcomes. A ‘prospective’ cohort study recruits participants before any intervention and follows them into the future. A ‘retrospective’ cohort study identifies subjects from past records describing the interventions received and follows them from the time of those records (Higgins 2011).
In order to be included, cohort studies had to report on a contemporary control group, and both groups needed to be described with sufficient detail to allow assessment of potential 'confounding by indication'. At least baseline characteristics, the treatment regimen and outcome data had to be reported separately for both groups.
Harms. RCTs, CCTs, open‐label extension (OLE), cohort and case‐control studies, population‐based registries, case‐series and case‐reports for each treatment for which benefit was assessed.
Types of participants
Patients over 13 years of age with a diagnosis of NBS (as first clinical manifestations of BS or as complication of BS), according to widely accepted diagnostic criteria, such as the International Study Group for Behcet's disease criteria (ISG 1990), or the International Criteria for Behçet's Disease (ICBD 2013), regardless of disease phase (first attack, recurrent or progressive NBS), patient gender and ethnicity, inpatient or outpatient setting.
Types of interventions
Any treatment compared with any other pharmacological treatment, placebo or no treatment.
-
Biologics: anti‐CD20 (rituximab), anti‐interleukin (IL)‐1 (anakinra, canakinumab, gevokizumab, rilonacept), anti‐IL‐2 (daclizumab), anti‐IL‐6 (tocilizumab), and anti‐IL‐17 (secukinumab), TNF inhibitors (adalimumab, etanercept, infliximab).
-
Colchicine.
-
Corticosteroids.
-
Immunosuppressants (azathioprine, cyclophosphamide, chlorambucil, cyclosporine‐A, methotrexate, micophenolate, mitoxantrone, levamisole, tacrolimus)
-
Interferon‐alpha.
Regimens were included irrespective of their duration and dose, as long as they were within therapeutic range.
Types of outcome measures
Primary outcomes
Benefit
-
Induction or maintenance of remission with disease activity considered as a dichotomous outcome, i.e. sustained‐remission rate and relapse‐free survival (RFS).
-
Change of patient‐reported outcomes (PROs, e.g. Short Form 36, Behcet disease quality of life (Gilworth 2004), or any other PRO validated measures as reported in primary studies).
Harms
-
Withdrawals due to serious adverse events (SAEs).
-
Proportion of patients with at least one of the following adverse events (AEs):
-
-
infections (pneumonia, fungal and opportunistic infections, tuberculosis reactivation);
-
cardiac disorders;
-
all cancers, including lymphomas and leukaemia;
-
mental disorders;
-
any other AE reported in the included studies.
-
Secondary outcomes
-
Time to remission.
-
Overall survival (OS).
-
Physical and cognitive disability measured with validated instruments, such as the Expanded Disability Status Scale (EDSS).
Disease activity, remission, relapse and disability measures could only be included if they had been assessed by validated instruments, as reported in primary studies. SAEs were defined according to the National Cancer Institute Common Terminology Criteria for AE (CTCAE 2003).
Search methods for identification of studies
No language restrictions were applied to the search.
Electronic searches
The Trials Search Co‐ordinator searched the following databases.
-
Trials Specialised Register of The Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (2014, issue 9).
-
MEDLINE (PubMed) (1966 to 30 September 2014).
-
EMBASE (EMBASE.com) (1974 to 30 September 2014).
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (1981 to 30 September 2014).
-
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 to 30 September 2014).
-
ORPHANET (http://www.orpha.net/consor/cgi‐bin/index.php).
-
Clinical trials registries (http://clinicaltrials.gov/).
-
World Health Organization (WHO) International Clinical Trials Registry Portal (http://apps.who.int/trialsearch/).
The keywords used to search for studies for this review are listed in (Appendix 1).
The search terms were adapted to each database, where appropriate.
Searching other resources
-
Screening of reference lists of review articles and primary studies found.
-
Contacted authors and researchers active in this field for additional data when necessary.
Data collection and analysis
Selection of studies
Two review authors (FN, FG) independently identified each potentially‐relevant study obtained from the electronic and manual searches. The first screening was done by reviewing titles and abstracts. The full‐text copies of the selected articles were evaluated during the second screening. We recorded 'excluded studies' and the reasons for exclusion. Disagreements were discussed and resolved by consensus among review authors. We used EndNote (https://www.myendnoteweb.com/) to store references.
Data extraction and management
We planned that two review authors (FN, FG) would independently extract data from the included studies using a standardised Excel form. We intended to contact principal investigators of included studies, when necessary, to request additional data or confirmation of methodological aspects of the study. We planned to extract the following data from the included studies.
-
Study design and year of publication.
-
Participants (sample size of any treatment arm, study setting, demographic and clinical characteristics of participants such as age, gender, ethnicity, presence of other BS manifestations).
-
Details of the experimental and control interventions (type, duration, schedules and dose).
-
Outcomes previously defined (see Types of outcome measures section).
Disagreements were discussed and resolved by consensus among review authors.
Assessment of risk of bias in included studies
We planned to assess the risk of bias for each included study using The Cochrane Collaboration criteria (Higgins 2011). Any discrepancies were to be resolved through discussions among review authors.
1) Criteria for assessing risk of bias in RCTs and CCTs.
For RCTs and CCTs, these criteria included the following domains: random sequence generation, allocation concealment, blinding of personnel, patients and outcome assessors, incomplete outcome data, selective outcome reporting and other biases. The classification of each criterion included three categories: low, high or unclear risk of bias. To summarise the overall risk of bias for a study, we decided to classify RCTs as being at high risk of bias if at least one of the following domains was judged to be at high risk of bias: allocation concealment, blinding of outcome assessors, and incomplete outcome data defined as >15% lost to follow‐up. For CCTs, we decided to classify them as being at high risk of bias if at least one of the following domains was judged to be at high risk of bias: selection of patients, blinding of outcome assessors, and incomplete outcome data defined as >15% lost to follow‐up. RCTs and CCTs had to be classified as being at unclear risk of bias if they presented insufficient information or uncertainty over the potential for bias.
2) Criteria for assessing risk of bias in non‐randomised studies (NRSs).
We planned to assess the risk of bias of non‐randomised studies (NRSs) using the new Cochrane 'Risk of bias' tool for NRSs (Sterne 2013). This tool covers seven domains related to pre‐ and post‐intervention potential biases. Pre‐intervention biases include baseline confounding, selection of participants into the study, and measurement of intervention. Post‐intervention biases include departures from intended interventions, missing data, measurement of outcomes, and selection of the reported result.
We planned to consider the following potential confounding factors: age, gender, ethnicity, clinical phenotypes, disease phase and severity, activity state, treatment phase, and previous treatments.
Each domain‐level 'Risk of bias' judgment includes five categories: low (if the study is comparable to a well‐performed RCT), moderate (if the study is sound for a NRS but cannot be considered comparable to a well‐performed RCT), serious (if the study has some important problems), critical (if the study is too problematic to provide any useful evidence), and no information on which to base a judgment about risk of bias.
The overall risk of bias is judged at low (if all the domains are judged to be at low risk of bias), moderate (if all the domains are judged to be at low or moderate risk of bias), serious (if at least one domain is judged to be at serious risk of bias), critical (if at least one domain is judged to be at critical risk of bias), or no information if there is a lack of information in one or more key domains of bias.
3) Criteria for assessing quality of harm data.
We planned to assess risk of bias for adverse events (AEs) for each included study using the following criteria.
-
Did the researchers actively monitor for AEs (low risk of bias), or did they simply provide spontaneous reporting of AEs that arose (high risk of bias)?
-
Did the authors define SAEs according to an accepted international classification and report the number of SAEs?
Measures of treatment effect
We planned to use dichotomous, continuous, and survival data, and to calculate risk ratios (RRs) for dichotomous, means and standard deviations for continuous outcomes, and hazard ratios (HRs) for time to event data, with the corresponding 95% confidence intervals (CI).
Unit of analysis issues
The unit of analysis was the individual patient.
Dealing with missing data
For missing data, when possible we planned to contact the authors of studies to obtain data, otherwise we planned to perform any appropriate sensitivity analysis with different scenarios (i.e., likely, worst and best‐case scenarios).
Assessment of heterogeneity
We planned to assess clinical homogeneity among studies with respect to age, gender, ethnicity, clinical phenotype, disease phase and severity, activity state, type of immunosuppressant and biologic drugs, concomitant drug including steroids use, treatment phase, previous treatments, and length of follow‐up. We planned to assess statistical heterogeneity using the Chi2 test, I2 statistics (Higgins 2011) and by graphical presentations (forest plot) (Egger 1997).
Assessment of reporting biases
We planned to examine reporting bias as a part of the overall 'Risk of bias' assessment, by comparing outcomes stated in protocols to those reported in article, or by comparing outcomes listed in the methods section to those in results where protocols are not available or cannot be retrieved. If some indications of reporting bias were found, we planned to contact study authors for clarification. Funnel plots (Egger 1997) were planned to be drawn if at least 10 studies were included in the review, to assess the possibility of publication bias. Possible asymmetry of the funnel plot found either by inspection or statistical tests would be taken into account in interpreting the overall estimate of treatment effects.
Data synthesis
We planned to analyse data using Review Manager software (Review Manager 2014) (version 5.3.3), and report them as specified in Chapter 9 (Deeks 2011) and Chapter 13 (Reeves 2011) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) . For NRSs, if they were judged reasonably resistant to bias and relatively homogeneous in this respect, we planned to meta‐analyse adjusted estimates (i.e., analyses that attempt to control for the predefined confounders), using the inverse‐variance weighted average (Reeves 2011).
We planned to perform separate meta‐analyses according to the study design: RCTs, CCTs, prospective and retrospective cohorts. The main analyses were planned to be of biologics, colchicine, corticosteroids, immunosuppressant and interferon‐alpha compared to placebo and to each other.
We planned to compute pooled estimates using a fixed‐effect model, or a random‐effects model in case of heterogeneity (I2 > 50%).
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses based on the following.
-
Age.
-
Gender.
-
Ethnicity.
-
Clinical phenotypes (first attack of NBS, recurrent NBS, progressive NBS, NBS complicating BS).
-
Categories of NBS involvement (parenchymal and non‐parenchymal).
-
Type of immunosuppressant and biologic drug.
-
Concomitant drug including steroids use versus no concomitant therapy.
-
Treatment phase (therapy for induction or maintenance of remission).
-
Previous treatments with immunosuppressants, biologics or interferon‐alpha.
-
Duration of study (< 12 months versus ≥ 12 months).
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the robustness of our review results by repeating the analyses with the exclusion of studies classified as being at high risk of bias.
For missing data, we planned to perform any appropriate sensitivity analysis as mentioned above (see Dealing with missing data section).
'Summary of Findings' table
We planned to present the main results of the review in a 'Summary of Findings' table, as recommended by The Cochrane Collaboration (Schünemann 2011a). Using the GRADE approach (Schünemann 2011b), we defined that the Summary of Findings' table had to include an overall grading of the quality of evidence related to each of the following major outcomes.
-
Induction or maintenance of remission.
-
Change of PROs.
-
Withdrawals due to SAEs.
Results
Description of studies
Results of the search
Flow charts describe the results of the electronic search and article screening for evaluation of benefit (Figure 1, Figure 2). Searches returned a total of 7270 references. After a first screening by reviewing titles and abstracts, the full‐text copies of 11 articles were evaluated to assess for eligibility, but no studies met the inclusion criteria (Figure 1).

Flow diagram.
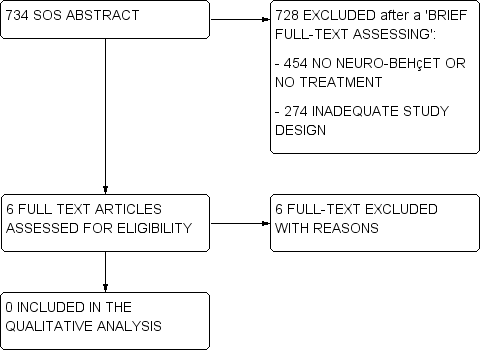
Flow diagram (2).
A total of 734 titles/abstracts did not provide enough information in order to verify the presence of at least the main eligibility criteria on study design, participants and treatment. For these studies (called 'SOS abstract') we performed a brief reading of the full‐text in order to define if they had to be excluded at the first screening (e.g., they did not include NBS patients, treatments of interest, or they had an inadequate study design as case‐series and registries), or if their full‐text had to be assessed for eligibility. Among these 734 SOS abstracts, the full‐text of six articles was assessed for eligibility, but no studies met the inclusion criteria (Figure 2).
Since no studies were included in the assessment of benefit, no further search was performed in order to collect data on harms.
Included studies
No studies were included in the present review, since no RCTs, CCTs or controlled cohort studies on the benefit of the treatments for NBS were found.
Excluded studies
Seventeen studies were excluded from the review (see Characteristics of excluded studies). Sixteen of these studies (Akman‐Demir 1996; Benamour 2006; Borhani Haghighi 2011; Desbois 2012; Dutra 2011; Essaadouni 2010; Farah 1998; Ideguchi 2010; Krupa 2011; Saadoun 2009; Serdaroğlu 1989; Tohmé 2009; Wechsler 1992; Whallett 1999; Yesilot 2007; Yesilot 2009) were excluded since they did not define a control group allowing to assess a causal relationship between intervention and outcome. Only one cohort study (Sbai 2003) defined a control group in order to examine associations between interventions and subsequent outcomes, but different intervention groups were not described with sufficient detail to allow assessment of potential bias due to confounding, and the corresponding data were not provided by the authors. Accordingly, this study (Sbai 2003) had the potential to meet the inclusion criteria: it was a retrospective cohort study on 109 consecutive NBS patients enrolled from 1968 to 2001 at the Pitié Salpetriere Hospital in Paris (France), in which treatment administration together with neurological attacks, disability and remissions were extracted from medical records. However, the authors merely reported that "all patients received colchicine after admission, if they had not before", "103 patients received corticosteroids and 6 did not", and "84 patients received immunomodulatory drugs (50 azathioprine, 57 cyclophosphamide, 12 chloraminophene, 6 methotrexate, 7 cyclosporine, 3 plasma exchange and 1 interferon)". They also reported odds ratios for the associations between treatments and attacks, disability and remissions, but how they managed potential 'confounding by indication', treatment duration, and treatment combinations were not provided.
Risk of bias in included studies
The searches retrieved no trials relevant to this review and thus no assessment of methodological quality was conducted.
Effects of interventions
No data were available for analysis.
Discussion
Summary of main results
No RCTs, CCTs or controlled cohort studies on the benefit of treatments for NBS could be included in this review. Only one potentially eligible study was identified, but since different intervention groups were not described with sufficient detail to allow assessment of potential confounding, and no additional data on treatments combinations were provided on request, this study was excluded (Sbai 2003). Hence, no benefit or harm assessment could be performed.
Overall completeness and applicability of evidence
In view of the lack of evidence available in the literature, well‐designed multicentre RCTs are needed that can inform and guide clinical practice.
Quality of the evidence
There is neither high or low level evidence to support or refute the benefit of biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha for the treatment of patients with NBS.
Potential biases in the review process
Each attempt to limit bias in the review process was made by ensuring a comprehensive search of potentially eligible studies. The authors’ independent assessments of eligibility of studies for inclusion in this review minimised the potential for additional bias.
Agreements and disagreements with other studies or reviews
As already pointed out, there have been no controlled or comparative studies on NBS treatment, thus scanty and not reliable information on treatments for NBS (mostly parenchymal NBS) were provided by case‐series and case‐reports, only. In particular, a few case‐series studies reported that two‐thirds of patients with brainstem or cerebral lesions appeared to benefit from steroids (Akman‐Demir 1999; Al‐Fahad 1999; Kidd 1999; Siva 2001). Some publications indicated advantages for serious manifestations of NBS potentially related to several disease‐modifying therapies, such as azathioprine (Hatemi 2008), micophenolate (Shugaiv 2011), methotrexate (Hirohata 1998; Kikuchi 2003), chlorambucil (O'Duffy 1984), and cyclophosphamide (Ait Ben Haddou 2012). Other studies supported the use of infliximab (Fasano 2011; Giardina 2011; Pipitone 2008; Sarwar 2005), adalimumab (Leccese 2010; Olivieri 2011), etanercept (Alty 2007), tocilizumab (Shapiro 2012), and interferon‐alpha (Nichols 2001) as potential effective alternatives for NBS patients. On the contrary, cyclosporine has been linked with higher risk of NBS development (Akman‐Demir 2008; Kato 2001; Kotake 1999; Kötter 2006).
Our findings are in agreement with recent international consensus recommendations for diagnosis and management of NBS (Kalra 2014). In the aforementioned study, the authors did not find any controlled or comparative trial on the treatment of NBS. Hence, treatment recommendations arising from this international consensus were based on case‐reports, small series of patients or retrospective data from relatively wide, although not controlled, cohorts of patients. Accordingly, they concluded that NBS management recommendations include the use of disease‐modifying therapies in general, although future studies are needed to provide evidence‐based treatments.

